Abstract
Modern cognitive neuroscientific theories and empirical evidence suggest that brain structures involved in movement may be related to action-related semantic knowledge. To test this hypothesis, we examined the naming of environmental sounds in patients with corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), two neurodegenerative diseases associated with cognitive and motor deficits. Subjects were presented with 56 environmental sounds: 28 of objects that required manipulation when producing the sound, and 28 that required no manipulation. Subjects were asked to provide the name of the object that produced the sound and also complete a sound-picture matching condition. Subjects included 33 individuals from four groups: CBD/PSP, Alzheimer disease, frontotemporal dementia, and normal controls. We hypothesized that CBD/PSP patients would exhibit impaired naming performance compared with controls, but the impairment would be most apparent when naming sounds associated with actions. We also explored neural correlates of naming environmental sounds using voxel-based morphometry (VBM) of brain MRI. As expected, CBD/PSP patients scored lower on environmental sounds naming (p<0.007) compared with the controls. In particular, the CBD/PSP patients scored the lowest when naming sounds of manipulable objects (p<0.05), but did not show deficits in naming sounds of non-manipulable objects. VBM analysis across all groups showed that performance in naming sounds of manipulable objects correlated with atrophy in the left premotor region, extending from area 6 to the middle and superior frontal gyrus. These results indicate an association between impairment in the retrieval of action-related names and the motor system, and suggest that difficulty in naming manipulable sounds may be related to atrophy in the premotor cortex. Our results support the hypothesis that retrieval of action-related semantic knowledge involves motor regions in the brain.
1. Introduction
Corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) are two neurodegenerative diseases characterized by cognitive and motor symptoms. Patients with CBD often have deficits in executive function, visuospatial abilities, and language (Graham, Bak, Patterson, & Hodges, 2003). Motor symptoms of CBD can include apraxia, myoclonus, asymmetric extrapyramidal syndrome, cortical sensory loss, and alien limb phenomenon (Gorno-Tempini et al., 2006; Graham et al., 2003). Patients with PSP also exhibit motor deficits, including falls, loss of balance, axial rigidity, and bradykinesia, in addition to eye movement abnormalities (Rampello et al., 2005). Patients with PSP often exhibit cognitive impairments, including deficits in planning, monitoring and language (Krishnan, Mathuranath, Sarma, & Kishore, 2006). Damage to both cortical and subcortical structures, particularly the basal ganglia and frontal cortex, are hypothesized to contribute to motor and cognitive dysfunction in both CBD and PSP.
Recent pathological and genetic observations suggest overlap between CBD and PSP. Both disorders are adult-onset neurodegenerative disorders with tau neuropathology (Scaravilli, Tolosa, & Ferrer, 2005). However, the tau pathology occurs in slightly different brain regions in PSP and CBD: in PSP, the basal ganglia and brainstem are most affected, while neuropathology in CBD occurs in the prefrontal and premotor cortices with the caudate nucleus (Scaravilli et al., 2005). In addition, the extended tau 1 haplotype (H1) in the tau gene is overrepresented in CBD and PSP patients (Scaravilli et al., 2005). Despite neuropathological differences, it is hypothesized that PSP and CBD are separate phenotypes of one nosological disorder (Kertesz & Munoz, 2004; Scaravilli et al., 2005). This possible overlap is still being debated, but these disorders provide an opportunity to study interactions between motor and cognitive systems.
As discussed above, changes in language function may occur in patients with CBD and PSP. However, relatively few studies of language function have been done, possibly due to the categorization of these disorders as primarily movement disorders. Patients with early-stage CBD often complain of difficulty finding words, effortful speech, and handwriting changes. Language comprehension of grammatically complex sentences is initially preserved but declines as CBD progresses. Clinically diagnosed cases of progressive nonfluent aphasia (PNFA), for example, are often associated with CBD and PSP pathology (Josephs et al., 2006). PNFA is characterized by effortful speech with relatively preserved comprehension, speech apraxia, anomia, phonemic paraphasias, and agrammatism (Neary et al., 1998). These language abnormalities in CBD are hypothesized to be due to frontal and parietal atrophy (Murray et al., 2007). In PSP, furthermore, general mental slowing and dysarthria contribute to difficulty with communication. Patients with PSP commonly have diminished fluency of speech and are impaired in confrontation naming of objects and describing pictures (Podoll, Schwarz, & Noth, 1991). More specifically, action naming and verb production are often impaired in patients with PSP and CBD (Bak et al., 2006; Cotelli et al., 2006; Daniele, Giustolisi, Silveri, Colosimo, & Gainotti, 1994). These studies suggest that the ability to name an object and the ability to physically manipulate an object are related (Cotelli et al., 2006). Thus, difficulty in naming action-related objects may reflect cortical and subcortical damage in CBD and PSP.
While the majority of these studies assess naming by asking patients to name pictures (Glaser, 1992), the ability to name sounds has not yet been examined in patients with CBD and PSP. The auditory system is another modality by which memory and language can be accessed. It is possible that visuospatial difficulties may influence performance on tests of visual object or picture naming. The perception of sound, in general, involves the ascending auditory system, including nuclei in the brainstem, midbrain, and thalamus (Recanzone & Sutter, 2008). Naming sounds also involves brain areas such as the left ventral infra-temporal region and the left frontal operculum (Tranel et al., 2003). Interestingly, one study of naming animal sounds found activation in the mesial occipital cortex, suggesting that the retrieval of conceptual knowledge depends on the production of ‘internal’ visual images (Tranel et al., 2003).
Different types of sounds may activate different brain areas. For example, sounds that evoke actions may activate different brain regions than sounds that do not evoke actions. They may evoke the image of action by activating visual areas such as the mesial occipital cortex (Tranel et al., 2003). The sound of a hammer pounding a nail, for example, may evoke the image of a person pounding a nail into a piece of wood with a hammer. Moreover, different parts of the auditory cortex respond to different categories of sounds. Human voices selectively activate the bilateral upper banks of the superior temporal sulcus using functional MRI methods (Belin, Zatorre, Lafaille, Ahad, & Pike, 2000). Tettamanti and colleagues (2005) found that when subjects listen to sentences describing actions involving the mouth, hand, or leg, areas of the premotor and motor cortex, including the inferior parietal lobule, intraparietal sulcus, and posterior middle temporal gyrus were activated in a functional MRI paradigm. This result suggests that semantic representation may be accessible by both visual and auditory pathways, and damage to different brain regions may differentially affect sound naming and identification.
Therefore, the purpose of this study was to examine naming of environmental sounds in patients with CBD and PSP. Specifically, we evaluated the effect of manipulability on sound naming. We hypothesized that patients with CBD/PSP would have deficits in naming sounds of objects but, in particular, naming sounds of manipulable objects because of the motor deficits. Finally, we explored the brain correlates of sound naming using voxel-based morphometry of brain MRI. We also included patients with Alzheimer disease (AD) and frontotemporal dementia (FTD) for comparison and also to provide anatomical variance for the MRI analysis.
2. Methods
2.1. Participants
All subjects were recruited from the University of California San Francisco’s (UCSF) Memory and Aging Center and provided informed consent to participate in the procedures. Surrogates provided consent for patients with a dementia diagnosis. Clinical diagnosis was determined after a detailed clinical history, neurological examination, one-hour neuropsychological battery (Kramer et al., 2003), laboratory screening, and brain MRI (which was used to exclude patients with stroke, tumor, or other brain abnormalities). The neurologist also rated the presence or absence of oralbuccal and limb apraxia.
We included 33 subjects consisting of 10 controls and 23 patients with neurodegenerative diseases. Six of the patients were diagnosed with probable AD (McKhann et al., 1984) nine with FTD (Neary et al., 1998), and eight with CBD or PSP palsy (Litvan et al., 1996) (age range = 49–80 years; 12 males / 11 females). Subjects were in the mild stage of dementia (operationally defined as a Mini-Mental State Examination score > 19). Control subjects (age range = 57–73 years; 3 males / 7 females) were recruited from the UCSF Alzheimer’s Disease Research Center and underwent an evaluation identical to the patients. None of the controls showed evidence of impairment on neuropsychological testing or had a history of a neurological or psychiatric disorder.
Subjects were excluded if they had a current psychiatric disorder, hearing aids or significant clinical hearing impairment, head trauma with loss of consciousness greater than 10 minutes, substance abuse, or an additional neurological disease apart from the diagnosis of interest.
2.2 Neuropsychological Battery
Subjects were administered a one-hour screening neuropsychological battery that measures multiple domains of cognition (Kramer JH, 2003). Memory was evaluated using the 10-minute delayed recall trial of the California Verbal Learning Test – Mental Status (CVLT-MS) (Delis DC., 2000) and 10-minute recall of the modified Rey-Osterrieth figure. The longest correct backward digit span was used as a measure of working memory. Executive function was assessed using modified versions of Trailmaking B, the interference trial from the Stroop task, and abstractions (i.e., providing interpretations of similar items and proverbs). Measures of verbal fluency included letter fluency (number of D words in one minute) and animals (number in 1 minute). Language was assessed using a 15-item Boston Naming Test (Kaplan E, 1983), 16 items from the Peabody Picture Vocabulary Test – Revised (Dunn LM, 1981), and sentence comprehension subtest from the Curtiss-Yamade Comprehensive Language Evaluation-Receptive (CYCLE-R) (Curitss, 1988). The copy trial of the modified Rey-Osterrieth figure (Kramer JH, 2003) and the Number Location condition from the Visual Object Spatial Perception battery (VOSP) (Warrington EK, 1991) were used to assess visuospatial abilities.
2.3 Environmental Sounds Battery
The environmental sounds were selected from a sound list by Marcell and colleagues (Marcell et al., 2000) that had norms for naming accuracy and familiarity ratings in young adults. Twenty-eight sounds associated with manipulation were selected. A sound was operationally defined as manipulable if its corresponding action results in a manual, goal-directed manipulation of an object by the hand (e.g., hammer, guitar). In addition, 28 sounds that were not associated with manipulation were selected and matched on ratings of naming accuracy. A sound was considered non-manipulable if hand movements are not typically associated with the action (Saccuman et al., 2006). The sounds had a mean duration of 2491 milliseconds and a mean naming accuracy of 87% correct in young adults. There were no differences (all p>0.05) between manipulable and non-manipulable sounds in naming accuracy, stimulus duration, or familiar ratings as reported by Marcell (Marcell et al., 2000).
The sound battery was administered in a quiet testing room using a laptop and portable speakers. Participants were instructed to listen to a sound and generate a name (presented a maximum of two times). Specifically, participants were instructed, “I will be playing some sounds. I want you to tell me the name of what makes the sound.” If subjects had difficulty naming the sound, the following probes were used, “Tell me more. What makes that sound?”. Scoring guidelines were provided by Marcell (Marcell et al., 2000). Following the sound naming condition (even if the participant was able to correctly name the sound), the subjects were presented with four black and white, line drawing pictures (Snodgrass & Vanderwart, 1980), and the sound was again presented in a sound-picture matching task. Subjects were asked to point to the picture that goes with the sound. In addition to the correct picture, there were two pictures from a similar living/non-living category (e.g., dog for cat) and one from a different living/non-living category (e.g., airplane for cat).
2.4 Statistical analysis of Behavioral Data
We used an analysis of variance (ANOVA) with Tukey posthoc pairwise comparisons to evaluate possible group differences on demographic, neuropsychological, and experimental variables. To examine whether there was a proportional difference in naming of manipulable versus non-manipulable sounds, we also performed an ANOVA on the ratio of manipulable to non-manipulable naming between the four groups.
2.5 Voxel Based Morphometry
Voxel-based morphometry (VBM) was used to investigate the correlation between grey matter volume and sound naming test scores. All subjects in the behavioral study who had a high-quality 1.5T MRI scan within one year of the experimental testing were included in the imaging study. This group included 27 subjects (9 controls, 6 AD, 5 FTD, and 7 CBD/PSP; mean age = 64.9±7.1 years; 9 males / 18 females). Six subjects were excluded because they did not have an MRI within one year of testing. All subjects were treated as a single group, and were not subdivided on the basis of diagnosis (Brambati et al., 2006; Rosen et al., 2005). Similar to other studies (Baron et al., 2001; Boxer et al., 2006; Brambati et al., 2006; Gorno-Tempini et al., 2004; Mummery et al., 2000), a range of subject scores with different patterns of gray matter atrophy were entered as a single-group in the statistical model in order to provide variability in the sample and thus increase the power of the correlation analysis.
2.5.1 Image Acquisition
MRI scans were obtained on a 1.5T Magnetom VISION system (Siemens, Iselin, NJ). A volumetric magnetization prepared rapid gradient-echo MRI (MPRG, TR/TE/TI = 10/4/300 milliseconds) was used to obtain T1-weighted images of the entire brain, 15-degree flip angle, coronal orientation perpendicular to the double spin-echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness, as described in a previous study (Brambati et al., 2006).
2.5.2 VBM analysis
VBM analysis included two steps: spatial preprocessing (normalization, segmentation, Jacobian modulation and smoothing) and statistical analysis. Both steps were implemented using the SPM2 software package (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm) running on Matlab 6.5.1 (MathWorks, Natick, MA).
Anatomical MRI images were spatially pre-processed using standard procedures (Good et al., 2002). Ad hoc template and a priori images were created by averaging 30 age-matched normal control scans that had been normalized and segmented in the Montreal Neurological Institute (MNI) stereotaxic space. All T1 structural images were segmented, bias corrected and spatially normalized to the MNI space using the unified segmentation procedure (Ashburner & Friston, 2005). The VBM analysis was based on modulated gray matter images, whereby the gray matter value in each voxel was multiplied by the Jacobian determinant derived from the spatial normalization in order to preserve the total amount of gray matter from the original images. These modulated gray matter images were smoothed with a Gaussian kernel (8 mm FWHM).
The scores for naming sounds of manipulable objects were entered in a ‘covariate-only’ statistical model as a covariate of interest. Smoothed gray matter images of all subjects, irrespective of their diagnosis, were entered as a single group in the statistical model. Age, gender and the accuracy in naming sounds of non-manipulable sounds were entered as nuisance covariates. Global nuisance effect was accounted for by scaling all images to the same global volume. The correlation was tested using a [1] t-contrast, assuming that decreased sound naming ability would be associated with decreased gray matter volumes. A threshold of significance of p<0.001 uncorrected for multiple comparisons was adopted. VBM results were overlaid on the T1 template and anatomical labels in Table 3 were determined based on visual inspection of the data with reference to the Duvernoy (1999) atlas.
Table 3.
Results of the VBM Correlation Analysis.
| Brain Regions (BA) | BA | x | y | z | T Value | Z Score |
|---|---|---|---|---|---|---|
| L Precentral Gyrus | 6 | −46 | 7 | 48 | 5.8 | 4.4 |
| 6 | −30 | −4 | 52 | 5.5 | 4.3 | |
| L Superior Frontal Gyrus | 8 | −20 | 35 | 57 | 4.3 | 3.6 |
| 9 | −21 | 50 | 42 | 4.0 | 3.4 | |
| L Middle Frontal Gyrus | 9 | −37 | 16 | 57 | 4.2 | 3.6 |
| 9 | −38 | 37 | 43 | 4.3 | 3.6 | |
| R Precentral Gyrus | 6 | 23 | −9 | 60 | 4.0 | 3.4 |
| R Superior Frontal Gyrus | 8 | 20 | 27 | 63 | 4.5 | 3.7 |
| 9 | 8 | 55 | 46 | 3.9 | 3.3 | |
| R Middle Frontal Gyrus | 6 | 45 | −2 | 61 | 3.9 | 3.3 |
3. Results
3.1. Demographics
A summary of group demographics is provided in Table 1. There were no group differences in age or years of education. However, there was a significant difference in gender distribution; the FTD patients were predominantly men, while the controls were predominantly women. As expected, there were group differences on the CDR, and all patient groups scored below controls. Five out of the six CBD/PSP patients had symptoms of apraxia on the neurological examination, while none of the controls had apraxia. Of the CBD/PSP patients who had apraxia, all had symptoms of apraxia in at least one extremity, and one had evidence of oralbuccal apraxia. Apraxia was not assessed in the AD and FTD patients.
Table 1.
Demographics.
| Controls | AD | FTD | CBD/PSP | p | |
|---|---|---|---|---|---|
| N | 10 | 6 | 9 | 8 | NA |
| Age (years) | 66.8 (4.4) | 62.3 (12.2) | 59.2 (6.4) | 58.5 (20.2) | .114 |
| Sex (males/females) | 3/7 | 2/4 | 7/2 | 3/5 | .043+ |
| Education (years) | 16.9 (2.6) | 13.8 (2.7) | 14.6 (1.4) | 15.3 (3.1) | .144 |
| CDR – sum of boxes | 0 (0) | 5.4 (2.0)* | 6.7 (2.7)* | 6.3 (2.7)* | <0.0001* |
1 case was excluded in this analysis for years of education and 6 for CDR
Abbreviation: CDR = Clinical Dementia Rating
Statistical results from an analysis of variance (ANOVA) test
different from controls, Tukey HSD post-hoc, p<0.05
p-value from a Chi-squared statistic
3.2. Neuropsychological Battery
Results from the neuropsychological battery are found in Table 2. Overall, there were group differences on the majority of neuropsychological tests. The AD and CBD/PSP, but not FTD, patients scored lower than controls on the MMSE. On the learning trials of the CVLT-MS, the AD and CBD/PSP, but not FTD patients, scored lower than controls. Only AD patients recalled fewer words than controls on the CVLT-MS delayed recall, but all patient groups scored below controls on the test of visual memory (delayed recall of the modified Rey-Osterrieth figure). There were group differences on all tests of executive function. Only the CBD/PSP patients scored lower than controls on the modified Trailmaking Test B. AD and CBD/PSP patients scored below controls on the letter fluency, and all groups scored below controls on the abstractions. On the tests of visuospatial abilities, the AD and CBD/PSP patients scored below controls on the copy of the modified Rey-Osterrieth figure, and only patients with CBD/PSP scored below controls on the VOSP number location task. On language tasks, all patient groups named fewer animals in one minute, but there were no group differences when asked to name pictures on the Boston Naming Test.
Table 2.
Neuropsychological Test Results.
| Controls | AD | FTD | CBD/PSP | P | ||
|---|---|---|---|---|---|---|
| Global | MMSE (max. 30) | 29.5 (1.0) | 23.4 (2.0)* | 27.3 (2.0) | 24.3 (3.7)* | <0.0001 |
| Memory | CVLT-MS Total of Trials 1–4 (max. 36) |
31.3 (1.5) | 15.8 (3.3)* | 24.1 (5.1) | 17.9 (7.0)* | 0.002 |
| CVLT-MS 10-min. recall (max. 9) | 7.7 (1.5) | 1.2 (1.1)* | 5.3 (2.9) | 4.0 (3.0) | 0.012 | |
| Modified Rey-Osterrieth figure recall (max. 17) |
14.0 (2.3) | 2.8 (6.3)* | 7.9 (5.5)* | 7.0 (3.8)* | 0.001 | |
|
Executive Function |
Modified Trailmaking Test B (max. 120 sec) |
42.3 (33.0) | 84.3 (42.3) | 72.3 (46.3) | 117.0 (7.5)* | 0.003 |
| Letter fluency (D words in one minute) |
17.7 (3.7) | 8.6 (4.0)* | 10.9 (8.8) | 4.5 (1.2)* | <0.0001 | |
| Abstractions (max. 6) | 5.9 (0.4) | 3.2 (1.7)* | 2.5 (1.9)* | 1.8 (1.4)* | <0.0001 | |
|
Visuo- spatial |
Copy of modified Rey-Osterrieth figure (max. 17) |
15.8(1.6) | 9.4 (8.4)* | 14.4 (1.7) | 11.3 (2.4)* | 0.292 |
| VOSP Number Location (max. 10) | 9.3 (0.7) | 6.5 (1.7) | 8.8 (0.9) | 6.4 (2.8)* | 0.016 | |
| Language | Modified Boston Naming Test (max. 15) |
14.6 (0.7) | 12.3 (3.2) | 13.1 (1.1) | 12 (4.0) | 0.176 |
| Animal Fluency | 25.0 (5.1) | 11.5(7.3)* | 13.6 (7.5)* | 6.4 (81.6)* | <0.0001 |
Abbreviations: MMSE = Mini-mental state exam; CVLT-MS = California Verbal Learning Test – Mental Status;
Statistical results from an analysis of variance (ANOVA) test
different from controls, Tukey HSD post-hoc, p<0.05
3.3 Environmental Sound Battery
Figure 1 summarizes the overall results on environmental sound naming. Overall, the controls named an average of 48 (SD=5) out of 56 sounds. The CBD/PSP patients were the only group who named significantly fewer sounds (mean=30, SD=11) compared to the controls. In contrast, the FTD (mean=42, SD=6) and AD patients (mean=41, SD=10) scored similarly to the controls.
Figure 1.
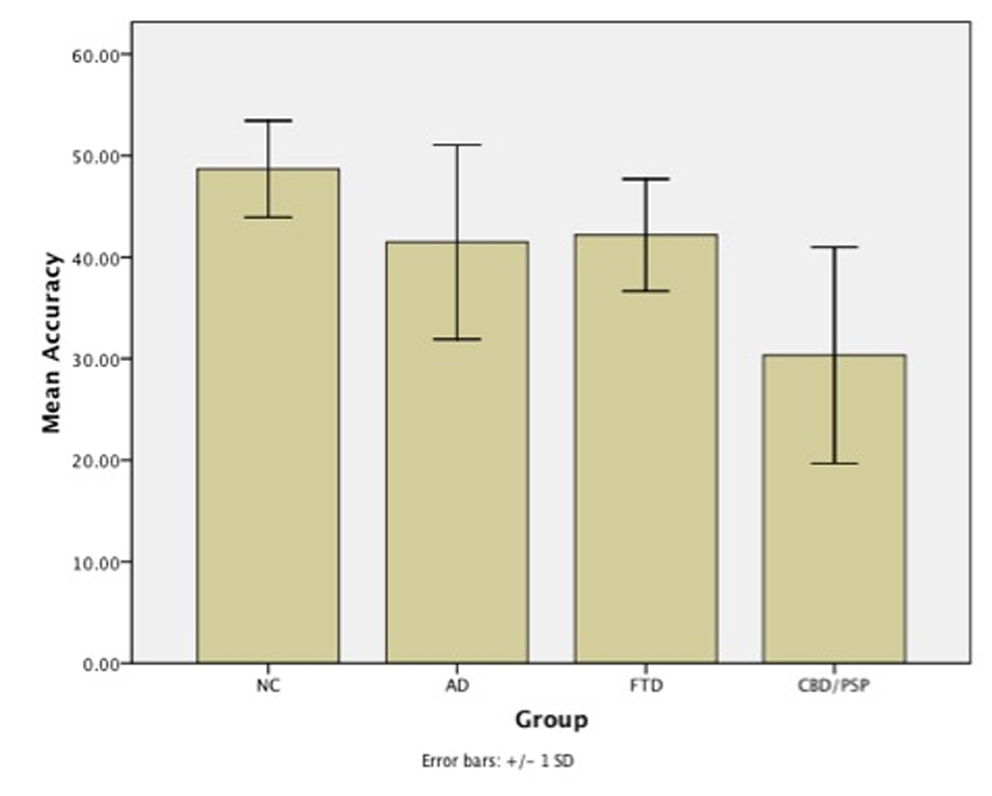
Summary of environmental sound naming accuracy across diagnostic groups. Error bars represent standard deviations.
Figure 2 shows the sound naming results according to the category of manipulable versus non-manipulable sounds. The controls named an average of 93% (SD=9) of the manipulable sounds, and patients with AD (mean=75%, SD=20) and FTD (mean=80%, SD=12) scored similarly to controls. However, patients with CBD/PSP scored significantly lower than controls and were only able to name 46% (SD=22) of the manipulable sounds.
Figure 2.
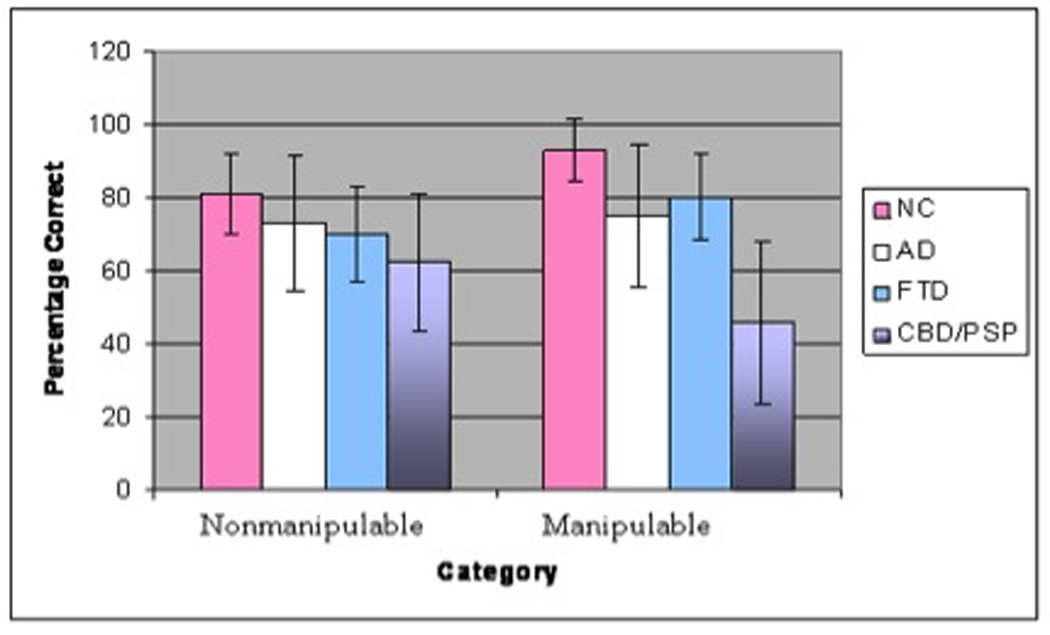
Sound naming across groups of manipulable versus non-manipulable sounds. Error bars represent standard deviation.
In contrast, there were no group differences on naming sounds of non-manipulable objects. The controls named 81% (SD=11) of non-manipulable sounds, and the patients with AD (mean=73%, SD=18), FTD (mean= 70%, SD=13), and CBD/PSP patients (mean=63%, SD=19) scored similarly to controls.
We also found a proportional difference between groups in the naming of manipulable and non-manipulable sounds. Compared with controls, the ratio of naming accuracy of manipulable to non-manipulable sounds of the CBD/PSP patients was significantly different (p<0.003). The AD and FTD group ratios, in contrast, were not different from that of the control group (p>0.05).
On the sound-picture matching task, controls performed at ceiling (mean=100%, SD=1). Compared with controls, both the AD (mean=87%, SD=9, p<0.05) and the CBD/PSP patients (mean=89%, SD=9, p<0.05) selected fewer correct pictures on the sound-picture matching task. The FTD group (mean=95%, SD=5) did not differ from the controls on this task.
3.4 Voxel-Based Morphometry
Results of the VBM analysis are found in Table 3 and Figure 3. The VBM analysis showed that low scores on naming sounds of manipulable objects correlated with gray matter volume bilaterally in the cortical pre-motor regions extending from the area 6 of the precentral gyrus to the middle and superior frontal gyri (p<0.001, uncorrected). Figure 3 shows a representation of the areas where atrophy correlates with difficulty in naming manipulable sounds.
Figure 3.
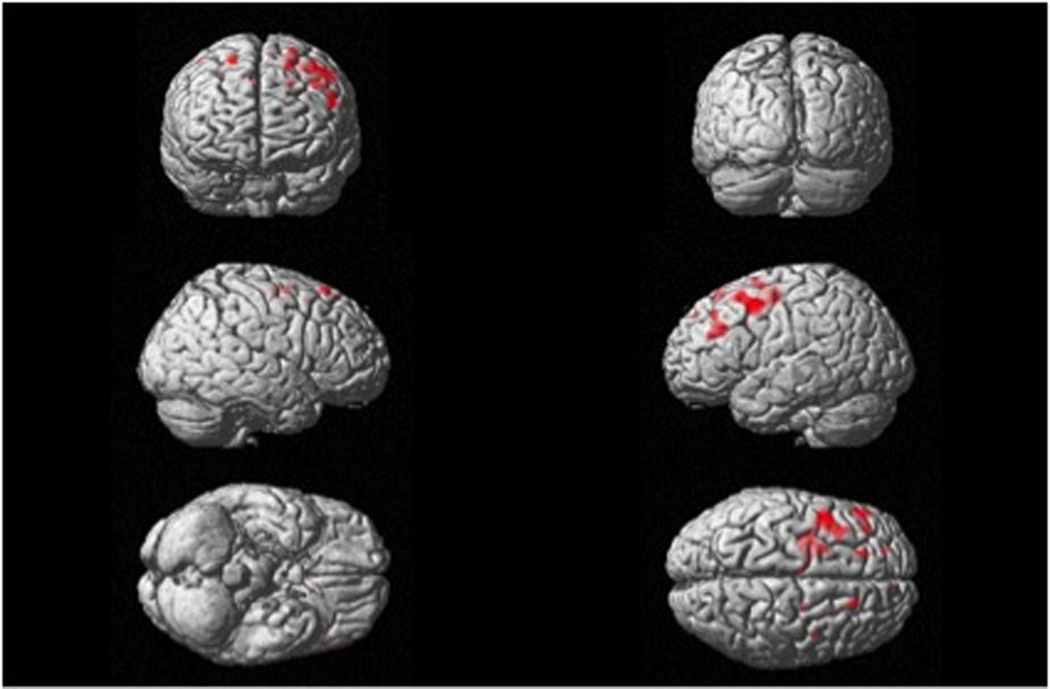
Brain areas that positively correlate with manipulable naming scores. The threshold for display is p < .001, uncorrected. Maps of significant correlation are superimposed on the 3-D rendering of the MNI standard brain.
4. Discussion
In this study, the relationship between cognitive and motor systems was investigated by examining the ability to name environmental sounds in patients with neurodegenerative diseases and controls. Overall, we found that patients with CBD / PSP had difficulty naming environmental sounds. In particular, the patients with CBD / PSP had a disproportionate impairment in the ability to name sounds of manipulable objects when compared with controls and other patient groups. Finally, a VBM analysis of MRI showed that atrophy in the premotor areas in the left hemisphere correlated with the difficulty in naming sounds of manipulable objects, independent of diagnosis. These results suggest that the naming of objects that require manipulation is influenced by damage to motor systems, such as with CBD or PSP.
It is interesting that the patients with CBD/PSP had disproportionate difficulty in naming sounds of manipulable objects (46% correct) when compared with non-manipulable objects (63% correct). The reverse pattern was evident with the controls and FTD patients who generally scored better on naming sounds of manipulable objects. Although the CBD/PSP patients scored the lowest on naming sounds of non-manipulable objects, there were no statistically significant group differences. The patients with CBD/PSP also scored similarly to patients with AD and FTD and controls when asked to name pictures on the Boston Naming Test. All but one of the pictures in the 15-item Boston Naming Test involved non-manipulable objects. The patients with CBD/PSP may have disproportionate difficulty naming sounds from objects that specifically involve movement. The motor deficits in CBD/PSP patients may, therefore, influence the naming of sounds involved with hand movement. This finding provides evidence for the relationship between cognition and motor systems in patients with CBD/PSP. This relationship may also be evident in other patients. For example, Buxbaum and colleagues (2002) found that patients with apraxia after a left hemisphere stroke had more difficulty naming body parts and tools than animals. Ideational apraxia is defined as a deficit in action sequencing (Poeck, 1983). Frontal lobe injury (Schwartz, 1995), dementia (Buxbaum, Sirigu, Schwartz, & Klatzky, 2003), and left hemisphere injury (Buxbaum, Glosser, & Coslett, 1999) may be associated with apraxia. Our results are also consistent with the findings of Cotelli and colleagues (2006), which asserted that patients with PSP and CBD were more impaired in oral production of action words than of words that name objects in an action-object picture-naming task. They concluded that disorders affecting frontoparietal-subcortical circuits involved in action knowledge and representation will impair action naming. Further, Silveri and Ciccarelli (2007) proposed a multimodal model of semantic memory where an integration of sensory and motor attributes is required for the concept of action, and that CBD prevents the integration of sensory and motor information. Our findings are also in line with this hypothesis.
The second major finding is that scores for naming sounds of manipulable objects correlates with atrophy in left, premotor areas and left dorsolateral prefrontal cortex. Several studies suggest that manipulable objects may be represented in the temporal cortex (Holdstock, 2005) and also other motor areas, such as the premotor cortex (Gerlach, Law, Gade, & Paulson, 2000). Atrophy in the left lateral temporal cortex correlated with naming accuracy in patients with neurodegenerative diseases (Grossman et al., 2004) indicating that naming deficits may have a common origin in different neurodegenerative diseases. CBD and PSP patients may have an additional disadvantage in naming objects in general compared to patients with atrophy localized to the left temporal cortex due to atrophy in the motor areas. The disadvantage may be manifested in the naming of manipulable objects. Our results support this idea because CBD/PSP patients, with possible atrophy in the motor areas, were impaired in naming manipulable objects. Moreover, our VBM analysis across groups found a correlation between atrophy in premotor areas and difficulty in naming a manipulable object. It is especially striking because although FTD and AD are best characterized by their cognitive deficits, these groups still exhibited similar ability for naming the sounds of manipulable objects as normal controls.
It is important to point out, however, that the brain areas associated with manipulable objects must be considered as part of a larger brain network. Buxbaum and colleagues (2002) suggested that frontoparietal structures, such as the areas found to be atrophied in the subjects in the current study that had difficulty naming sounds of manipulable objects, in the classical dorsal processing stream are important for spatiomotor action coding and semantic storage of information of actions with body parts and objects (Buxbaum, 2001; Gainotti, Silveri, Daniele, & Giustolisi, 1995). The current study cannot support the complete hypothesis, since only a portion of the fronto-parietal region was identified as being related to manipulable object naming, but supports that recognition of manipulable objects such as tools and musical instruments utilizes part of this pathway. Our results also suggest that auditory perception require similar routes for recognition as visual pathways. It is logical that manipulation knowledge should be intimately coupled with spatial knowledge, because manipulation of objects often requires directionality and thus perception of space. In isolation, the left premotor areas may affect manipulable object naming, and additional studies may reveal further downstream effects of atrophy originating from neurodegenerative disorders such as CBD and PSP.
Acknowledgements
Supported by NIMH Summer Research Training Program R25-MH18910 and NIH/NIA P50-AG0300601.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bak TH, Yancopoulou D, Nestor PJ, Xuereb JH, Spillantini MG, Pulvermuller F, et al. Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain. 2006;129(Pt 2):321–332. doi: 10.1093/brain/awh701. [DOI] [PubMed] [Google Scholar]
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18(10):1644–1653. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: a call to action. Neurocase. 2001;7(6):445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Glosser G, Coslett HB. Impaired face and word recognition without object agnosia. Neuropsychologia. 1999;37(1):41–50. doi: 10.1016/s0028-3932(98)00048-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Saffran EM. Knowledge of object manipulation and object function: dissociations in apraxic and nonapraxic subjects. Brain Lang. 2002;82(2):179–199. doi: 10.1016/s0093-934x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41(8):1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Alberici A, Calabria M, Agosti C, et al. Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology. 2006;20(5):558–565. doi: 10.1037/0894-4105.20.5.558. [DOI] [PubMed] [Google Scholar]
- Curitss SYJ. Curtiss-Yamada Comprehensive Language Evaluation. 1988. (unpublished test) [Google Scholar]
- Daniele A, Giustolisi L, Silveri MC, Colosimo C, Gainotti G. Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia. 1994;32(11):1325–1341. doi: 10.1016/0028-3932(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Delis DC, K. J, Kaplan E, Ober BA. California Verbal Learning Test. Second ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Dunn LM, DL . Peabody PIcture Vocabulary Test-Revised. Minneapolis, Minn: American Guidance Service; 1981. [Google Scholar]
- Duvernoy HM. The Human Brain. Surface, Blood Supply, and Three-Dimensional Sectional Anatony. New York: Springer Wien; 1999. [Google Scholar]
- Gainotti G, Silveri MC, Daniele A, Giustolisi L. Neuroanatomical correlates of category-specific semantic disorders: a critical survey. Memory. 1995;3(3–4):247–264. doi: 10.1080/09658219508253153. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Law I, Gade A, Paulson OB. Categorization and category effects in normal object recognition: a PET study. Neuropsychologia. 2000;38(13):1693–1703. doi: 10.1016/s0028-3932(00)00082-8. [DOI] [PubMed] [Google Scholar]
- Glaser WR. Picture naming. Cognition. 1992;42(1–3):61–105. doi: 10.1016/0010-0277(92)90040-o. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17(1):29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Ogar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006;67(10):1849–1851. doi: 10.1212/01.wnl.0000237038.55627.5b. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004;40(4–5):631–644. doi: 10.1016/s0010-9452(08)70159-x. [DOI] [PubMed] [Google Scholar]
- Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61(4):493–499. doi: 10.1212/01.wnl.0000081230.09863.ed. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(Pt 3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Holdstock JS. The role of the human medial temporal lobe in object recognition and object discrimination. Q J Exp Psychol B. 2005;58(3–4):326–339. doi: 10.1080/02724990444000177. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66(1):41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Kaplan EGH, Wintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kertesz A, Munoz D. Relationship between frontotemporal dementia and corticobasal degeneration/progressive supranuclear palsy. Dement Geriatr Cogn Disord. 2004;17(4):282–286. doi: 10.1159/000077155. [DOI] [PubMed] [Google Scholar]
- Kramer JH, JJ, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Mathuranath PS, Sarma S, Kishore A. Neuropsychological functions in progressive supranuclear palsy, multiple system atrophy and Parkinson's disease. Neurol India. 2006;54(3):268–272. doi: 10.4103/0028-3886.27150. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Marcell MM, Borella D, Greene M, Kerr E, Rogers S. Confrontation naming of environmental sounds. J Clin Exp Neuropsychol. 2000;22(6):830–864. doi: 10.1076/jcen.22.6.830.949. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45. [PubMed] [Google Scholar]
- Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68(16):1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Podoll K, Schwarz M, Noth J. Language functions in progressive supranuclear palsy. Brain. 1991;114(Pt 3):1457–1472. doi: 10.1093/brain/114.3.1457. [DOI] [PubMed] [Google Scholar]
- Poeck K. Ideational apraxia. J Neurol. 1983;230(1):1–5. doi: 10.1007/BF00313591. [DOI] [PubMed] [Google Scholar]
- Rampello L, Butta V, Raffaele R, Vecchio I, Battaglia G, Cormaci G, et al. Progressive supranuclear palsy: a systematic review. Neurobiol Dis. 2005;20(2):179–186. doi: 10.1016/j.nbd.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Sutter ML. The biological basis of audition. Annu Rev Psychol. 2008;59:119–142. doi: 10.1146/annurev.psych.59.103006.093544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccuman MC, Cappa SF, Bates EA, Arevalo A, Della Rosa P, Danna M, et al. The impact of semantic reference on word class: an fMRI study of action and object naming. Neuroimage. 2006;32(4):1865–1878. doi: 10.1016/j.neuroimage.2006.04.179. [DOI] [PubMed] [Google Scholar]
- Scaravilli T, Tolosa E, Ferrer I. Progressive supranuclear palsy and corticobasal degeneration: lumping versus splitting. Mov Disord. 2005;20 Suppl 12:S21–S28. doi: 10.1002/mds.20536. [DOI] [PubMed] [Google Scholar]
- Schwartz MF. Re-examining the role of executive functions in routine action production. Ann N Y Acad Sci. 1995;769:321–335. doi: 10.1111/j.1749-6632.1995.tb38148.x. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Ciccarelli N. The deficit for the word-class "verb" in corticobasal degeneration: linguistic expression of the movement disorder? Neuropsychologia. 2007;45(11):2570–2579. doi: 10.1016/j.neuropsychologia.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, et al. Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci. 2005;17(2):273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Eichhorn GR, Grabowski T, Ponto LL, Hichwa RD. Neural correlates of naming animals from their characteristic sounds. Neuropsychologia. 2003;41(7):847–854. doi: 10.1016/s0028-3932(02)00223-3. [DOI] [PubMed] [Google Scholar]
- Warrington EK, JM . The visual object and space perception battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]


