Abstract
The discovery of microRNAs as a novel class of gene expression regulators has led to a new strategy for disease diagnostics and therapeutics. Cell cycle, cell proliferation, and tumorigenesis are all regulated by microRNAs. Several general principles linking microRNAs and cancer have been recently reviewed; therefore, the current review focuses specifically on the perspective of microRNAs in control of cell cycle, stem cells, and heterotypic signaling, as well as the role of these processes in breast cancer. Altered abundance of cell cycle regulation proteins and aberrant expression of microRNAs frequently coexist in human breast cancers. Altered microRNA expression in breast cancer cell lines is associated with altered cell cycle progression and cell proliferation. Indeed, recent studies have demonstrated a causal role for microRNA in governing breast tumor suppression or collaborative oncogenesis. This review summarizes the current understanding of the role for microRNA in regulating the cell cycle and summarizes the evidence for aberrant microRNA expression in breast cancer. The new evidence for microRNA regulation by annotated genes and the involvement of microRNA in breast cancer metastasis are discussed, as is the potential for microRNA to improve breast cancer diagnosis and therapy.
microRNA (miR) are a new class of multifunctional small molecules that regulate the stability or translational efficiency of targeted messenger RNAs. According to the miRBase Sequence Database (Release 13.0 in March 2009), 706 miRs have been identified in humans and 547 in mice to date. Mature miRs are assembled into a ribonucleoprotein complex known as RNA-induced silencing complex (RISC) that includes Argonaute protein Ago2.1 The miR-RISC complex may lead to base-pairing interactions between miRs and the 3′ untranslated region (3′UTR) of their target mRNAs, often repressing the gene translation or cleaving the target mRNA, depending on the base-pairing features between the miR and the target mRNA.2,3 Because each vertebrate miR may bind to as many as 200 gene targets, and each gene may contain multiple binding sites for different miRs, miRs potentially could regulate the expression of about one-third of human mRNAs.4 miRs often target various components of cellular networks or signaling pathways.5,6 As such, miRs have been predicted to play a prominent role in regulating a broad range of biological processes including development, apoptosis, cell cycle progression, cellular proliferation, cancer initiation, and cancer metastasis.7–14
Altered expression of miRs has been demonstrated in different types of human cancer. Thus, the potential of miRs to be robust biomarkers for cancer diagnosis, prognosis, and pathogenesis has been predicted.14,15 miR-encoding genes are frequently located at fragile sites and in minimal regions of loss of heterozygosity, minimal regions of amplification, and in common breakpoint regions involved in cancers.11
Altered miR expression in human breast cancer was first demonstrated in 2005.16 In breast cancer, abnormalities of the cell cycle are frequently observed, including loss of retinoblastoma (Rb) function, reduced cyclin-dependent kinase (CDK) inhibitor p21 (Waf1/Cip1) and p27 (Kip1) abundances, as well as increased abundance of D and E type cyclins. Cyclin D1 encodes a key regulator of the cell cycle transition from G1 to the DNA synthetic phase and is overexpressed in more than 50% of breast cancers, functioning as a rate-limiting factor for human breast cancer cell proliferation in vivo and in vitro.17,18 Cyclin E, another important cell cycle regulator, is overexpressed in more than 10% of breast cancers and is a powerful prognostic predictor in early stage breast cancer as well as a significant determinant of tumor aggressiveness.19 miRs interact with E2F, Rb, cyclins, CDKs, and CDK inhibitors, providing the potential to regulate cellular division and cell cycle progression.20–23
miR Regulation of Cell Cycle Progression Genes
The mechanisms governing cell cycle control by miRs are increasingly well understood. Five groups of miRs, including the miR-15a/16 cluster, the miR-17/20 cluster, the miR-221/222 cluster, and the let-7 and miR-34 families, can regulate cell cycle progression by directly targeting cell cycle regulators (Figure 1).
Figure 1.
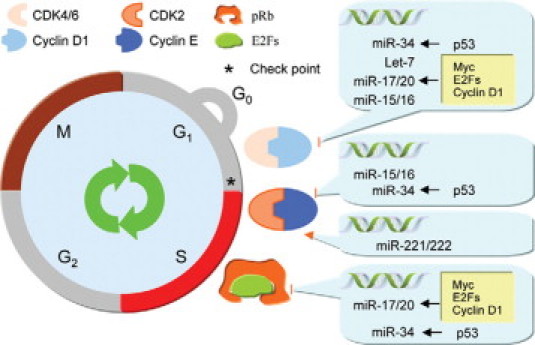
miRNA–cell cycle regulation network. The miR-34 family is involved in cell cycle regulation by repressing E2F, cyclin D1, and cyclin E expression. miR-34 itself is a direct transcriptional target of p53. The miR-17/20 cluster is transcriptionally regulated by myc, E2Fs, and cyclin D1, and it in turn regulates E2F, pRb, and cyclin D1 expression at the translation level. miR-15/16 regulates cell cycle control by inhibiting cyclin D1, cyclin E, and CDK4/6.
miR-15a/16 Cluster
The miR-15a/16 cluster is deleted and/or down-regulated in ≈70% of chronic lymphocytic leukemia patients,24 as well as in pituitary adenomas25 and in a gastric cancer cell line,26 suggesting an important role in cell proliferation and tumor growth. The miR-15a/16 cluster induces cell cycle arrest at the G1 phase by targeting cyclin D1, cyclin E1, cyclin D3, and CDK6.27 In addition, through a high-throughput profiling analysis Calin et al identified a gene signature regulated by miR-15a/16 in a leukemic cell line that included cell cycle and apoptosis signaling genes.28
miR-17/20 Cluster
The miR-17/20 cluster, which encodes six mature miRs within a 1-kb genomic region, inhibits tumor growth in a human B cell line20 and breast cancer cell lines.22 miR-17/20 coordinates the timing of cell cycle by targeting multiple cell cycle regulators (E2F, c-myc, Rb, and cyclin D1) as shown in Figure 1. In the G1 phase of a cell cycle, c-myc and cyclin D1 are induced while E2F1 is inactivated by binding to Rb. The miR-17/20 cluster is involved in the G1 to S transition of the cell cycle. Previous publications have indicated that miR-17/20 dampens the reciprocal activation of E2F by c-myc through inhibiting E2F translation.20 A Rb family member, Rbl2, is also a target of miR-17-5p.21 The first studies demonstrating that miRs directly inhibit cyclin D1 showed that miR-17/20 targeted the cyclin D1 3′UTR in the MCF-7 breast cancer cell line, resulting in cell cycle arrest and suppression of cell proliferation.22 miR-17-5p also inhibits the estrogen receptor α (ER-α) coactivator AIB1 in breast cancer cells.29 Collectively, these studies suggested the miR-17/20 cluster may function as a breast tumor suppressor through the regulation of cell cycle progression genes.
It has been hypothesized that the miR-17/20 cluster regulates cell cycle progression via E2F, c-Myc, and cyclin D1, as these proteins can both regulate and be regulated by miR-17/20 (Figure 2, A–D). These regulatory loops provide mechanisms to tightly control cell cycle progression and cell proliferative signals. In contrast with the mammary gland,22 the expression of this miR cluster was increased and cell growth was enhanced in both lung cancer and lymphomas,30,31 indicating that miR-17/20 function is cell type–dependent.
Figure 2.
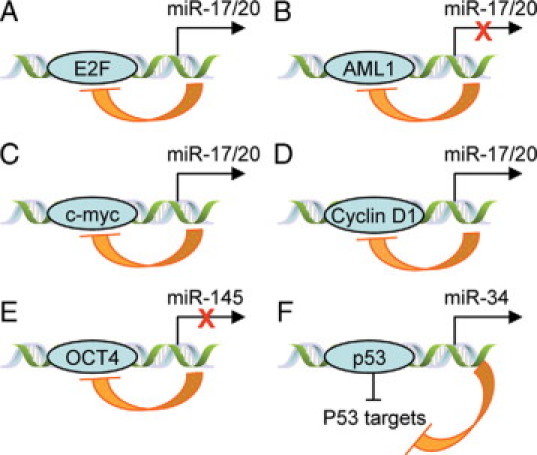
miR-target regulatory loop. A: The miR-17/20 cluster, which is transcriptionally induced by E2F, in turn translationally represses E2F expression. B: AML1 transcriptionally inhibits miR-17/20 expression, and miR-17/20 inhibits AML1 translation. C: Myc induces the miR-17/20 cluster, and miR-17/20 inhibits myc expression. D: Cyclin D1 induces miR-17/20 expression, and miR-17/20 translationally inhibits cyclin D1 expression. E: OCT4 inhibits miR-145 expression, and miR-145 in turn inhibits OCT4 by 3′UTR binding. F: p53 transcriptionally induce the expression of the miR-34 family, and miR-34 negatively targets the downstream genes of p53.
miR-221/222
miR-221/222 regulates the cell cycle by targeting CDK inhibitors. Ectopic expression of the miR-221/222 cluster activates CDK2, facilitates G1/S phase transition, and enhances tumor growth by negatively regulating p27kip1 and p57kip2.32 This has been demonstrated in both the MCF-7 cell line and HER2/neu-positive primary human breast cancer tissues.23 Furthermore, increased miR-221/222 cluster expression is associated with tamoxifen resistance in breast cancer.23,33
let-7
let-7 controls the timing of cell cycle exit and terminal differentiation in C. elegans.34 The abundance of let-7 family members is reduced in several types of cancer including lung and breast cancer.35,36 let-7 overexpression in lung cancer cell lines suppressed cell cycle progression and reduced cell division.37 The mechanism by which let-7 regulates tumor growth is via targeting the Ras, HMGA2, and caspase-3 genes.36,38–40 Multiple important cell cycle control genes are repressed by let-7 including cyclin D1, cyclin D3, cyclin A, CDK441 and CCNA2, CDC25A, CDK6, and CDK8.37
miR-34
The miR-34 family (miR-34a, miR-34b, and miR-34c) is an important component of the p53 tumor suppressor network.42 DNA damage and oncogenic stress activate p53. p53 binds to the promoter of miR-34a and miR-34b/c, inducing their expression at the transcriptional level (Figure 2F). Ectopic expression of the miR-34 family induces cell cycle arrest and apoptosis by down-regulating cyclin D1, cyclin E2, E2Fs, and CDK4/6.42,43,44 miR-34b/c overexpression inhibits cell proliferation and colony formation in soft agar.45 Two additional miRs, miR-192 and miR-215, are also involved in the p53 network, and upregulation of these two miRs suppresses carcinogenesis via p21CIP1 accumulation.46
miRs in Breast Cancer
Liu et al16 first reported the aberrant expression of miRs in human breast cancers compared with normal tissue. They identified 29 miRs with aberrant expression in human breast cancer by microarray and Northern blot analyses on 76 breast tumor samples and 14 human breast cell lines. Of the altered miRs in breast cancers, a substantial proportion has been aligned to genomic fragile sites or regions associated with cancers.11,47 Zhang and colleagues performed an analysis on 283 known human miR genes by array-based comparative genomic hybridization in 73 breast cancer specimens (55 primary tumors and 18 cell lines), demonstrating the high-frequency gene copy number abnormality of miR-containing regions throughout the genome in human breast cancers.47 Approximately 73% (206 of 283) of miR genes were located in regions that exhibited DNA copy number abnormalities.47 The location of miRs in a genomic region amenable to alterations is not a random event, suggesting that the loss or the gain of genomic regions including miRs in a specific type of cancer participates in the cause of the malignancy.48
The miR distribution in breast tumor tissues from more than 100 patients using in situ hybridization49 shows a different distribution and expression of many miRs in the breast cancer tissues compared with normal tissues. miR-145 and miR-205 were restricted to the myoepithelial/basal cell compartment of normal mammary ducts and lobules. However, their accumulation was reduced in matched tumor specimens. Compared with luminal epithelial cells in normal tissue, expression of miR-21 was frequently increased, whereas let-7a was decreased in malignant cells. Careful comparison of normal tissue and tumorous tissue in the same patient demonstrated altered expression of miR-17/20 in breast cancer. In normal tissue, the relative abundance of miR-17/20 is higher compared with matched tumor tissue from the same patient.22
Suppressor miRs in Breast Cancer
Tumor suppressor miRs can inhibit tumorigenesis by repressing oncogenes (Table 1). The ErbB family plays an important role in organismal development, cellular proliferation, and survival in human epithelial malignancies.50 ErbB2 is amplified and/or overexpressed in about 20% to 30% of human breast cancers. miR-125 targets the ErbB2 gene in breast cancer cells.51 miR-125a and mir-125b overexpression in SKBR3 cells decreased ErbB2 protein level ≈40% to 65% and decreased ErbB3 level ≈60% to 80%. SKBR3 cells overexpressing miR-125a or miR-125b were impaired in their anchorage-dependent growth, migration, and invasion capacities.51
Table 1.
miRs in Breast Cancer
The human miR-17/20 cluster's genomic location, chromosome 13q31, correlates with loss of heterozygosity in a number of different cancers including breast cancer.52,53 miR-17/20 decreased cyclin D1 abundance, suppressed MCF-7 cell proliferation, and inhibited G1/S phase transition of cell cycle.22 In human breast cancer cell lines, reduced miR-17/20 expression was inversely correlated with high cyclin D1 abundance. In human breast cancer specimens, decreased miR-17/20 expression correlated with high cyclin D1 abundance compared with matched normal breast tissues. Targeted gene deletion revealed that mice deficient for the miR-17/20 cluster die shortly after birth,54 therefore conditional gene deletion or tissue-specific transgenic techniques will be required to determine the function of miR-17/20 in mammary tumorigenesis.
Estrogen, which binds ER-α, is a risk factor for breast cancer development. ER-α is overexpressed in approximately 75% of primary breast cancers. It was reported that miR-206 expression is strongly inhibited by ER-α agonists, and miR-206 levels are higher in ER-α–negative MB-MDA-231 cells than ER-α–positive MCF-7 cells.55 miR-206 overexpression reduces ER-α level in MCF-7 cells, indicating an autoregulatory feedback loop between ER-α and miR-206. Decreased expression of miR-206 occurs in ER-α–positive human breast cancer tissue. miR-206 expression and ER-α protein level are inversely correlated in human breast cancer.56 In addition, miR-145 targets RTNK and inhibits breast tumor growth.57 miR-205 targets HER3 receptor, thereby inhibiting HER-mediated proliferative signaling.58
Oncogenic miRs in Breast Cancer
Several groups have noticed the frequent overexpression of miR-21 in breast tumors compared with the matched normal breast tissues (Table 1).49,59,60 miR-21 knock down inhibits MCF-7 cell growth in vitro and suppresses MCF-7 cell–derived breast tumor growth in a murine xenograft model.59 The tumor suppressor tropomyosin 1 (TPM1) was a target of miR-21 in MCF-7 cells. A genome-wide screen for miR-21 targets identified several p53-regulated mRNAs, including FAM3C, ACTA2, APAF1, BTG2, FAS, CDKN1A (p21), PDCD4, and SESN160 in MCF-7 cells, suggesting a functional link between miR-21 and the p53 tumor suppressor pathway.60
miR-27a was reported as a breast cancer oncomiR that regulates the zinc finger ZBTB10 gene,61 a putative repressor of oncogene specificity proteins (Sp). Inactivation of miR-27a in MDA-MB-231 cells induced ZBTB10 expression and reduced abundance of the Sp genes.62 miR-27a suppressed the expression of cdc2/cyclin B inhibitor Myt-1 in MDA-MB-231 cells, thereby increasing the cdc2/cyclin B activity and inducing breast cancer cell proliferation. Thus miRs regulate distinct signaling cascades in breast cancer cells.
miRs in Breast Cancer Metastases
Metastasis represents a complex process by which primary solid tumor cells invade adjacent tissue and grow into secondary tumor (Figure 3, A and B).63 Breast cancer cells have the potential to spread to almost any region of the body where they continue to grow and multiply. Expression of a metastasis suppressor gene, CD44, is reduced during progression of breast cancer to the metastatic phenotype.64 miR-373 and miR-520c stimulated human breast cancer cell migration and invasion in vitro and in vivo by suppressing the expression of CD44.65 miR-21 promotes breast cancer cell invasion and metastasis by targeting multiple tumor/metastasis suppressor genes.66 miR-10b is markedly upregulated in breast cancer cells compared with either primary human mammary epithelial cells or MCF-10A cells.67 miR-10b promotes cell migration in vitro and initiates breast tumor invasion in vivo by targeting gene HOXD10.67 Twist promotes epithelial to mesenchymal transition (EMT) and mammary tumor metastasis,68 and miR-10b is directly regulated by Twist through a transcriptional manner.67
Figure 3.
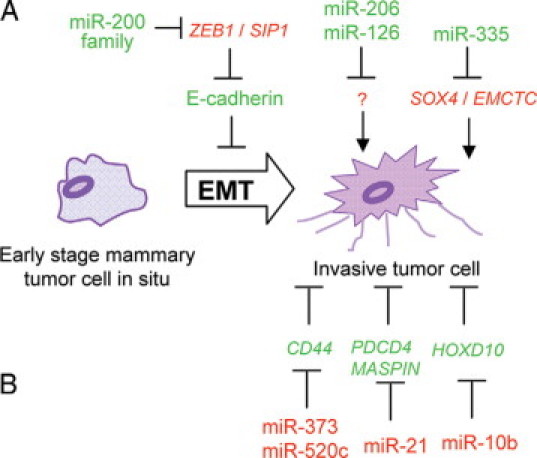
miRNA regulation of breast cancer metastasis. A: Metastasis inhibitor miRs in breast cancer. B: Metastasis inducer miRs in breast cancer.
In contrast, miR-335, miR-206, and miR-126 have been identified as human breast cancer metastasis suppressor miRs.63 miR-335 or miR-206 overexpression reduced cell migration of lung metastatic LM2 cells and bone metastatic BoM1 cells. The expression of miR-335 and miR-126 in human mammary tumors is inversely associated with metastatic relapse. Breast cancer metastasis and migration are inhibited by miR-335 through targeting the transcription factor SOX4, which is known to regulate progenitor cell development and migration.63
miR-200 family and miR-205 regulate EMT, which is thought to be an essential early step in tumor metastasis.69 Expression of these miRs is reduced in invasive breast cancer cell lines with a mesenchymal phenotype. Enforced expression of the miRs prevents TGF-β–induced EMT. Conversely, overexpression of these microRNAs in mesenchymal cells initiated mesenchymal to epithelial transition. The miR-200 family repressed ZEB1 and SIP1, which have been implicated in EMT induction and tumor metastasis.70 A ZEB1-miR200 family-feed-forward loop may stabilize EMT and hereby promote cancer cell invasion.71 Because most of the evidence for miRs in regulating metastasis has been performed in cultured cells, in vivo studies of miR using transgenic and knockout mice will be important.72
Regulation of miRs by Annotated Genes
miRs are also regulated by products of annotated genes, which opens the possibility of regulating miRs expression by external agents. For instance, a number of miRs are induced by all-trans-retinoic acid, and the promoters of several miRs have putative RARα receptor binding sites.73 p53 is known to induce miR-34,42 which in turn targets several genes whose expression is decreased by p53. The induction of miR-34 family by p53 allows p53 to regulate a large number of genes that are downstream targets of miR-34. The targeting of p53-regulated mRNAs by miR-34 may contribute to the fine tuning of the p53 response.74 This finding suggests a dual model for gene down-regulation of gene expression by p53, one by direct action on the target genes transcription or stability, and a second action via a specific miR (Figure 2F). Increased miR-223 expression in differentiating myeloid cells occurs via the binding of C/EBPα (an inducer of differentiation) to the miR-223 promoter.75 Another example of feedback regulation by annotated genes is the finding by Yu et al,22 in which cyclin D1 is targeted to the miR-17/20 cluster, which in turn regulates the expression of the same cluster. Thus cyclin D1, like C/EBPα and RARα, binds to the promoter region of miR to regulate their expression. The finding that cyclin D1 regulates miR promoter is consistent with previous finding that cyclin D1 targets gene promoter elements in the context of local chromatin.76,77 Feedback regulation of miR and annotated genes may be quite frequent, and a feedback between OCT4 and miR-145 will be discussed below.
miRs in Human Stem Cell Renewal and Differentiation
miRs were discovered as modulators of differentiation in embryos of lower animals. miR-145, which was reported to act as a tumor suppressor,16,78 also plays an important role in human stem cell growth and differentiation by targeting the 3′UTR of OCT4, SOX2, and KLF4.79 OCT4 in turn represses miR-145 expression (Figure 2E). Shi et al6 showed that miR-145 inhibits human cancer cell growth in vitro via the 3′UTR of the type 1 insulin-like growth factor receptor and its docking protein, the insulin receptor substrate-1 (IRS-1). Rubin et al80 found IRS-1 was highly expressed in undifferentiated murine embryonic stem cells (mESC), with decreased expression when the cells differentiated. Forced expression of IRS-1 inhibited mESC differentiation suggesting miR-145 and IRS-1 function in stem cell differentiation.
It is believed that only a small proportion of cancer cells display the stem/progenitor cell characteristics and retain the ability to form new tumors. These cells are termed tumor-initiating cells (T-ICs) or cancer stem cells.81 Breast tumor-initiating cells (BT-ICs) have been identified and isolated as CD44(+)CD24(−/low)Lineage(−) cells by Al-Hajj and colleagues.82 The miR let-7, which is reduced in BT-ICs and increased with differentiation, can regulate self renewal and tumorigenicity of breast cancer cells.36 Let-7 overexpression in BT-ICs inhibited cell proliferation and mammosphere formation, blocking tumor formation and metastasis in NOD/SCID mice.36 Two targets of let-7, RAS39 and HMGA2,38 showed high expression in BT-ICs and were silenced during differentiation.
Concluding Remarks
The regulation of miR expression by annotated genes indicates the importance of miR promoter regions and new possibilities for therapeutic intervention by using compounds to regulate miR expression. miRs have been identified that arrest cell cycle progression and suppress cell proliferation by negatively regulating cell cycle modulators in breast cancer cells. miRs that are overexpressed in breast cancers can limit the proliferation of tumor cells by targeting oncogenic genes. Because miRs are involved in breast cancer from the onset of the malignant state through the progression to metastasis, miR may be ideal targets for the development of new drug therapies for treating breast cancer patients.
Although the up- or down-regulation of a miR expression in cancers depends on the individual miR itself and tumor type, the global decrease of miRs in cancers reflects the tendency in the direction of the differential expression of miRs in human cancers.83 This finding suggests that miRs may serve a general tumor suppressor function. The regulation of miRs may therefore form the basis for a therapeutic strategy for cancers. Synthetic miR mimics, DNA expression vectors containing artificial miR precursor sequences and/or miR inhibitors (modified miR antisense oligonucleotides), have strong potential as new therapeutic tools.83
Acknowledgements
We thank Atenssa L. Cheek for the preparation of this manuscript.
Footnotes
Supported in part by R01CA70896, R01CA75503, R01CA107382 (to R.G.P.), and R01CA120876 (to M.P.L.). The Kimmel Cancer Center was supported by the NIH Cancer Center Core grant P30CA56036 (to R.G.P.). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from Pennsylvania Department of Health (to R.G.P.). The Department specifically disclaims responsibility for an analysis, interpretations or conclusions.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University or Albert Einstein College of Medicine was involved in the peer review process or final disposition for this article.
References
- 1.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell. 2004;16:861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 5.Cui Q, Yu Z, Purisima EO, Wang E. Principles of microRNA regulation of a human cellular signaling network. Mol Syst Biol. 2006;2:46. doi: 10.1038/msb4100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem. 2007;282:32582–32590. doi: 10.1074/jbc.M702806200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–289. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Rajewsky N. Deep conservation of microRNA-target relationships and 3′UTR motifs in vertebrates, flies, and nematodes. Cold Spring Harb Symp Quant Biol. 2006;71:149–156. doi: 10.1101/sqb.2006.71.039. [DOI] [PubMed] [Google Scholar]
- 9.Tili E, Michaille JJ, Gandhi V, Plunkett W, Sampath D, Calin GA. miRNAs and their potential for use against cancer and other diseases. Future Oncol. 2007;3:521–537. doi: 10.2217/14796694.3.5.521. [DOI] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 14.Cummins JM, Velculescu VE. Implications of micro-RNA profiling for cancer diagnosis. Oncogene. 2006;25:6220–6227. doi: 10.1038/sj.onc.1209914. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed FE. Role of miRNA in carcinogenesis and biomarker selection: a methodological view. Expert Rev Mol Diagn. 2007;7:569–603. doi: 10.1586/14737159.7.5.569. [DOI] [PubMed] [Google Scholar]
- 16.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 17.Lee RJ, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, Haines GK, 3rd, Siegel PM, Hung MC, Yarden Y, Horowitz JM, Muller WJ, Pestell RG. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 19.Hunt KK, Keyomarsi K. Cyclin E as a prognostic and predictive marker in breast cancer. Semin Cancer Biol. 2005;15:319–326. doi: 10.1016/j.semcancer.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T, McCue P, Pestell RG. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16–1 down-regulation in pituitary adenomas. J Cell Physiol. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- 26.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI, Alder H, Volinia S, Rassenti L, Liu X, Liu CG, Kipps TJ, Negrini M, Croce CM. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 32.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, Kim VN. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 35.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 36.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 37.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 38.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Tsang WP, Kwok TT. Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008;13:1215–1222. doi: 10.1007/s10495-008-0256-z. [DOI] [PubMed] [Google Scholar]
- 41.Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–557. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 42.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 44.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 45.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 46.Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Orntoft TF, Andersen CL, Dobbelstein M. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68:10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O'Brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, Schwartz G, Wells W, Kauppinen S, Cole CN. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 50.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 51.Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem. 2007;282:1479–1486. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- 52.Eiriksdottir G, Johannesdottir G, Ingvarsson S, Bjornsdottir IB, Jonasson JG, Agnarsson BA, Hallgrimsson J, Gudmundsson J, Egilsson V, Sigurdsson H, Barkardottir RB. Mapping loss of heterozygosity at chromosome 13q: loss at 13q12–q13 is associated with breast tumour progression and poor prognosis. Eur J Cancer. 1998;34:2076–2081. doi: 10.1016/s0959-8049(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 53.Lin YW, Sheu JC, Liu LY, Chen CH, Lee HS, Huang GT, Wang JT, Lee PH, Lu FJ. Loss of heterozygosity at chromosome 13q in hepatocellular carcinoma: identification of three independent regions. Eur J Cancer. 1999;35:1730–1734. doi: 10.1016/s0959-8049(99)00205-1. [DOI] [PubMed] [Google Scholar]
- 54.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 56.Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. miR-206 Expression is down-regulated in estrogen receptor alpha-positive human breast cancer. Cancer Res. 2008;68:5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, Zhou H, Zhao RC. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34:1461–1466. [PubMed] [Google Scholar]
- 58.Iorio MV, Casalini P, Piovan C, Di Leva G, Merlo A, Triulzi T, Menard S, Croce CM, Tagliabue E. microRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69:2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- 59.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 60.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 61.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 62.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 63.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 65.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Pure E, Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 66.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 67.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 69.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 70.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 71.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma L, Weinberg RA. Micromanagers of malignancy: role of microRNAs in regulating metastasis. Trends Genet. 2008;24:448–456. doi: 10.1016/j.tig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H, Calin GA, Liu CG, Andreeff M, Croce CM. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 74.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 75.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–831. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 76.Wang C, Pattabiraman N, Zhou JN, Fu M, Sakamaki T, Albanese C, Li Z, Wu K, Hulit J, Neumeister P, Novikoff PM, Brownlee M, Scherer PE, Jones JG, Whitney KD, Donehower LA, Harris EL, Rohan T, Johns DC, Pestell RG. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma expression and transactivation. Mol Cell Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu M, Rao M, Bouras T, Wang C, Wu K, Zhang X, Li Z, Yao T-P, Pestell RG. Cyclin D1 inhibits PPARgamma -mediated adipogenesis through HDAC recruitment. J Biol Chem. 2005;280:16934–16941. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- 78.Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 79.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4. SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 80.Rubin R, Arzumanyan A, Soliera AR, Ross B, Peruzzi F, Prisco M. Insulin receptor substrate (IRS)-1 regulates murine embryonic stem (mES) cells self-renewal. J Cell Physiol. 2007;213:445–453. doi: 10.1002/jcp.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ponti D, Zaffaroni N, Capelli C, Daidone MG. Breast cancer stem cells: an overview. Eur J Cancer. 2006;42:1219–1224. doi: 10.1016/j.ejca.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]


