Abstract
ThioTEPA is a chemotherapeutic agent used in the treatment of cancers, and more recently has been proposed as a component of high-dose therapy for young patients with recurrent malignant brain tumors. We previously demonstrated a significant dose-dependent reduction of cell proliferation in the dentate gyrus of the hippocampus in mice immediately following a 3-day regiment of thioTEPA. The aim of this study was to evaluate the long-term effects of thioTEPA treatment on hippocampal cell proliferation and potential effects on memory deficit or depression-related behavior in C57BL/6J mice.
A 3-day regimen of thioTEPA (10 mg/kg/d, i.p.) yielded a significant reduction in cell proliferation immediately after treatment as assessed by BrdU incorporation, and none of the labeled progeny that initially survived the treatment were detectable one week later. Following a 3-week rebound in proliferation following treatment, a significant deficit in proliferation reappeared and persisted for at least 21 weeks following treatment.
ThioTEPA-treated mice subjected to an object recognition test 1,2,3,4,8,12, 20 or 30 weeks following treatment demonstrated significant memory deficits at 12 and 20 weeks. Mice demonstrated a similar deficit in an object placement test when tested 20 weeks following thioTEPA treatment. However, no observable effects on performance in the Porsolt forced swim test or the tail suspension test were observed in thioTEPA-treated mice.
Together, these studies suggest that cumulative long-term negative effects of thioTEPA treatment on proliferation of new cells in the dentate gyrus may contribute to cognitive impairments associated with its use in the treatment of cancer.
Keywords: chemotherapy, thioTEPA, dentate, neurogenesis, mice
Introduction
The chemotherapeutic agent thioTEPA is an agent that reduces cell proliferation through alkylation of DNA, thereby reducing cell proliferation. It has been used clinically since the 1950s in the treatment of ovarian, breast and bladder cancers, and more recently is being investigated as high-dose treatment for aggressive cancers in both adult and pediatric patients [12, 31, 35]. Recent literature has addressed the possibility that cognitive and neurological deficits (i.e., memory function, processing speed, executive function, depression) may result from treatment with chemotherapeutic drugs (for reviews, see [1, 37, 53]), a condition referred to commonly as “chemo brain,” and it has been suggested that chemotherapeutic drugs may induce these negative cognitive or affective consequences in part by altering neurogenesis in the brain.
Neurogenesis in the adult mammalian central nervous system occurs in a restricted number of sites, including the dentate gyrus of the hippocampus. In rodents, the generation of cells in the dentate gyrus is prolific, yielding thousands of cells per day. This proliferation is accompanied by varied degrees of subsequent cell degeneration over the following several weeks, such that only a subset survive and integrate into the existing neuronal network [16, 24, 26, 52].
While an exact contribution of hippocampal neurogenesis to cognition or behavior has not conclusively been determined, a putative role in associative learning processes has been proposed. Chronic exposure to factors that inhibit cell proliferation such as stress, corticosteroids, or aging [4, 13, 29], or targeted inhibition of hippocampal cytogenesis by X-irradiation [40], genetic ablation of progenitors [23] or with antimitotic drug treatment [6, 44, 45, 49, 50] also impair acquisition of hippocampal-dependent learning tasks. Some types of learning stimuli enhance survival of new neurons [51], and environmental enrichment, which enhances hippocampal neurogenesis, actually recruits new neurons to spatial circuits in the dentate gyrus [27]. It has recently been proposed that the dentate gyrus contributes fine spatial discrimination or metric aspects of spatial memory formation [14, 20], which may corroborate the impairment of contextual but not cued fear conditioning observed in rodents with impaired neurogenesis [19, 21, 43, 45] or lesions of the dentate gyrus [17, 18].
There is also correlative evidence suggesting a link between neurogenesis and depression. Hippocampal size is decreased in human patients with major depression [5], and this reduction is correlated with the length of depressive illness [32, 47, 48]. In animal studies, neurogenesis appears to be required for the behavioral effects of antidepressants [41], and the timecourse for behavioral effects of antidepressants corresponds to the timecourse of restored levels of neurogenesis by these agents [34]. Antidepressants are also thought to influence cell survival in the dentate gyrus [30, 55], and have also been shown to reverse the inhibitory effects of stress on hippocampal cell proliferation in rodents [33]. Stress and glucocorticoids play a major role in depression [10, 22, 28, 42], and their effects on neurogenesis are also well-documented.
Previously, we demonstrated potent inhibition of proliferation of new cells in the dentate gyrus of the hippocampus following treatment with thioTEPA [36], which has proven to be both a useful tool for manipulating cell proliferation in order to elucidate its role in brain function and a useful animal model for studying potential cognitive and behavioral deficits associated with clinical use of chemotherapy in humans. Given that histopathology [9, 15] and cognitive deficits [44, 45] associated with chemotherapy can be delayed or long-lasting, we examined the effect of thioTEPA on cell proliferation in the dentate gyrus of mice for 2-30 weeks following administration. We tested separate groups of similarly treated mice over the same timecourse for depression-related behavior using two measures of behavioral despair, the tail suspension test and the forced swim test, for which our automated scoring procedures were validated using positive control groups treated with the tricyclic antidepressant, desipramine. In addition, we tested yet other thioTEPA treated mice for learning and memory deficits using the object recognition test and the object placement test.
2. Materials and Methods
2.1. General
Subjects were male C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) at 5 weeks of age. Mice were housed 2-4 per cage under 12:12 light-dark cycle, with food and water provided ad libitum. At 8-9 weeks of age (the peak of postnatal hippocampal cell proliferation), groups of mice (n=4) received three daily injections of either thioTEPA (10 mg/kg, i.p.) or phosphate buffered saline (PBS) vehicle.
2.2. Long-term effects of thioTEPA on hippocampal cell proliferation
Proliferative capacity in the dentate gyrus was measured in groups of mice (n=4/group) immediately and at 1, 2, 3, 4, 8, 12, and 30 weeks following the 3-day thioTEPA regimen. In order to detect newborn cells, mice were administered a single injection of BrdU (50 mg/kg i.p., dissolved in PBS), 30 minutes prior to perfusion with heparinized saline followed by 4% paraformaldehyde. Brains were post-fixed in paraformaldehyde overnight at 4°C then immersed in 30% sucrose in PBS for 1-2 days, and 30μm coronal sections were cut using a cryostat and stored in PBS at 4°C. Immunohistochemistry was performed on every 7th section throughout the rostrocaudal extent of the dentate gyrus in order to estimate total labeled cell counts for the dentate gyrus [54]. Slices were subjected to 2N HCl at 37°C for 30 mins, neutralized with 1.0M boric acid (pH 8.5) for 10 mins, rinsed, and incubated for 48 hours in PBS containing rat anti-BrdU antiserum (1:250, Abcam, Cambridge, MA), 3% donkey serum, 0.1% Triton X-100 and 0.1% TWEEN-20. Sections were then rinsed and incubated for 4 hours at room temperature in rhodamine-conjugated donkey anti-rat IgG (1:250; Jackson Immunoresearch, West Grove, PA). Following final rinse, sections were mounted on coated slides and coverslipped, and fluorescently labeled cells counted by an investigator unaware of treatments of the donor mice.
In order to measure progenitor survival, mice received 3 daily thioTEPA or vehicle injections followed immediately by a single BrdU injection (50 mg/kg, i.p.), as above. Mice (n=4/group) were then sacrificed weekly for 4 weeks, and processed for BrdU staining as above.
2.3. Effects of thioTEPA on depression-related behavior
In order to evaluate the effects of thioTEPA treatment on depression-related behavior, different groups of control and thioTEPA-treated mice (n=10-12/group) were subjected to the tail suspension test or the Porsolt forced swim test. Activity for both tests was measured using the Biobserve FST video tracking System (Biobserve, Bonn, Germany) running on a Dell Inspiron laptop computer using a CCD camera connected through a USB analog-digital converter and mounted to obtain a side view of the subject, using settings for mobility and immobility established empirically for each of the two tests.
In the tail suspension test, mice were suspended by the distal 1.5cm end of the tail from the bottom of a vertically oriented metal bar (6 mm diameter) using medical tape. Duration of immobility was assessed over a 5-minute trial. To ensure that differences in behavior were detectable using the software settings, performance in a positive control group of mice treated with the tricyclic antidepressant desipramine (30 mg/kg i.p., 24 and 1 hour prior to testing, n=4/group) was compared to vehicle-treated controls [2, 7]. Separate groups of thioTEPA and vehicle-treated controls were then tested at 8, 13 or 21 weeks after drug treatment.
In the Porsolt forced swim test, mice were placed in transparent 17-cm diameter plastic tanks filled to a depth of 20 cm with 23-25°C water, and time spent mobile versus immobile scored by the FST software during the last four minutes of 6-minute trials. To ensure that differences in behavior were detectable by the system, performance in a positive control group of mice treated with desipramine (16 mg/kg i.p., 24 and 1 hour prior to testing, n=10/group) was compared with vehicle treated mice [2, 7]. Separate groups of thioTEPA and vehicle-treated controls were then tested at 3, 6, 8, 13 or 21 weeks after drug treatment.
2.4. Effects of thioTEPA on learning & memory
In order to evaluate the effects of thioTEPA treatment on learning and memory, different groups of control and thioTEPA-treated mice (n=10-12/group) were subjected to the object recognition test or the object placement test. Groups of mice were subjected to both tests, but only at different time points at least 4 weeks apart, and using different objects between the tests. Investigation times in both tests were measured manually with stopwatches by investigators unaware of subject treatment. Tests were performed in a 40 × 50 cm plastic arena under normal room light (400-500 lux). Objects used in the tests included miniature ceramic teapots, ceramic salt shakers, and miniature glass teacups similar in size but different in shape and color, and for which no differential affinity or aversion was measured in preliminary experiments (data not shown). Investigation was defined as directly contacting, sniffing, looking at or whisking an object within 2 cm of it. Objects and arena were cleaned with 70% ethanol between trials.
In the object recognition test, two identical objects were placed equidistant from the ends of the arena, 25 cm apart. Mice (n=8-10/group) were placed into the center of the area perpendicular to the objects, and investigation time for each object was measured during a 5-minute trial. After a 24 hour retention interval, the subject was again placed in the arena with one of the familiar objects and a novel object. The order of presentation of the object used in the first trial was reversed for half the subjects in each group. Time spent investigating the familiar object to be replaced (trial 1) or the novel object (trial 2) were then compared for separate groups of control and thioTEPA-treated mice at 2, 4, 8, 12, 20 or 30 weeks following treatment.
In the object placement test, mice (n=8-10/group) were introduced into an arena with two identical objects placed in opposite corner quadrants and time spent investigating each object during a 5-minute trial was measured by stopwatch by an investigator unaware of subject treatment. In the second trial 24 hours later, one object was moved to a new location and investigation measured again during a 5-minute trial. Time spent investigating the familiar object to be relocated (trial 1) or the relocated object (trial 2) relative to total investigation time were compared for separate groups of control and thioTEPA-treated mice at 2, 4, 13, 20 or 30 weeks following treatment.
In both object recognition and object placement tests, preference for the novel object during Trial 2 is expressed graphically in Figs. 5 and 6 as a ratio of investigation time of the novel object relative to total investigation time, with 0.5 representing chance (equal) investigation of the two objects.
Fig. 5.
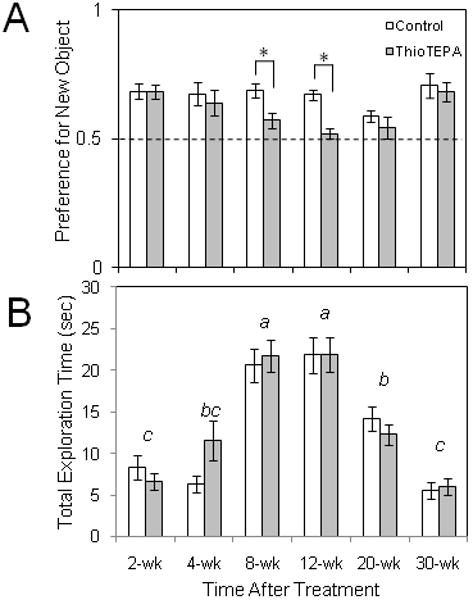
Effect of thioTEPA on object preference in the object recognition test. Subjects were exposed to a novel object and a familiar object after a 24 hour retention interval. (A) A preference for the novel object was observed in both mice treated with vehicle and mice treated with thioTEPA (10 mg/kg/d × 3d, i.p.) in the 2-4 weeks following treatment, but preference for the novel object was significantly lower in thioTEPA-treated mice tested 8 to 12 weeks following thioTEPA treatment, suggesting an impairment of memory of the original object (*p<.01, two-way ANOVA followed by Tukey analysis). Dashed line represents equal preference for either of the objects. (B) Total exploration times were significantly different among the time points sampled following treatment (a-d, two-way ANOVA followed by Tukey analysis), but at no time point were total investigation times different between control and thioTEPA-treated mice.
Fig. 6.
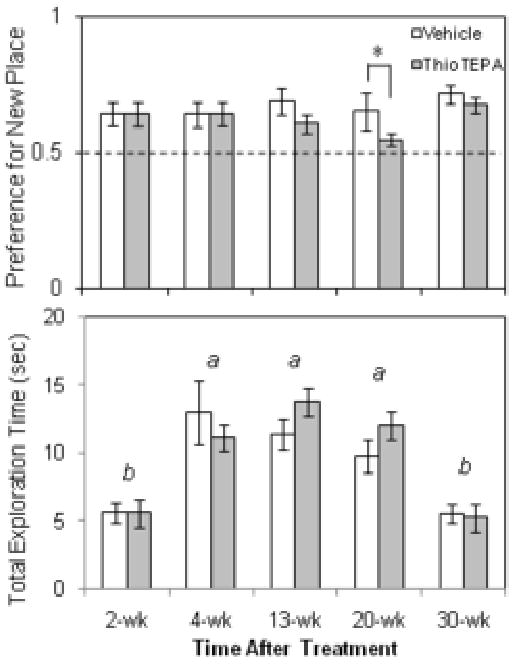
Effect of thioTEPA on object preference in the object placement test. Subjects were exposed to an object with a novel location and a familiarly placed object after a 24 hour retention interval. (A) A preference for the novel placement was observed in both mice treated with vehicle and mice treated with thioTEPA (10 mg/kg/d × 3d, i.p.) in the 2-8 weeks following treatment, but preference for the novel object was significantly lower in thioTEPA-treated mice tested 20 weeks following thioTEPA treatment, suggesting an impairment of memory of the original object location (*p<.05, two-way ANOVA followed by Tukey analysis). Dashed line represents equal preference for either of the objects. (B) Total exploration times were significantly different among the timepoints sampled, but at no time point were investigation times different for control and thioTEPA-treated mice (a-b, two-way ANOVA followed by Tukey analysis).
2.5. Statistics
Fluorescent microscope counts of BrdU-labeled cells and behavioral data on thioTEPA and control-treated mice were subjected to two-way ANOVA, followed by Tukey post hoc analysis when appropriate. Data from positive control (desipramine versus vehicle) experiments for the forced swim test and tail suspension test were subjected to Student's t-test. Probability values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Long-term effects of thioTEPA on hippocampal cell proliferation & survival
Proliferation of new cells in the dentate gyrus was inhibited by approximately 50% relative to controls when measured immediately after a 3-day regimen of thioTEPA (Fig. 1), as previously reported [36]. A rebound in proliferation to control levels was evident in the 1-3 weeks following thioTEPA treatment, after which thioTEPA-treated mice demonstrated significantly lower cell proliferation than control mice at the 4, 8 and 12-week time points sampled (two-way ANOVA, Treatment × Week interaction, (F(7,52)=4.050; p=0.002), followed by Tukey post-hoc tests). Reductions of cell proliferation by thioTEPA occurred in addition to an age-related decline in cell proliferation observed in vehicle-treated control mice over the 30 weeks of the study described previously in C57BL/6J mice [3]. Proliferation was lower in thioTEPA-treated mice even at 30 weeks following treatment, although by this age, baseline levels of proliferation are diminished, and the difference did not reach statistical significance.
Fig. 1.
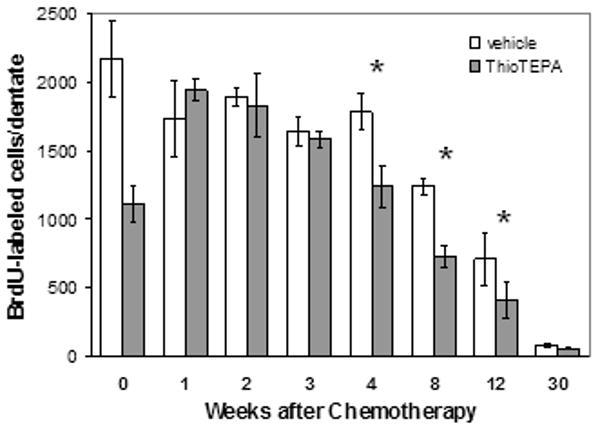
Effect of thioTEPA on cell proliferation rates in the dentate gyrus. Cell proliferation rates (assessed as BdrU incorporation following a 30-minute exposure to BrdU) decrease immediately (week 0) following a 3-day regimen of thioTEPA (10 mg/kg/day, i.p.), rebound to control levels for three weeks, but then fall again to significantly lower levels than those in control mice for up to 12 weeks. The thioTEPA-induced impairment occurs on top of a significant age-related decline in proliferation. *p<.05, two-way ANOVA followed by Tukey analysis.
Survival of newborn cells labeled with BrdU at the time of treatment remained constant for 2 weeks in control mice but decreased significantly 3 weeks after treatment with vehicle, whereas in thioTEPA-treated mice, no labeled cells survived even 1 week (treatment × week interaction, F(4,29)=6.230; p=.002)(Fig. 2).
Fig. 2.
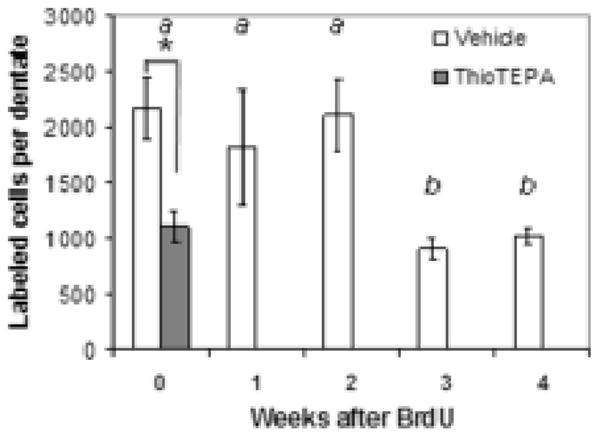
Effect of thioTEPA on survival of labeled newborn cells in the dentate gyrus. ThioTEPA treament (10 mg/kg/d × 3 days) immediately reduces the proliferation of newborn cells in the dentate gyrus (week 0), as assessed by BrdU labeling. In vehicle-treated animals, a significant reduction of the population of cells labeled immediately after treatment occurs three weeks later. The reduced number of newborn cells labeled in thioTEPA-treated mice at the time of treatment (week 0) have been lost within one week. *, significant treatment effect; a,b: significant effect of time after treatment; p<.05, two-way ANOVA followed by Tukey analysis.
3.2. Effects of thioTEPA on depression-related behavior
In the tail suspension test, mice injected with desipramine showed a significant decrease in immobility compared to control-treated mice (p<.05, Student's t-test)(Fig. 3A). However, no significant differences were observed between control and thioTEPA-treated mice at any of the time points sampled (Fig. 3B), although the interaction of treatment by time point approached statistical significance (F(2,68)=.055).
Fig. 3.
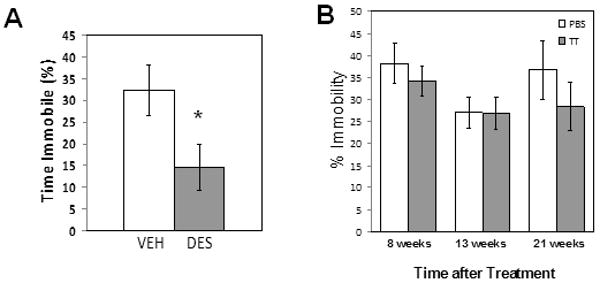
Effect of thioTEPA on depression-related behavior in the tail suspension test. (A) Treatment of mice with the tricyclic antidepressant desipramine (DES; 30 mg/kg, i.p., 24 and 1 hr prior to the test; n=10/group) reduces immobility and increases struggling in the test compared to vehicle-treated mice, as reported by other investigators, validating the sensitivity of the Biobserve tracking software to changes in behavior in our experiments. (B) At none of the time points sampled was time spent immobile altered in thioTEPA-treated mice (shaded bars) relative to PBS-treated control mice (open bars)(p>.05, two-way ANOVA, n=10-12/group).
In the forced swim test, desipramine-treated mice showed a significant decrease in immobility compared to control-treated mice (p<.001, Student's t-test)(Fig. 4A), thus validating that differences in behavior were detectable by the settings used by the video tracking system. In the comparison of performance in control and thioTEPA-treated mice at various times following treatment, a significant effect of time (F(5,109)=5.08, p<.01), but not of treatment or an interaction of time by treatment (p>.05), was observed.
Fig. 4.
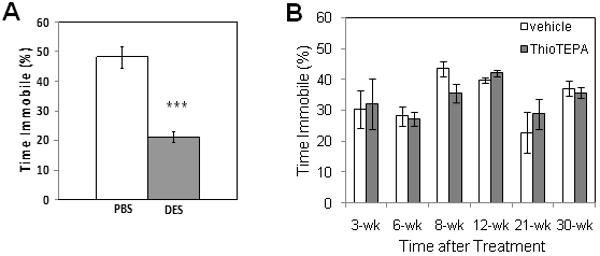
Effect of thioTEPA on depression-related behavior in the forced swim test. (A) Treatment of mice with the tricyclic antidepressant desipramine (DES, 16 mg/kg, i.p., 24 and 1 hr prior to the test; n=10/group) reduces immobility and increases struggling in the test compared to vehicle-treated mice (p<.001, Student's t-test), as reported by other investigators, validating the sensitivity of the Biobserve tracking software to changes in behavior in our experiments. (B) At none of the time points sampled was time spent immobile altered in thioTEPA-treated mice (shaded bars) relative to vehicle-treated mice (open bars)(p>.05, two-way ANOVA, n=10-12/group).
3.3. Effects of thioTEPA on learning & memory
While vehicle treated subjects displayed intact object recognition memory at all time points, assessed as a preference for the novel object, thioTEPA-treated mice showed significant deficits in object recognition memory at 8 and 12 weeks after thioTEPA treatment (Fig. 5A). A significant interaction of Object by Treatment was observed on the 2nd day trials for the 8-week (F(1,42)=5.41, p=.025) and 12-week samples (F(1,47)=7.53, p=.009), with Tukey analysis indicating a lack of preference for the novel object in thioTEPA-treated mice. At all other time points sampled, a significant preference for the novel object was observed in the 2nd day trials, independent of treatment. Total exploration time varied significantly at various weeks following treatment (F(5,135)= 38.52, p<.001, Fig. 5B). No preference for either object was observed during first day trials at each time point (p>.05, two-way ANOVA; data not shown).
ThioTEPA treatment also produced deficits in spatial memory 20 weeks after administration (time point by treatment interaction, F(1,42)=3.92, p=.044) reflected by a lack of preference for the relocated object by thioTEPA-treated mice (Fig. 6A). Total exploration time varied significantly at various weeks following treatment (F(4,108)= 13.07, p<.001; Fig 6B). No preference for either object location was observed during first day trials at each time point (p>.05, two-way ANOVA; data not shown).
4. Discussion
We have used the antimitotic chemotherapy drug thioTEPA to impair proliferation of newborn cells in the dentate gyrus of the hippocampus in young adult C57BL/6J mice. This effect is considerable in magnitude and relatively long-lasting, with significant reductions in cytogenesis for up to 12 weeks following a single regimen consisting of 3 daily injections. Moreover, we have demonstrated impairment of recognition memory at 8-12 weeks following treatment, and spatial memory after 20 weeks, but no measureable effects on depression-related behavior using behavioral despair models of depression. Our results are consistent with studies of other cytostatic agents with similar (∼40-50%) degrees of inhibition of dentate cell proliferation and associated cognitive or behavioral deficits [6, 44, 45, 49, 50].
An age-related decline in proliferation of new cells occurs in the dentate gyrus of both vehicle and thioTEPA-treated mice, consistent with similar published observations in mice and rats [3]. Following thioTEPA treatment, a reduction in cytogenesis to approximately 50% of control levels is followed by a rebound in proliferation to normal levels lasting several weeks before falling once again to 50-60% of control levels for the next 8-12 weeks. It is possible that this post-treatment rebound represents a surge in neurogenesis [11] or in proliferation of astrocytic cells suggested to be precursors to the neuronal population of the dentate gyrus [46] as a result of insult to the tissue following chemotherapy administration or through specific cell signaling mechanisms regulating levels of cell proliferation in the dentate gyrus. The rebound could also reflect an elevated level of gliogenesis [8] or specifically of microglial proliferation shown to precede neurogenesis following insult to the hippocampus [38]. Further examination of phenotypic markers in cells generated during the rebound will be required to elucidate the exact nature of this response.
A decline in survival of newborn cells in control animals was observed three weeks after labeling, consistent with the timecourse of migration and integration of these cells into the granule cell layer and coincident loss of a significant portion of these cells during that process [16, 24, 26, 52]. That all of the cells in the population born (and labeled) immediately treatment were lost within one week after thioTEPA treatment indicates an acute loss of the pool of candidate cells for integration in the several weeks following treatment. Further experiments examining the survival of cohorts of cells born at later time points after thioTEPA treatment, presumably in conjunction with phenotypic identification using type-specific markers, will be necessary to determine the extent to which addition of neurons to the existing network is impaired.
Age-related declines in cognitive performance have been associated not only with alterations in cell proliferation but also in differentiation, maturation, integration and survival of newborn cells into the functional circuitry of the dentate gyrus [39]. Altering proliferation is an appealing manipulation with which to study the putative role of neurogenesis in cognition and behavior as it limits the number of candidate cells available to undergo these other subsequent processes. In our experiments, the onset of memory impairment appears four weeks or more after thioTEPA treatment, coinciding roughly with the re-appearance of diminished cytogenesis following the post-treatment rebound in cell proliferation. The parallel of these two time courses is consistent with the observation that newborn cells do not achieve functional connectivity within the granule cell layer until approximately four weeks following their production [52] before which time their absence would presumably not be functionally relevant. However, it is difficult to reconcile the apparent recovery of object memory by thioTEPA-treated mice at the latest time points in our experiments given that the basal level of cytogenesis at 30 weeks post-treatment is greatly diminished even in the aged controls. That control mice are capable of object recognition at that age despite a severe lack of cell proliferation suggests that either ongoing cell proliferation is not required for object recognition memory, at least at this later age, or alternative mechanisms compensate for this age-related decline in cell proliferation. It must be considered that thioTEPA may be altering other neurological processes [9, 15] in addition to its effects on cytogenesis in the dentate gyrus that are responsible for the cognitive deficits documented here and in other published reports. Nevertheless, our results showing impaired object recognition following thioTEPA treatment in mice corroborate other recently published observations of impaired object recognition or contextual fear conditioning following impairment of neurogenesis by similar or alternative means in rats or mice [6, 23, 44, 45].
The effect of thioTEPA treatment in the object placement test mirrored the results of the object recognition test, with a decline in performance several months following treatment, although these differences achieved statistical significance only at 20 weeks post-treatment. The effect may have been more robust if the experiments had been performed with shorter retention intervals or with increased training times, or with additional spatial cues that reduce the difficulty of the task rather than relying solely on the objects and the entry point for the mouse as the subjects' reference for spatial location within the primarily featureless arena used in the experiment.
Despite strong correlative evidence in the literature linking impaired neurogenesis and depression, no effect of impaired cell proliferation on depression-related behaviors were observed in our experiments. It is possible that cell proliferation was not diminished sufficiently by thioTEPA treatment to produce measurable depression-related behavior in our assays. Alternatively, diminished cell proliferation may be necessary but not sufficient in and of itself to induce depression-related behavior. We are currently addressing these possibilities in experiments to determine whether diminished cell proliferation in the dentate gyrus might instead render subjects more susceptible to the effects of other factors that produce depression-related behavior using several mouse models of depression in addition to the behavioral despair models used in these experiments.
In addition to the utility of thioTEPA as an experimental agent for studying potential links between cell proliferation in the dentate gyrus and cognitive and behavioral consequences using mice, our study indicates potential clinical ramifications associated with the use of thioTEPA in the treatment of cancers in humans. Cognitive impairments have been documented in a subset of chemotherapy patients in a variety of functions including memory, concentration and information processing which can last for relatively long periods of time [1, 25]. It is imperative, given the rising incidence of cancers and the prevalent use of chemotherapy in their treatments, that the neurological and behavioral effects of chemotherapy are understood. Furthermore, this and other recent studies in rodents underscore the need to consider the time courses associated with these impairments when evaluating and elucidating mechanisms by which these agents affect cognitive behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armario A, Gavalda A, Marti O. Forced swimming test in rats: effect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility and defecation rate. Eur J Pharmacol. 1988;158(3):207–12. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- 3.Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 31(1):151–61. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15(1 Pt 1):61–9. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115–8. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21(2):513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 7.Crowley JJ, Jones MD, O'Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav. 2004;78(2):269–74. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24(2):476–88. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duman RS, Malberg J, Nakagawa S, D'Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48(8):732–9. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 11.Ernst C, Christie BR. Temporally specific proliferation events are induced in the hippocampus following acute focal injury. J Neurosci Res. 2006;83(3):349–61. doi: 10.1002/jnr.20724. [DOI] [PubMed] [Google Scholar]
- 12.Finlay JL, Goldman S, Wong MC, Cairo M, Garvin J, August C, Cohen BH, Stanley P, Zimmerman RA, Bostrom B, Geyer JR, Harris RE, Sanders J, Yates AJ, Boyett JM, Packer RJ. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors. The Children's Cancer Group. J Clin Oncol. 1996;14(9):2495–503. doi: 10.1200/JCO.1996.14.9.2495. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher M, Pelleymounter MA. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol Aging. 1988;9(56):549–56. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- 14.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122(1):16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- 15.Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413(1):146–54. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Rabaza V, Barcia JA, Llorens-Martin M, Trejo JL, Canales JJ. Spared place and object-place learning but limited spatial working memory capacity in rats with selective lesions of the dentate gyrus. Brain Res Bull. 2007;72(46):315–23. doi: 10.1016/j.brainresbull.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Rabaza V, Hontecillas-Prieto L, Velazquez-Sanchez C, Ferragud A, Perez-Villaba A, Arcusa A, Barcia JA, Trejo JL, Canales JJ. The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem. 2008;90(3):553–9. doi: 10.1016/j.nlm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, Barcia JA, Canales JJ. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159(1):59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 20.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18(10):1064–73. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 21.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5(3):262–9. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 23.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18(10):2707–12. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 25.Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20(6):719–25. doi: 10.1097/WCO.0b013e3282f1a06e. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–4. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 27.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 28.Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar Disord. 2002;4(1):17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- 29.Krugers HJ, Douma BR, Andringa G, Bohus B, Korf J, Luiten PG. Exposure to chronic psychosocial stress and corticosterone in the rat: effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus. 1997;7(4):427–36. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Lucassen PJ, Fuchs E, Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry. 2004;55(8):789–96. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Lucidarme N, Valteau-Couanet D, Oberlin O, Couanet D, Kalifa C, Beaujean F, Lapierre V, Hartmann O. Phase II study of high-dose thiotepa and hematopoietic stem cell transplantation in children with solid tumors. Bone Marrow Transplant. 1998;22(6):535–40. doi: 10.1038/sj.bmt.1701395. [DOI] [PubMed] [Google Scholar]
- 32.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 34.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massimino M, Gandola L, Luksch R, Spreafico F, Riva D, Solero C, Giangaspero F, Locatelli F, Podda M, Bozzi F, Pignoli E, Collini P, Cefalo G, Zecca M, Casanova M, Ferrari A, Terenziani M, Meazza C, Polastri D, Scaramuzza D, Ravagnani F, Fossati-Bellani F. Sequential chemotherapy, high-dose thiotepa, circulating progenitor cell rescue, and radiotherapy for childhood high-grade glioma. Neuro Oncol. 2005;7(1):41–8. doi: 10.1215/S1152851704000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mignone RG, Weber ET. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thioTEPA. Brain Res. 2006;1111(1):26–9. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 37.Minisini A, Atalay G, Bottomley A, Puglisi F, Piccart M, Biganzoli L. What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol. 2004;5(5):273–82. doi: 10.1016/S1470-2045(04)01465-2. [DOI] [PubMed] [Google Scholar]
- 38.Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31(2):218–29. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyffeler M, Yee BK, Feldon J, Knuesel I. Abnormal differentiation of newborn granule cells in age-related working memory impairments. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 41.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 42.Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56(3):137–9. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186(2):168–75. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FS, Koolhaas JM, Buwalda B. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res. 2009;201(2):279–84. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 48.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 50.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–9. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–63. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 54.Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278(3):344–52. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- 55.Wu X, Castren E. Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol Psychiatry. 2009;66(1):5–8. doi: 10.1016/j.biopsych.2009.01.023. [DOI] [PubMed] [Google Scholar]


