Abstract
Background
We have developed a novel miniature robotic device (HeartLander) that can navigate on the surface of the beating heart through a subxiphoid approach. This study investigates the ability of HeartLander to perform in vivo semiautonomous epicardial injections on the beating heart.
Methods and Results
The inchworm-like locomotion of HeartLander is generated using vacuum pressure for prehension of the epicardium and drive wires for actuation. The control system enables semiautonomous target acquisition by combining the joystick input with real-time 3-dimensional localization of the robot provided by an electromagnetic tracking system. In 12 porcine preparations, the device was inserted into the intrapericardial space through a subxiphoid approach. Ventricular epicardial injections of dye were performed with a custom injection system through HeartLander’s working channel. HeartLander successfully navigated to designated targets located around the circumference of the ventricles (mean path length=51±25 mm; mean speed=38±26 mm/min). Injections were successfully accomplished following the precise acquisition of target patterns on the left ventricle (mean injection depth=3.0±0.5 mm). Semiautonomous target acquisition was achieved within 1.0±0.9 mm relative to the reference frame of the tracking system. No fatal arrhythmia or bleeding was noted. There were no histological injuries to the heart due to the robot prehension, locomotion, or injection.
Conclusions
In this proof-of-concept study, HeartLander demonstrated semiautonomous, precise, and safe target acquisition and epicardial injection on a beating porcine heart through a subxiphoid approach. This technique may facilitate minimally invasive cardiac cell transplantation or polymer therapy in patients with heart failure.
Keywords: computers, heart failure, minimally invasive robotic surgery, myocardium
Myocardial injection therapy, which is based on the rationale of improving cardiac function in situ by introducing tissue-engineered materials (eg, stem cells or biopolymers) into an infarct area, is emerging as a therapeutic strategy for post-myocardial infarct heart failure.1–4 Although this therapy is currently dominated by transcatheter endocardial approaches, direct epicardial injection offers some advantages such as easy detection of target myocardial infarct lesions, decreased likelihood of cerebrovascular complications,5 and superior site specific efficacy.6 These advantages do not apply when making injections into the septal wall, which would require puncturing the healthy ventricular wall from an epicardial approach as opposed to an endocardial approach. The other major drawback to direct epicardial injection is the lack of dedicated minimally invasive access technology, causing it to be performed only in conjunction with other procedures using a full sternotomy or thoracotomy. This approach introduces high associated morbidity despite the intrinsically simple and noninvasive nature of the injection procedure. Ott et al have reported successful robotic-assisted minimally invasive cell transplantation to the epicardia of porcine hearts.7 The DaVinci robotic surgical system, however, requires multiport placement and lung deflation and does not readily facilitate the precise placement and depth of injections with its rigid endoscopic instrumentation. A dedicated technology for precise interaction with the heart from within the intrapericardial space that balances treatment efficacy and minimal invasiveness would benefit direct myocardial injection therapy and the development of intrapericardial therapies in general.
To address this need, we have developed a novel miniature robotic device (HeartLander) that navigates over the epicardial surface to facilitate the delivery of minimally invasive intrapericardial therapy through a subxiphoid approach. Previous prototypes have been tested using both in vitro and in vivo animal preparations. These devices have demonstrated safe remote-controlled navigation over the beating heart8–10 and epicardial lead placement to the anterolateral ventricle.11 To improve the interaction for the surgeon, the HeartLander system now features a real-time 3-dimensional visualization display and semiautonomous navigation to selected targets. This report presents the design of the robotic system and a preliminary evaluation of its ability to perform semiautonomous epicardial injections in a precise pattern on a beating porcine heart through a subxiphoid approach.
Materials and Methods
HeartLander System Overview
The HeartLander system consists of a surgical user interface and patient-side instrumentation. The surgeon interacts with the robotic system through the user interface, which features a 3-dimensional visualization display and both joystick and computer mouse input devices. The patient-side instrumentation translates the commands of the surgeon into the appropriate robotic actions through the support system, which consists of the external motors, vacuum pumps, and computer control system. This instrumentation drives a miniature tethered crawling device located within the intrapericardial space of the patient. This tethered design allows the crawler, the therapeutic portion of the robotic system, to be miniature, lightweight, passive, and disposable.
Design of the HeartLander Crawler
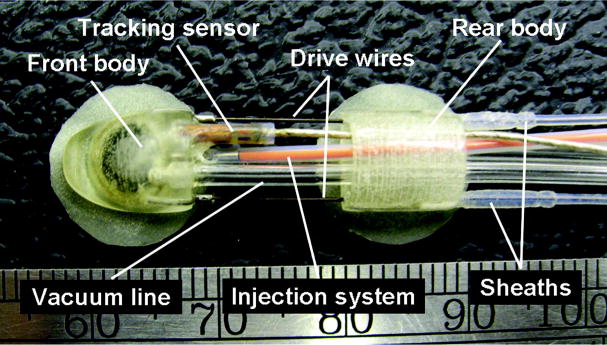
The tethered crawler consists of 2 bodies (front and rear) that each contain an independent suction pad for prehension of the epicardium using vacuum pressure (Figure 1). Each suction pad has a cylindrical shape with a diameter of 6.0 mm and a depth of 3.5 mm, which is integrated into the body (Figure 2). Thin latex skirts surround the periphery of the suction pads to help create a vacuum seal with the epicardium. The crawler bodies are each 5.5×8.0×8.0 mm (height×width×length) and are made of a strong plastic using stereolithography fabrication. These dimensions include a 2-mm diameter working channel and allow the robot to fit through an 8-mm diameter port. The drive transmission that actuates the crawler is comprised of 2 superelastic nitinol wires (0.3 mm in diameter) that are attached to the front body and sheathed within lengths of low-friction plastic tubing that are attached to the rear body (Figure 1). The wires slide freely within the plastic sheaths when driven by the motors located in the support system, modifying the distance and angle between the crawler bodies. Vacuum pressure is regulated by computer-controlled valves in the support system and is supplied to the suction pads via vacuum lines. The vacuum pressure is adjustable up to 600 mm Hg and was set within the range of 500 to 550 mm Hg during in vivo animal testing. The proximal portion of each vacuum line is equipped with a pressure sensor, which measures the pressure in the corresponding suction pad.
Figure 1.
The tethered HeartLander crawler is the therapeutic portion of the robotic system and is driven by offboard instrumentation in the support system. Each body contains a suction pad for gripping the epicardium that is supplied with vacuum pressure through a vacuum line. Two drive wires actuate the crawler. An electromagnetic tracking sensor is mounted to the front body.
Figure 2.
A, The custom remote needle injection system located in the retracted position for safe locomotion over the heart surface. B, The needle (highlighted by the arrow) is now in the extended position for injection into the myocardium.
The locomotion of the HeartLander crawler is a cyclic, inchworm-like process that is coordinated through the computer control system by regulating the wire lengths between the crawler bodies and the vacuum pressure in the corresponding suction pads. To move forward, the wires are extended to advance the front body while the rear body is under active suction. Retracting the wires after active suction has been transferred to the front body then causes the rear body to advance toward the front body. To move backward, this process is reversed. Turning is achieved by advancing the drive wires in different lengths to achieve the desired heading orientation. This low-level coordination is maintained by the computer control system, and thus the details are transparent to the surgeon. Throughout the locomotive cycle, the control system also monitors the data from the vacuum line pressure sensors to ensure that at least one suction pad maintains prehension of the epicardial surface at all times. The driving forces of the motors on the wires are also monitored by the control system using force sensors located within the crawler drive transmission in the support system.
Remote Injection System
A remote injection system for performing epicardial injections from beneath the pericardium has been developed to fit within the working channel of the HeartLander crawler. During locomotion, the 27-gauge needle is safely housed inside the working channel of the front body (Figure 2A). When the crawler reaches the desired target location, the needle is extended into the tissue that has been drawn into the active front body suction pad (Figure 2B). The proximal end of the needle injection system is connected to a syringe. The depth of the needle penetration into the tissue is set by an adjustable mechanical constraint within the range of 1 to 5 mm.
Electromagnetic Tracking System
The 3-dimensional visualization display in the surgeon’s user interface is generated using the data from an electromagnetic tracking system (microBIRD; Ascension Technologies, Burlington, Vt).12 The position and orientation of a miniature tracking sensor, located on the front body of the crawler (Figure 1), are measured in real time with respect to a magnetic transmitter attached to the operating table. This method of tracking does not require a line of sight between the sensor and the transmitter and is thus well suited for tracking tools located inside the body. Due to the fact that HeartLander passively moves with the portion of the epicardium to which it is attached at any given time, the heart surface motion is captured in the tracking data. However, we are primarily concerned with the locomotion of the robot relative to the heart surface, and therefore the physiological motion is filtered out of the tracking data in real time. The user interface software generates a 2-dimensional projection of the 3-dimensional location of the robot and its locomotion trail for the current view angle, which is specified by the surgeon based on his or her desired view (Figure 3A).
Figure 3.

A, A photograph of the interventional suite for a porcine trial. The support system instrumentation box (arrow 1) and HeartLander crawler are mounted on the table above the pig. Real-time feedback of the 3-dimensional visualization is displayed on the surgeon’s monitor (arrow 2), which shows the registered heart surface model, the target, the target path, and the trajectory of the robot. The engineer (E) manages the HeartLander control interface and supports the surgeon (S) by adjusting the control and display parameters under the surgeon’s commands (eg, changing the view angle of the visualization display, decreasing locomotion speed). B, A registered heart surface model. After the target (red dot) has been designated, the target path (gray line) is automatically created along the surface of the heart.
Epicardial Injection Trials
We decompose the acquisition of any general target pattern into 2 distinct sequential tasks: navigating from the initial location to the general vicinity of the targets (navigation task) and performing a series of short, precise motions to each of the individual targets (fine-positioning task). By separating a target pattern acquisition into these 2 tasks, the control system is able to reduce the total procedure time by adjusting the balance between speed and accuracy as warranted by the tasks. Due to the distinct natures of these tasks, we also evaluate each separately in in vivo animal testing.
Animal Preparation
Healthy Yorkshire swine (N=12, body weight of 40 to 50 kg) were anesthetized and placed in a supine position. A small subxiphoid incision (40 mm) and pericardiotomy (15 mm) were created to access the apex of the left ventricle. The HeartLander patient-side instrumentation was mounted on a rack next to the operating table while the user interface was positioned according to the request of the surgeon (Figure 3A). Blood pressure and electrocardiograpms were continuously monitored through the trials. All of the in vivo animal testing was performed with the chest closed and pericardium intact through a subxiphoid approach.
The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (1996).
Construction of the Heart Surface Model
A stationary wire frame computer model of the heart surface was generated for the 3-dimensional visualization display in the user interface. This model provided a reference to localize the robot on the heart during the closed chest animal testing. To capture the structure of the heart surface in the reference frame of the electromagnetic tracking system, the surgeon traced the heart with a tracking probe through the subxiphoid incision. The position data captured from the tracking probe were then used by the HeartLander 3-dimensional visualization software to create a wire frame computer model of the heart surface (Figure 3B). The apex of the left ventricle was marked on the model according to its anatomic location on the porcine heart, which was visible through the subxiphoid incision. Because the heart surface model was defined with respect to the tracking system reference frame, it was automatically registered properly to the robot tracking data. Targets for both the navigation and fine-positioning testing were also defined with respect to the tracking system reference frame and were thus registered to both the heart surface model and robot tracker.
Navigation Testing Protocol
Navigation testing was performed to evaluate the ability of HeartLander to reach general target regions located around the circumference of the ventricles from an initial location proximal to the apex. Before each trial, the surgeon designated the navigation target using the tracked probe while viewing its location on the heart surface model in real time on the 3-dimensional visualization display. The control system then generated a direct navigation path from the registered apex location to the navigation target and displayed the path over the heart surface model (Figure 3A–B). The apex was selected as the origin for all navigation paths because it was easily accessible through the subxiphoid approach. Direct paths to the navigation targets were generated because they minimize the travel distance and the curvature of the tether. The HeartLander crawler was then placed proximal to the origin of the navigation path through the subxiphoid incision by the surgeon. The navigation control system enabled semiautonomous navigation by advancing the robot along the path toward the current target when the surgeon pressed the joystick. The low-level calculations to steer the robot along the path and coordinate the locomotion cycle were handled completely by the control system, whereas the high-level command to proceed toward the target was issued by the surgeon. The 3-dimensional visualization display allowed the surgeon to monitor the progress of the robot in real time by showing the crawler location and trail, the navigation path, and the navigation target over the wire frame heart surface model (Figure 4). When the navigation target was acquired within the distance specified by the surgeon through the user interface, the crawler stopped and locked onto the epicardium with active suction. The surgeon also had the option to override the semiautonomous control by switching to a remote control mode, in which hard-coded locomotion commands were executed according to the joystick input (eg, walk straight, turn left). This alternative control mode was made available for use in the case that the surgeon decided to deviate from the planned navigation path.
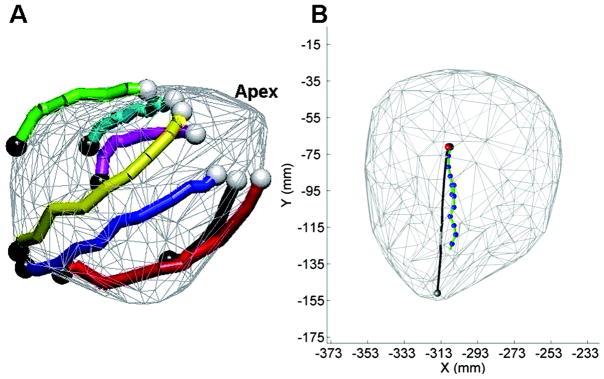
Figure 4.
A, Successful navigation paths over the heart surface model from porcine testing. Access to the entire surface of the heart has been illustrated. The white dots indicate the starting locations, whereas the black dots show the target locations. B, A representative image of a semiautonomous navigation path to a target. The gray dot indicates the heart apex, the gray line indicates the target path, and the red dot shows the target location. The trail of the robot toward the target is shown by the green line and blue dots, whereas the current robot location is shown by the black dot (which is difficult to see because it is aligned with the target location).
Fine-Positioning Testing Protocol
The fine-positioning experiments tested the ability of HeartLander to quickly and accurately acquire multiple targets located in a user-defined pattern. The left ventricle was selected as the location for the testing for all animals due to its clinical significance with regard to heart failure. The surgeon first selected the size and shape of the target pattern and its position and orientation on the left ventricle. The library of injection pattern templates included 2-point linear, 3-point triangular, 4-point rectangular, and 8- or 9-point circular shapes. After the parameters of the target pattern were defined by the surgeon, the targets were fixed in space with respect to the tracking system reference frame. In the fine-positioning mode, the control system advanced the front body of the crawler to align with the current target while the rear body remained fixed to the epicardium. The calculations to align the robot and the target were performed by the control system based on the 3-dimensional location of the robot measured from the tracking sensor and the fixed location of the current target. Target acquisition proceeded as long as the surgeon depressed the joystick until the target was acquired within the distance specified by the surgeon though the user interface. The surgeon monitored the progress of the robot toward the each of the targets in the pattern with the 3-dimensional visualization display. This semiautonomous control paradigm allowed the surgeon to control the entire system at a high level while allowing the computer control system to perform the low-level calculations for the motions for the robot. After each successful target acquisition, the surgeon locked the front body onto the epicardium using active suction and performed an injection of dye into the myocardium with the remote injection system. The surgeon proceeded in this fashion until all targets in the pattern were acquired and marked with dye on the heart surface.
Postmortem Study
The animals were euthanized at the end of each trial. The hearts were first examined in situ to assess damage to noncardiac structures and were then removed. Gross visual inspection was performed from the epicardial vantage to surrounding structures in the mediastinum. The portions of heart tissue along the navigation paths and at the injection sites were stained with hematoxylin and eosin to assess histological injuries.
Statistical Analysis
All values are expressed as the mean±SD.
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Navigation Over the Heart Surface
The HeartLander system took an average of 45 minutes to set up, which was performed by the engineer while the surgeon performed the animal preparation. The creation of the heart surface model for the 3-dimensional visualization display took an additional 15 minutes to prepare. Under semiautonomous control, HeartLander navigated to designated targets located around the circumference of the ventricles (Figure 4A). The average number of navigation trials was 8±1 per animal. The navigation paths of the crawler over the heart averaged 51±25 mm in length with an average navigation speed of 38±28 mm/min. Mean suction pressure and drive wire force over all trials were 562±26 mm Hg and 4.1±0.9 N, respectively. The 3-dimensional visualization display proved sufficient to monitor the progress of the robot during all trials (Figure 4B). Semiautonomous acquisition of the navigation targets was achieved within 1.1±0.9 mm, which was expressed as the final distance between the center of the target and the tracking sensor mounted on the front body. The average duration for the entire navigation testing period was 20 minutes. Thus, the average total experiment time, including setup, was 80 minutes.
Acquisition of Injection Target Patterns Using Fine-Positioning
The fine-positioning control system successfully guided HeartLander in the precise acquisition of the injection target patterns. The injection targets were acquired within an average of 1.0±0.4 mm. The average acquisition time for each individual target within the pattern was 16±12 seconds, excluding injection time. After each successful target acquisition, an injection of 0.1 mL of oil-based dye was injected into the myocardium using the remote injection system through the working channel of the HeartLander crawler. The average number of injections was 5±2 per animal. Injections were successfully demonstrated on the anterior, posterior, and lateral ventricular walls of the beating heart through a subxiphoid approach (Figure 5). With the needle penetration depth set to 3 mm, the average dye penetration depth was 3.0±0.5 mm. The average duration for the entire injection testing period, including the administration of the dye injections, was 25 minutes. Thus, the average total experiment time, including setup, was 85 minutes.
Figure 5.
A–B, The 3-point injection pattern shown on the 3-dimensional visualization display (A) and the corresponding injection sites on the lateral left ventricle of the porcine heart (B). C–D, The 8-point circular pattern shown on the 3-dimensional visualization display (C) and the corresponding injection sites on the posterior left ventricle of the porcine heart.
In the animal model testing of both navigation and fine positioning, we did not observe any occurrences of HeartLander coming loose from the epicardium, failing to engage the epicardium, or requiring repositioning.
Operative Notes and Postmortem Study
All animals tolerated the testing well until planned euthanasia. No adverse hemodynamic or electrophysiological events (eg, hypotension, fatal arrhythmia, bleeding) were noted during any trial, except for occasional self-limiting premature ventricular contractions. The surrounding mediastinal structures (ie, pericardium, phrenic nerve, lungs, pulmonary vein) were intact on postmortem examinations. There were no gross and histological injuries (ie, epicardial delamination, hematoma) to the hearts due to the robot locomotion, suction prehension, or injections.
Discussion
There are myriad opportunities for therapeutic applications within the intrapericardial space, including, but not limited to, cell transplant therapy for heart failure, left atrial appendage ligation, epicardial ablation, device-based mitral valve repair, and epicardial pacemaker lead placement for cardiac resynchronization therapy2,13,14. Recently, several minimally invasive approaches such as traditional and robotic-assisted thoracoscopy have been reported for these therapies7,15,16. These approaches, however, require multiple port placements under general anesthesia with double lumen ventilation. In addition, commercially available rigid endoscopic instruments intrinsically limit the operative field; specifically, it is difficult to access the posterior of the heart, and changing operative sites may require additional incisions and reinsertion of instrumentation. Alternatively, the insertion of HeartLander uses a subxiphoid approach. Our group previously used this approach for intrapericardial access to accomplish endoscopic ligation of the left atrium appendage, pacing lead implantation, and epicardial mapping using a rigid shaft subxiphoid videopericardioscopy device.17–19 The subxiphoid approach is a useful method to access the intrapericardial space because there are no significant anatomic barriers, and it requires only a single port. Deploying a robot with locomotion capabilities through this approach has the potential to obviate general endotracheal anesthesia, lung deflation, and full sternotomy without sacrificing treatment efficacy. In this manner, HeartLander has the potential to enable cardiac surgery on an outpatient basis.
In the present study, HeartLander demonstrated precise, stable, semiautonomous acquisition of predefined target patterns followed by injections on the beating heart through a subxiphoid approach with no adverse events. HeartLander also illustrated the ability to navigate to locations around the circumference of the ventricles, including the posterior ventricular wall, a location that surgeons have some difficulty accessing even under full sternotomy. The combination of the accuracy of the fine positioning within 1 mm of the target, coupled with HeartLander’s ability to navigate to any general region on the heart, results in a complete system for the delivery of precise epicardial injections through a single subxiphoid port. The size, shape, and density of the injection target patterns can be easily adjusted through the user interface according to clinical needs (ie, the shape of myocardial infarct lesions).
The electromagnetic tracking system plays an important role in the HeartLander system. In the present study, the 3-dimensional tracking data were used to generate the real-time 3-dimensional visualization display of the location of the robot with an adjustable viewing angle. This display was adequate for the surgeon to monitor the location of the robot, relative to the heart surface model, as it proceeded toward the targets. This 3-dimensional display technique was easily able to generate any arbitrary viewing angle of the heart surface according to the desire of the surgeon at any given time, which is not possible using traditional 2-dimensional camera-based visualization. Furthermore, the 3-dimensional tracking data from the robot were used by the control system during the calculation of the semi-autonomous navigation and fine positioning.
One of the most important advantages of HeartLander, in addition to providing minimally invasive access and precise intrapericardial therapeutic delivery, is the benefit of passive compensation of the heartbeat motion. By adhering directly to the epicardium, the HeartLander crawler is located in the moving reference frame of the beating heart. By passively synchronizing with the heart, the 3-dimensional locations of the tip of the injection needle and the target remain aligned. Stabilization of the distance between the needle and the heart surface results in reproducible needle penetration depths, whereas stability tangential to the heart surface ensures that the injection is precisely located. Injection methods not synchronizing with the heart motion (eg, manual injection, endoscopic tool injection) suffer with regard to both of these issues. In this manner, HeartLander does not require cardiac stabilization for injection, which significantly reduces the risks of hemodynamic impairment and fatal arrhythmia that can result from the use of commercial mechanical stabilizers to immobilize the surface of the heart.
Nevertheless, there are several limitations in this study that need to be addressed. First, all animal studies were acute and therefore do not address the possibility of delayed mortality and morbidity. Long-term studies will be needed to further prove the safety of the HeartLander system. Second, we used a healthy porcine heart model due to the preliminary nature of this study. Given the irritability of the myocardial infarct heart when subjected to epicardial stimulation, a study using a myocardial infarct model, which better represents the patients targeted for future clinical applications, will be indispensable. HeartLander will also need to cope with adhesions between the pericardium and the epicardium and the presence of epicardial fat in these disease models. Finally, the major limitation of the current real-time 3-dimensional visualization display is that it does not provide detailed anatomic information of the surface of the heart (eg, coronary artery distribution). By integrating technologies that provide high-quality dynamic imaging (eg, 3-dimensional reconstructed CT), the precise injection capability of HeartLander will be greatly improved by allowing the surgeon to recognize anatomic targets and sites that must be avoided during injection (eg, the coronary arteries).
Conclusion
HeartLander demonstrated precise, stable, and safe semiautonomous navigation, target acquisition, and epicardial injection on a beating porcine heart through a subxiphoid approach. This novel paradigm represents a potential advance for the delivery of minimally invasive intrapericardial interventions.
Acknowledgments
The expert assistance of Stacy Cashman, LVT, and David Fischer, BS is gratefully acknowledged.
Sources of Funding
The project described was supported in part by the National Institutes of Health under grant R01 HL078839, the National Aeronautics and Space Administration under grant NNG05GL63H, and the National Science Foundation under grant EEC-9731748.
Footnotes
Presented at the American Heart Association Scientific Sessions, November 4–7, 2007, Orlando, Fla.
Disclosures
N.A.P., C.N.R., and M.A.Z. are coinventors of HeartLander and hold equity in HeartLander Surgical, Inc. (licensee of the IP from Carnegie Mellon University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 2.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 5.Segal AZ, Abernethy WB, Palacios IF, BeLue R, Rordorf G. Stroke as a complication of cardiac catheterization: Risk factors and clinical features. Neurology. 2001;56:975–977. doi: 10.1212/wnl.56.7.975. [DOI] [PubMed] [Google Scholar]
- 6.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 7.Ott HC, Brechtken J, Swingen C, Feldberg TM, Matthiesen TS, Barnes SA, Nelson W, Taylor DA. Robotic minimally invasive cell transplantation for heart failure. J Thorac Cardiovasc Surg. 2006;132:170–173. doi: 10.1016/j.jtcvs.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Riviere CN, Patronik NA, Zenati MA. Prototype epicardial crawling device for intrapericardial intervention on the beating heart. Heart Surg Forum. 2004;7:E639–643. doi: 10.1532/HSF98.20041057. [DOI] [PubMed] [Google Scholar]
- 9.Patronik NA, Zenati MA, Riviere CN. Preliminary evaluation of a mobile robotic device for navigation and intervention on the beating heart. Comput Aided Surg. 2005;10:225–232. doi: 10.3109/10929080500230197. [DOI] [PubMed] [Google Scholar]
- 10.Ota T, Patronik NA, Riviere CN, Zenati MA. Percutaneous subxiphoid access to the epicardium using a miniature crawling robotic device. Innovations Phila Pa. 2006;1:227–231. doi: 10.1097/01.IMI.0000240673.14388.fc. [DOI] [PubMed] [Google Scholar]
- 11.Ota T, Patronik NA, Schwartzman D, Riviere CN, Zenati MA. Subxiphoid epicardial pacing lead implantation using a miniature crawling robotic device. J Surg Res. 2007;137:242–243. [Google Scholar]
- 12.Schneider M, Stevens C. Development and testing of a new magnetic-tracking device for image guidance. Proceedings of SPIE Medical Imaging 2007: Visualization and Image-Guided Procedures; San Diego, Calif. February 17–22, 2007. [Google Scholar]
- 13.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 14.Rivero-Ayerza M, Theuns DAMJ, Garcia-Garcia HM, Boersma E, Simoons M, Jordaens LJ. Effects of cardiac resynchronization therapy on overall mortality and mode of death: a meta-analysis of randomized controlled trials. Eur Heart J. 2006;27:2682–2688. doi: 10.1093/eurheartj/ehl203. [DOI] [PubMed] [Google Scholar]
- 15.Pruitt JC, Lazzara RR, Dworkin GH, Badhwar V, Kuma C, Ebra G. Totally endoscopic ablation of lone atrial fibrillation: initial clinical experience. Ann Thorac Surg. 2006;81:1325–1330. doi: 10.1016/j.athoracsur.2005.07.095. [DOI] [PubMed] [Google Scholar]
- 16.Zenati MA. Robotic heart surgery. Cardiol Rev. 2001;9:287–294. doi: 10.1097/00045415-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zenati MA, Schwartzman D, Gartner M, McKeel D. Feasibility of a new method for percutaneous occlusion of the left atrial appendage. Circulation. 2002;106:II–619. [Google Scholar]
- 18.Zenati MA, Bonanomi G, Chin AK, Schwartzman D. Left heart pacing lead implantation using subxiphoid videopericardioscopy. J Cardiovasc Electrophysiol. 2003;14:949–953. doi: 10.1046/j.1540-8167.2003.03255.x. [DOI] [PubMed] [Google Scholar]
- 19.Zenati MA, Shalaby A, Eisenman G, Nosbisch J, McGarvey J, Ota T. Epicardial left ventricular mapping using subxiphoid video-pericardioscopy. Ann Thorac Surg. 2007;84:2106–2107. doi: 10.1016/j.athoracsur.2007.07.032. [DOI] [PubMed] [Google Scholar]