Abstract
Background
Progressive myelination during adolescence implicates an increased vulnerability to neurotoxic substances and enduring neurocognitive consequences. This study examined the cognitive manifestations of altered white matter microstructure in chronic marijuana and alcohol-using (MJ+ALC) adolescents.
Methods
Thirty-six MJ+ALC adolescents (ages 16-19) and 36 demographically similar controls were evaluated with diffusion tensor imaging (Bava et al., 2009) and neurocognitive tests. Regions of group difference in fractional anisotropy (FA) and mean diffusivity (MD) were analyzed in relation to cognitive performance.
Results
In users, lower FA in temporal areas related to poorer performance on attention, working memory, and speeded processing tasks. Among regions where users had higher FA than controls, occipital FA was positively associated with working memory and complex visuomotor sequencing, whereas FA in anterior regions was negatively associated with verbal memory performance.
Conclusions
Findings suggest differential influences of white matter development on cognition in MJ+ALC using adolescents than in non-using peers. Neuroadaptation may reflect additive and subtractive responses to substance use that are complicated by competing maturational processes.
Keywords: Marijuana, Alcohol, DTI, Adolescence, White matter, Neuropsychology, Cognition
Introduction
Adolescent substance use continues to be a prevalent concern, stimulating widespread initiatives aimed at better understanding the developmental consequences of early and chronic exposure. Marijuana and alcohol are often used in tandem, and are consistently the most frequently used substances among teens (Schweinsburg, Brown, & Tapert, 2008) (SAMSHA, 2007). Harmful effects of marijuana and alcohol span physiological, social, and psychological functioning (Macleod et al., 2004; Tucker, Ellickson, Collins, & Klein, 2006a, 2006b). Given the extent of neuromaturation occurring during this time, the neurobiological and neurocognitive vulnerabilities associated with combined marijuana and alcohol use are of great interest.
The principal active component of marijuana, delta9-tetrahydrocannabinol (delta9-THC), produces complex alterations in cognition and behavior (Fant, Heishman, Bunker, & Pickworth, 1998; Johns, 2001; Solowij et al., 2002). Brain regions with high densities of cannabinoid receptors include the frontal cortex, hippocampus, basal ganglia, cerebellum, amygdala, and striatum (Eggan & Lewis, 2006; Freedland, Whitlow, Miller, & Porrino, 2002; Pontieri, Conti, Zocchi, Fieschi, & Orzi, 1999; Quickfall & Crockford, 2006). Human studies provide evidence for increased metabolism (Block et al., 2002; Mathew et al., 2002), decreased gray matter density (Matochik, Eldreth, Cadet, & Bolla, 2005) and atypical activation within these regions (Eldreth, Matochik, Cadet, & Bolla, 2004; Kanayama, Rogowska, Pope, Gruber, & Yurgelun-Todd, 2004). Similarly, chronic alcohol exposure is associated with cortical and white matter (WM) volume loss in the hippocampus, cingulate, corpus callosum, cerebellum, and frontal brain regions (De Bellis et al., 2000; De Bellis et al., 2005; Harris et al., 2008; Medina et al., 2008; Nagel, Schweinsburg, Phan, & Tapert, 2005; Pfefferbaum, Adalsteinsson, & Sullivan, 2006a).
Indication that chronic marijuana and alcohol use may detrimentally influence the developing brain comes from neuroimaging studies showing a more distributed functional network and recruitment of alternate neural pathways (Jacobsen, Pugh, Constable, Westerveld, & Mencl, 2007; Schweinsburg, Nagel et al., 2008; Schweinsburg et al., 2005; Tapert et al., 2001; Tapert et al., 2004; Tapert et al., 2007); weaknesses in neurocognitive functioning especially attention, visuospatial functioning, and learning and retrieval of verbal and nonverbal information (Brown, Tapert, Granholm, & Delis, 2000; Medina et al., 2007; Tapert & Brown, 1999, 2000; Tapert, Granholm, Leedy, & Brown, 2002); morphological changes (Medina et al., 2008; Nagel et al., 2005); and anisotropic differences in WM (De Bellis et al., 2008; McQueeny et al., 2009). Vulnerability to marijuana use has been suggested in WM pathways within and connecting superior medial, and inferior frontal areas (Arnone et al., 2008; Bonekamp et al., 2007; Gruber & Yurgelun-Todd, 2005; Kanayama et al., 2004), temporal and parietal lobes (Ashtari, Cervellione, Cottone, Ardekani, & Kumra, 2009; Ashtari et al., 2007; Grant, Gonzalez, Carey, Natarajan, & Wolfson, 2003) and areas of the cerebellum including the tonsil (Ashtari et al., 2007; Chang, Yakupov, Cloak, & Ernst, 2006; Schweinsburg et al., 2006).
Heavy alcohol use during adolescence is similarly associated with smaller prefrontal WM volumes (De Bellis et al., 2005; Medina et al., 2008). Increased anisotropy in the genu and isthmus of the corpus callosum in alcohol-using teens lends further support to atypical developmental trajectories (De Bellis et al., 2008). Correlates of these changes are seen in attenuated frontal response during spatial working memory (Tapert et al., 2001; Tapert et al., 2004) and deficits on neuropsychological measures of attention, memory retrieval, and visuospatial functioning (Brown et al., 2000; Tapert & Brown, 1999, 2000; Tapert et al., 2002).
Given the frequency of comorbid marijuana and alcohol use and their potential interaction, the extent of underlying circuitry disruptions and neurocognitive consequences needs further elucidation. Using diffusion tensor imaging (DTI), we previously characterized the relationship between marijuana and alcohol use and WM integrity among adolescent users and age-matched controls (Bava et al., 2009). Decreased fractional anisotropy (FA) was found most prominently in frontal-parietal circuitry comprising fibers of the inferior frontal region, splenium of the corpus callosum, postcentral gyrus, and left superior longitudinal fasciculus (SLF). Although mean diffusivity (MD) was similar between groups in regions of FA discrepancy, MD in WM adjacent the lingual gyrus was higher in users but lower in the inferior longitudinal fasciculus (ILF) as compared to controls. These findings suggest the presence of selective aberrancies in cerebral WM in adolescent marijuana and alcohol use, and are the basis for neurobehavioral correlation in the current study.
Based on findings of reduced neurocognitive functioning in marijuana and alcohol using adolescents (Brown et al., 2000; Medina et al., 2007; Tapert & Brown, 1999, 2000; Tapert et al., 2002), we predicted that regions of decreased anisotropic diffusion would be associated with poorer performance on neuropsychological measures. Considering that increased FA in users may be associated with compensatory mechanisms, we hypothesized that FA in these brain areas would be associated with improved performance. In marijuana and alcohol using adolescents, scores on verbal learning and memory were expected to correlate positively with temporal FA, speeded processing and visuomotor sequencing with bilateral crus cerebri FA, and complex sequencing with FA in frontal regions and frontal association tracts such as the SLF.
Methods
Participants
Participants were 72 adolescents ranging in age from 16 through 19 years. Thirty-six adolescents were heavy marijuana and alcohol (MJ+ALC) users, and 36 were demographically similar controls with very limited substance use histories (see Table 1). Adolescents were recruited from San Diego area schools from 2005 to 2007. Inclusionary criteria required participants and their parents or legal guardians to provide consent and comprehensive medical history. Adolescents and their parents were screened with separate, private interviews to ascertain eligibility. Exclusionary criteria for both users and controls were: DSM-IV Axis I disorder; nicotine dependence (e.g., Fagerstrom Test for Nicotine Dependence (FTND; (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) score ≥ 3), use of psychoactive medications; chronic medical illness, history of neurological disorder, head trauma with loss of consciousness >2 minutes, learning disability or mental retardation, complicated/premature birth (<33 weeks gestation); evidence of maternal drinking (>7 drinks in a week or >4 drinks in a day) or illicit drug use during pregnancy; parental history of bipolar I or psychotic disorder; left handedness; non-fluency in English; MRI contraindications; and clinically abnormal brain anatomy. Having surpassed these exclusionary criteria, participants were classified as: (1) Controls with limited substance use history (i.e., <5 lifetime occurrences of marijuana use, <50 lifetime drinks, <2 lifetime episodes of other drug use); or (2) MJ+ALC users with history of lifetime marijuana use between 180 to 1800 times and lifetime drinks between 50 and 700. The most recent marijuana use occurred 24 hours prior to imaging and last heavy alcohol use (4 or 5 alcoholic beverages in one sitting for females and males, respectively) was 3 days prior (see Table 1), verified by breathalyzer and urine toxicology. MJ+ALC users were excluded if they met criteria for a DSM-IV Axis I disorder other than a marijuana or alcohol use disorder. Informed assent and consent were obtained from participants and legal guardians in accordance with UCSD Human Research Protections Program procedures.
Table 1.
Demographic and substance use characteristics of participants.
| MJ+ALC (n = 36) M (SD) or % |
Controls (n = 36) M (SD) or % |
|
|---|---|---|
| Years of age (range 16.3-19.0) | 17.9 (0.9) | 17.8 (0.8) |
| Female | 27.8 % | 27.8 % |
| Caucasian | 61.1 % | 62.9 % |
| Annual household income (thousands) | 141.7 (128.3) | 115.9 (60.2) |
| Hollingshead socioeconomic level | 28.8 (13.7) | 30.4 (16.2) |
| WRAT-3 Reading standard score | 106.7 (8.0) | 109.7 (6.6) |
| WASI Vocabulary T-score | 58.4 (8.7) | 58.7 (8.7) |
| Beck Depression Inventory Total | 3.2 (3.2) | 2.6 (2.7) |
| Spielberger State Anxiety T-score | 39.5 (6.7) | 37.7 (7.0) |
| Child Behavior Checklist Internalizing T-score | 46.1 (10.0) | 44.8 (7.9) |
| Child Behavior Checklist Externalizing T-score | 49.7 (9.4) | 45.2 (10.3) |
| Age of first weekly marijuana use | 14.7 (3.1) | - |
| Lifetime marijuana use episodes ** | 551.7 (481.2) | 1.4 (2.3) |
| Marijuana use days per month ** | 11.6 (8.4) | 0.1 (0.3) |
| Days since last marijuana use ** | 52.1 (69.6) | 355.4 (330.3) a |
| Age of first weekly alcohol use | 15.5 (1.7) | 16.0 b |
| Lifetime alcohol drinks ** | 195.3 (152.7) | 25.0 (38.6) |
| Drinks per month ** | 52.9 (52.4) | 8.2 (12.4) |
| Days since last alcohol use | 43.0 (68.6) | 86.9 (118.2) a |
| Cigarettes per smoking day * | 1.2 (3.8) | 0.0 (0.2) |
| Fagerstrom Test for Nicotine Dependence score * | 0.3 (0.7) | 0.0 (0.0) |
| Lifetime other drug use instances ** | 10.5 (11.2) | 0.5 (2.2) |
p<.05
p<.001
For controls with history of any marijuana (n=14) or alcohol (n=12) use
(n=1)
Notes: MJ+ALC, Marijuana and alcohol user. WASI, Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). WRAT-3, Wide Range Achievement Test, 3rd edition (Wilkinson, 1993).
Measures
Substance use
The Customary Drinking and Drug use Record (Brown et al., 1998) collected from all teens detailed information on quantity and frequency of lifetime and past 3-month alcohol, marijuana and other drug use (including misuse of prescription and over-the-counter medications), as well as abuse/dependence, withdrawal, and negative consequences. The Timeline Followback (Sobell & Sobell, 1992) assessed youth and parent report of teens' chronicity and intensity of substance use for each of the 28 days preceding the scan session (see Tapert et al., 2007).
Neuropsychological functioning
Based on findings of neurocognitive decrements in adolescent substance users (Tapert & Brown, 1999; Tapert et al., 2002), including users with primary marijuana and alcohol use (Brown et al., 2000; Medina et al., 2007; Tapert & Brown, 2000), we examined indices from measures that previously differentiated users from controls: Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) Digit Symbol total scaled score, Digit Span backward score and Digit Span total scaled score (Wechsler, 1997b); Paced Auditory Serial Addition Test (PASAT) 2-second and 3-second trial total scores (Gronwall, 1974); Delis-Kaplan Executive Function System (D-KEFS) TMT Number Sequencing, Letter Sequencing, and Switching scaled scores and TMT total errors (Delis, Kaplan, & Kramer, 2001); California Verbal Learning Test-II (CVLT-II) List A Trial 1 standard score (Delis, Kramer, Kaplan, & Ober, 2000); and Wechsler Memory Scale-Third Edition (WMS-III) Logical Memory first, immediate and delayed recall scaled scores and recognition total score (Wechsler, 1997a). Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary (Wechsler, 1999) and Wide Range Achievement Test – 3 (WRAT-3) Reading (Wilkinson, 1993) provided estimates of premorbid intellectual functioning.
Mood and psychopathological syndromes
The Beck Depression Inventory (Beck, 1978) and Spielberger State Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970) assessed mood prior to scanning. The Child Behavior Checklist (Achenbach & Rescorla, 2001) was completed by parents to assay internalizing and externalizing psychopathological symptoms.
Image acquisition and DTI quantification
Image acquisition, pre-processing and calculation of DTI indices are provided in detail in (Bava et al., 2009). Briefly, participants were imaged in a 3T General Electric Excite MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA). Diffusion-weighted images were generated with a single-shot dual spin echo excitation and collected along 15 non-collinear directions in addition to a reference image with no diffusion weighting (b=0). The following sequence parameters were applied and averaged over four volumes: TE/TR=93/12,000 ms, FOV=240 mm, matrix =128×128, 36 contiguous slices, 3 mm slice thickness, b-value=2000 s/mm2. Two field maps were collected (TE/TR=3.8/1,000 ms) to correct for signal loss and geometric distortion due to B0 field inhomogeneities (Andersson & Skare, 2002; Jezzard & Balaban, 1995).
Data were corrected for motion, eddy current, and field distortions using FMRIB Software Library (FSL; Oxford, United Kingdom; (Smith et al., 2004)). Corrected images were subjected to tensor decomposition to derive scalar diffusion indices, FA and MD (Le Bihan et al., 2001). Computations were performed in native coordinate space using Analysis of Functional NeuroImages' (Cox, 1996) diffusion plug-in 3dDWItoDT (Cox & Glen, 2006). FA and MD were examined with whole-brain voxelwise analysis using Tract-Based Spatial Statistics (TBSS; (Smith et al., 2006)).
Statistical analyses
Voxelwise statistics of FA and MD data were carried out in AFNI using independent sample t-tests. Type I error control for multiple comparisons was achieved with a combination of individual voxel probability and cluster size thresholding, requiring that any significant group difference was comprised of 54 contiguous voxels, each differing at p <.05. Monte Carlo simulations determined that this yielded a brain-wise probability of a false positive at p <.01. Thirteen such FA clusters and 2 MD clusters represented significant differences between users and controls (Bava et al., 2009). Clusters showing significant group differences in FA or MD were correlated with neuropsychological test scores using Pearson's r correlation coefficients (α=.05). Significant correlations were subjected to hierarchical regressions to assess the unique influence of FA on neuropsychological performance for each group, with mean FA and group status entered on step 1 and their interaction term on step 2. If the interaction was nonsignificant (p>.05), results from the first step were interpreted.
Follow-up analyses
For FA and MD clusters that significantly predicted neuropsychological test scores (α=.05), hierarchical regressions examined mean FA/MD and substance use (i.e., lifetime marijuana use, marijuana use days per month, lifetime alcohol use, drinks per month) and their interaction as predictors of neuropsychological test score to determine whether the extent of substance use moderated the relationship between WM quality and neuropsychological performance in users (n=36). Further, to evaluate the potential influence of premorbid characteristics on neuropsychological performance, mean FA/MD and parental history of SUD were examined with regression analyses, where parental SUD was entered on step 1, average FA or MD of the significant cluster entered on step 2, and neuropsychological performance as the dependent variable.
Results
Groups did not significantly differ on demographic variables including age, gender distribution, ethnic composition, and socioeconomic status (Hollingshead, 1965) (see Table 1). Measures of emotional functioning and psychopathology were similar between groups and within normal limits. Estimated premorbid IQ and academic reading achievement were also comparable between groups, typically falling in the average to high average range. As expected, MJ+ALC youths were more likely to have a parental history of substance use disorder (p<.05), and more nicotine (p<.05) and other drug (p<.001) use than controls, so these variables were included as covariates in statistical analyses.
FA and MD Group Differences
As reported in our previous study, independent samples t-tests, corrected for multiple comparisons with intensity and cluster-based thresholding, revealed 10 clusters (≥ 54 μl) in which MJ+ALC teens showed significantly lower FA than controls (Bava et al., 2009). The most prominent areas of decreased FA for users were found in the left SLF, left postcentral sensory gyrus, and bilateral crus cerebri (p<.001). Temporal regions including the right superior temporal gyrus (p<.001), projection fibers of the left temporo-thalamic tract (p<.01), and right ILF (p<.01) also showed lower FA in MJ+ALC users, as did association fibers in right inferior frontal (p<.001), left occipital-frontal (p<.01), and splenium (p<.01) regions (p-values refer to group difference in FA averaged across each cluster). Interestingly, in three right hemisphere clusters, MJ+ALC users had higher FA than controls (p<.001): the cuneus region of the occipital lobe, anterior limb of the internal capsule, and arcuate portion of the right SLF.
Within clusters of significant FA discrepancy, MD values did not differ between groups. However, a whole-brain analysis of MD revealed significant differences in two areas: Inferior to the right occipital-cuneus and adjacent the lingual gyrus (users had higher MD than controls, p<.01) and in the left ILF (users showed lower MD than controls, contrary to hypotheses, p<.01).
To evaluate the influence of potential confounds, group differences were examined in an ANCOVA (N = 72). Specifically, lifetime cigarettes smoked, Fagerstrom Test for Nicotine Dependence score, lifetime other drug use instances, and parental history of SUD were greater in MJ+ALC users than controls. Group differences in FA and MD reported above persisted after controlling for these variables (F(12,54) = 6.50, p <.001). Mean FA and MD did not relate to estimated premorbid IQ or gender in either group.
Neurocognitive Correlates of White Matter Anomalies
Neuropsychological performance
Groups did not significantly differ on measures of neurocognitive functioning, with the exception of WAIS-III Digit Symbol, where users evidenced poorer performance than controls (t(70) = 2.39, p = .02).
Fractional anisotropy
For users (n=36), FA in the right ILF, a cluster where FA was lower than that of controls, was positively correlated with Digit Span total scaled score (r = .38, p = .02) and Digit Symbol total scaled score (r = .35, p = .03) (see Table 2). Conversely, clusters in which users' FA exceeded that of controls showed bidirectional associations. CVLT-II List A Trial 1 (r = .47, p = .004) and D-KEFS TMT Switching (r = .55, p = .001) were positively associated with FA in the occipital-cuneus region, whereas Digit Span total (r = -.33, p = .04) showed a negative relationship with FA in the anterior limb of the internal capsule.
Table 2.
Correlations of fractional anisotropy (FA) and mean diffusivity (MD) to neuropsychological test performance in marijuana+alcohol users and controls
| D-KEFS TMT Number Sequencing | D-KEFS TMT Switching | CVLT-II Trial 1 | WAIS-III Digit Symbol | WAIS-III Digit Span | WAIS-III Digits Backward | WMS-III Logical Memory II | WMS-III Logical Memory Recognition | PASAT 2-second Trial | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Difference | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | MJ+ ALC | CT | |
| FA | |||||||||||||||||||
| Occipto-frontal tract L | MJ+ALC<CT | ||||||||||||||||||
| Inferior frontal gyrus (opercular/insular) R | MJ+ALC<CT | ||||||||||||||||||
| Postcentral gyrus L | MJ+ALC<CT | -.36* | -.34* | ||||||||||||||||
| Superior longitudinal fasciculus L | MJ+ALC<CT | -.37* | |||||||||||||||||
| Corpus callosum (splenium) R | MJ+ALC<CT | ||||||||||||||||||
| Inferior longitudinal fasciculus R | MJ+ALC<CT | .35* | .38* | ||||||||||||||||
| Superior temporal gyrus R | MJ+ALC<CT | .41* | |||||||||||||||||
| Temporo-thalamic tract L | MJ+ALC<CT | ||||||||||||||||||
| Crus cerebri R | MJ+ALC<CT | ||||||||||||||||||
| Crus cerebri L | MJ+ALC<CT | ||||||||||||||||||
| Anterior limb internal capsule R | MJ+ALC>CT | -.33* | -.34* | ||||||||||||||||
| Superior longitudinal fasciculus (arcuate) R | MJ+ALC>CT | -.33* | |||||||||||||||||
| Occipital-cuneus R | MJ+ALC>CT | .55** | .47** | ||||||||||||||||
| MD | |||||||||||||||||||
| Inferior longitudinal fasciculus L | MJ+ALC<CT | -.43** | -.45** | -.44** | -.34* | ||||||||||||||
| Occipital-lingual R | MJ+ALC>CT | .39* | .35* | .34* | |||||||||||||||
p<.05
p<.01
L=Left; R=Right; MJ+ALC=marijuana+alcohol user; CT=Control; TMT=Trail Making Test; CVLT-II=California Verbal Learning Test-II (Delis et al., 2000); PASAT=Paced Auditory Serial Addition Test (Gronwall, 1974); D-KEFS=Delis-Kaplan Executive Function System (Delis et al., 2001); WAIS-III= Wechsler Adult Intelligence Scale – III (Wechsler, 1997b); WMS-III, Wechsler Memory Scale – III (Wechsler, 1997a).
Hierarchical regression analyses of FA in the right occipital region (MJ+ALC FA > Control FA) indicated a significant FA × group interaction in predicting performance on D-KEFS TMT Switching (β = 3.85; p <.001). Simple effects analyses revealed that higher FA was associated with better performance on TMT Switching in the MJ+ALC group (β = .55; p <.01) (see Figure 1). Concomitant with this finding, an interesting trend emerged suggesting that higher FA in this region was associated with poorer performance on TMT Switching in controls (β = -.32; p=.06). When predicting CVLT-II List A Trial 1 scores, a significant FA × group interaction (β = 2.16; p<.05) indicated that for users only, increased FA in the right occipital region was associated with better Trial 1 performance (β = .47; p <.01). Controls did not show a significant relationship between FA in this region and Trial 1 performance. FA in the right anterior limb of the internal capsule, another cluster in which FA in MJ+ALC users exceeded that of controls, predicted performance on WMS-III Logical Memory II delayed recall for both groups (β = -.31; p<.05). The group main effect and FA × group interaction were nonsignificant.
Figure 1.
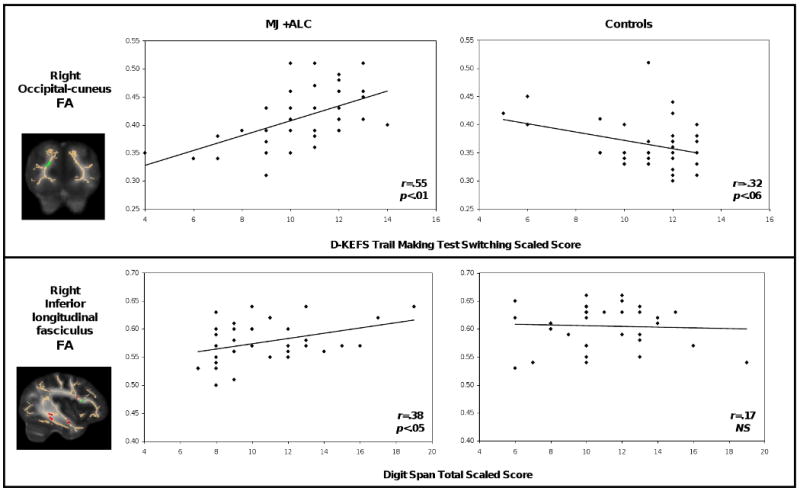
Neuropsychological correlates of fractional anisotropy (FA) in marijuana+alcohol users (MJ+ALC) (n=36) and controls (n=36). Significant clusters are superimposed on the fiber skeleton (beige) and overlaid on a standardized FA template. Red indicates decreased FA in MJ+ALC users. Green indicates increased FA in MJ+ALC users. D-KEFS=Delis-Kaplan Executive Function System Inventory
Regression analyses also revealed important findings in temporal brain regions. FA in the right superior temporal gyrus (β = .30; p<.05) and the right ILF (β = .31; p<.01) predicted performance on WAIS-III Digit Symbol total scaled score in both groups. The group main effects and FA × group interactions were nonsignificant. In addition, a significant FA × group interaction (β = 3.8; p <.05) indicated that increased FA in the right ILF predicted better performance on WAIS-III Digit Span total scaled score for MJ+ALC users (β = .38; p <.05), whereas controls showed no relationship between FA and Digit Span performance (see Figure 1).
Mean diffusivity
Correlation analyses indicated consistent and strong negative relationships between MD in the ILF (MJ+ALC < Control) and performance on measures of complex attention (CVLT-II List A Trial 1, Digit Span total scaled score, Digit Span backward) and verbal retention (Logical Memory II delayed recall) for users only. In contrast, MD in the occipital-lingual region (MJ+ALC > Control) was positively associated with performance on measures of complex attention (Digit Span total scaled score, Digits Backward, PASAT 2-second trial) in controls only (see Table 2). Hierarchical regression analyses of MD did not reveal significant findings.
Follow-up Analyses
To examine whether the extent of substance use moderated the relationship between FA and neuropsychological performance, hierarchical regressions examined mean FA and substance use (i.e., lifetime marijuana use, marijuana use days per month, lifetime alcohol use, drinks per month), and their interaction as predictors of neuropsychological test score. Analyses were conducted after outliers (>2.5 SD) on substance use were removed. Regression analyses examining the significance of FA in the right anterior limb of the internal capsule and lifetime marijuana use in predicting performance on WMS-III Logical Memory delayed recall (n = 32) indicated that increased use was associated with decreased performance on delayed recall (β = -.53; p <.01). The main effect of FA and the FA × use interaction were nonsignificant. Extent of marijuana use did not significantly moderate the relationship between FA and test performance in other regions. In addition, there were no significant main effects of alcohol use (lifetime or drinks per month) or FA × alcohol use interactions in predicting test performance.
The influence of premorbid characteristics on neuropsychological performance was examined using bivariate correlations of parental history of SUD with FA and MD and entered into regression analyses if significant (p <.05). FA in the right ILF was positively correlated with parental history of SUD (r = .39, p = .02). Regression analyses indicated that parental history of SUD did not predict neuropsychological performance beyond FA. No significant findings were found between MD and parental history of SUD.
Discussion
The goal of the present study was to examine the neurocognitive correlates of WM quality differences in MJ+ALC users. Previous findings showed decreased FA in frontal-parietal circuitry comprising fibers of the inferior frontal region, splenium of the corpus callosum, postcentral gyrus, and left SLF as well as areas of increased FA in three right hemisphere regions, including the cuneus region of the occipital lobe, anterior limb of the internal capsule, and arcuate portion of the SLF.
A number of findings supported our initial hypotheses that regions of decreased FA in users would be associated with lower performance on neurocognitive measures, whereas areas of increased FA would be linked to better performance, presumably due to compensatory processes. In temporal brain pathways comprising the right ILF and superior temporal gyrus, where FA was lower in the user group, performance on measures of speeded processing declined as FA decreased. Similarly, low FA in the ILF was associated with poorer scores on a measure of attention and working memory in users. Considering that this tract shows significant increases in FA from childhood to adolescence (Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008; Schmithorst, Wilke, Dardzinski, & Holland, 2002), the current findings implicate weaknesses in cognitive functioning in users that may be linked to diminished fiber integrity. By comparison, in the right occipital region, where users demonstrated higher FA than controls, visuomotor switching performance and first trial verbal list learning improved with increasing FA, and suggests the possibility of a coincident compensatory process occurring in MJ+ALC users during this developmental period.
Similar compensatory mechanisms are suggested in previous studies (Tapert et al., 2007), where adolescents with histories of chronic marijuana use show increased BOLD response compared to controls in areas connected to the occipital-cuneus (i.e., occipital gyri). Likewise, findings of greater brain activation in adult MJ+ALC users is thought to be associated with regional brain compensation (Chang & Chronicle, 2007; Pfefferbaum et al., 2001).
One unexpected finding indicated that FA in the anterior limb of the internal capsule, a region of higher FA in users, was associated with poorer performance on a measure of contextual verbal memory. This relationship departs from the compensatory model and suggests that elevated anisotropic diffusion may have adverse impact on brain function and neurocognitive performance. Higher FA in adolescent substance users, with primary MJ+ALC dependence, has been reported previously and related to premature myelination (De Bellis et al., 2008). The mechanism proposed is one wherein accelerated development could interfere with the formation or efficiency of typical functional networks. As such, increased FA in the anterior limb of the internal capsule may hinder connections with prefrontal, cingulate and thalamic fibers (Parent, 1990) and prove disadvantageous for cognitive performance.
The specific relationships between FA in regions such as the temporal lobe or crus cerebri to performance on measures of verbal learning and memory and visuomotor processing, respectively were not supported by the current findings. It is possible that traditional structure-function relationships may be altered by ongoing maturational processes, or that anisotropic changes have not reached the extent to which these cognitive functions would be impacted.
In this study, we explored the relationship between adolescent marijuana and alcohol use, WM quality, and neurocognitive performance. The present results link performance on standardized neurocognitive measures with WM diffusion properties in substance users. We found decreased FA in temporal brain areas in adolescents with histories of marijuana and alcohol use that was related to poorer attention, working memory, and speeded processing. These findings implicate substance-related alterations in WM with corresponding functional weakness. Conversely, in occipital brain regions, we found evidence for possible compensatory mechanisms in the user group. Specifically, users displayed higher FA that was associated with better working memory and complex sequencing performance. Higher FA and lower MD in this region did not correlate with enhanced cognitive performance in the control group, suggesting that changes in this region may optimize performance in the user group only. Another area of increased FA in users, in the internal capsule, was associated with poorer verbal memory performance indicating that neuroadaptive processes may not simply be additive or subtractive, but may be complicated by interfering or competing maturational processes.
A number of study limitations should be addressed in future work. Composite analysis of neuropsychological domains and WM quality may yield more power in assessing areas of neurocognitive vulnerability. In addition, future research should assess neurocognitive functioning in adolescents before they initiate MJ+ALC use to better understand the contribution of premorbid characteristics to substance-related WM changes. Although structural abnormalities appear to persist after sustained abstinence, delineation of the acute and chronic effects of MJ+ALC on brain structure is of interest in future work. The frequent comorbidity of marijuana and alcohol use makes it difficult to examine the separate and combined effects of these substances on brain structure and function. It is possible that the use of one substance can mitigate or potentiate the neurotoxic effects of the other and this mechanism should be further explored in follow-up studies. Finally, protocols that employ higher angular resolution (Frank, 2002) can provide more detailed assessment for exploration of circuitry disruption.
Marijuana and alcohol are two of the most widely used substances in adolescence. Understanding their role in neurodevelopment may help inform assessment of substance-related cognitive weaknesses and contribute to skill-based training and interventions to optimize cognitive development. The current findings suggest differential influences of white matter development on cognition in MJ+ALC using adolescents than in non-using peers. Neuroadaptation may reflect additive and subtractive responses to substance use that are complicated by competing maturational processes. Additional studies are needed to determine the long-term impact of MJ+ALC use on neurobehavioral functioning, particularly in light of preliminary work indicating a possible reversibility of WM changes with long-term abstinence (Delisi et al., 2006). Longitudinal studies of WM quality in adolescent substance users will also be important for assessing how neurobiological adaptations influence cognitive functioning throughout adolescence and into early adulthood.
Acknowledgments
This research was supported by grant R01 DA021182 to S.F. Tapert and F32 DA024476 to S. Bava. We extend our appreciation to our participants and their families, as well as to Dr. Sandra Brown, Christina Burke, Amanda Gorlick, Tim McQueeny, Dr. MJ Meloy, Ann Park, Anthony Scarlett, and Jennifer Winward whose support was vital to the completion of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Andersson JL, Skare S. A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage. 2002;16(1):177–199. doi: 10.1006/nimg.2001.1039. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41(3):1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatry Research. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35(2):501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) San Antonio, TX, USA: Psychological Corporation; 1978. [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. American Journal of Neuroradiology. 2001;22(10):1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology Biochemistry and Behavior. 2002;72(12):237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24(2):164–171. [PubMed] [Google Scholar]
- Cannistraro PA, Nakris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, et al. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depression and Anxiety. 2007;24(6):440–446. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- Chang L, Chronicle EP. Functional imaging studies in cannabis users. Neuroscientist. 2007;13(5):422–432. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129(Pt 5):1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cox R. Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R, Glen D. Efficient, Robust, Nonlinear, and Guaranteed Positive Definite Diffusion Tensor Estimation. International Society of Magnetic Resonance in Medicine; Seattle, WA. ISMRM 14th Scientific Meeting.2006. [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism. 2009;44(2):115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, et al. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32(3):395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test. 2nd. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, et al. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd PR, Foley PF, Buckley ST, Eckert AL, Innes DJ. Genes and gene expression in the brain of the alcoholic. Addictive Behaviors. 2004;29(7):1295–1309. doi: 10.1016/j.addbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical Distribution of the Cannabinoid CB1 Receptor in the Primate Neocortex: A Regional and Laminar Analysis. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23(3):914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, et al. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biological Psychiatry. 2005;58(12):974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, et al. Atrophy of the corpus callosum in chronic alcoholism. Journal of Neurological Sciences. 1997;146(2):145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacology Biochemistry and Behavior. 1998;60(4):777–784. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance in Medicine. 2002;47(6):1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Whitlow CT, Miller MD, Porrino LJ. Dose-dependent effects of Delta9-tetrahydrocannabinol on rates of local cerebral glucose utilization in rat. Synapse. 2002;45(2):134–142. doi: 10.1002/syn.10089. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. Journal of the International Neuropsychological Society. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced Auditory Serial-Addition Task: A measure of recovery from concussion. Perceptual and Motor Skills. 1974;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research: Cognitive Brain Research. 2005;23(1):107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences USA. 1998;95(14):8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Annals of the New York Academy of Sciences. 2000;899:274–282. [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, et al. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcoholism: Clinical and Experimental Research. 2008;32(6):1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, et al. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996;53(4):359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. Journal of Neuroscience. 2007;27(49):13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61(1):31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magnetic Resonance in Medicine. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Johns A. Psychiatric effects of cannabis. British Journal of Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176(34):239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathologica. 1989;79(2):200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363(9421):1579–1588. doi: 10.1016/S0140-6736(04)16200-4. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR, et al. Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Research. 2002;116(3):173–185. doi: 10.1016/s0925-4927(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered White Matter Integrity in Adolescent Binge Drinkers. Alcoholism: Clinical and Experimental Research. 2009 doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32(3):386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends in Neuroscience. 1990;13(7):254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, et al. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine & Tobacco Research. 2008;10(1):137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006a;27(7):994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial Profile of White Matter Microstructural Integrity in Recovering Alcoholic Men and Women. Biological Psychiatry. 2006b;59(4):364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14(1 Pt 1):7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerated with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage. 2002;15(3):708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30(2):423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24(8):1214–1221. [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Conti G, Zocchi A, Fieschi C, Orzi F. Metabolic mapping of the effects of WIN 55212-2 intravenous administration in the rat. Neuropsychopharmacology. 1999;21(6):773–776. doi: 10.1016/S0893-133X(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(3):318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic Resonance in Medicine. 2003;49(1):177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- SAMSHA. Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies, DHHS; 2007. [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008 doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Theilmann RJ, Eyler LT, T SF. Adolescent marijuana use and fMRI response during verbal learning. Journal of the International Neuropsychological Society. 2006;12(S1):210. [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, et al. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcoholism: Clinical and Experimental Research. 2001;25(6):924–934. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Palo Alto, CA, USA: Consulting Psychologists Press; 1970. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. Journal of the International Neuropsychological Society. 1999;5(6):481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Yeh CH, Calamante F, Cho KH, Connelly A, Lin CP. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42(2):617–625. doi: 10.1016/j.neuroimage.2008.05.002. Epub 2008 May 2009. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Are drug experimenters better adjusted than abstainers and users?: a longitudinal study of adolescent marijuana use. Journal of Adolescent Health. 2006a;39(4):488–494. doi: 10.1016/j.jadohealth.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Does solitary substance use increase adolescents' risk for poor psychosocial and behavioral outcomes? A 9-year longitudinal study comparing solitary and social users. Psychology of Addictive Behaviors. 2006b;20(4):363–372. doi: 10.1037/0893-164X.20.4.363. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Hallock K, Ducros M, Kim DS, Ronen I. Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. Neuroimage. 2008;39(1):1–9. doi: 10.1016/j.neuroimage.2007.08.046. Epub 2007 Sep 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-III. Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. WAIS-III Manual. New York: Psychological Corporation; 1997b. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The Wide Range Achievement Test-3 Administration Manual. Wilmington, DE, USA: Jastak Associates; 1993. [Google Scholar]


