Abstract
Autoimmune regulator (AIRE) modulates the expression of tissue-restricted antigens (TSAs) and promotes central tolerance in the thymus. However, few autoreactive T cells escape negative selection and reach the periphery, where peripheral tolerance is required to avoid autoimmunity. Murine lymph nodes (LNs) have been shown to contain “stromal” cells expressing AIRE and TSAs. Here we report the occurrence of AIRE-expressing cells in human peripheral lymphoid tissues, including LNs, tonsils, and gut-associated lymphoid tissue, with the exception of the spleen. Notably, AIRE+ cells are absent in fetal LNs and, in postnatal life, they are more numerous in abdominal than in superficial LNs, thus suggesting that their development in periphery may depend on instructive signals from microenvironment and antigen challenge. Extrathymic AIRE+ cells show a dendritic morphology, consistently express human leukocyte antigen-DR (HLADR) and fascin, and are largely positive for CD11c and S100 and for the dendritic cell-activation markers CD40, CD83, DC-LAMP/CD208, and CCR7. Lymphoid, myelomonocytic, mesenchymal, and epithelial cell lineage markers are negative. The HLADRhigh/AIRE+ cell fraction isolated from mesenteric LNs expressed TSAs (insulin, CYP17A1, and CYP21A2), as well as molecules associated with tolerogenic functions, such as interleukin-10 and indoleamine 2,3-dioxygenase. Data indicate that AIRE+ cells in human peripheral lymphoid tissues correspond to a subset of activated interdigitating dendritic cells expressing TSAs and the tolerogenic molecules indoleamine 2,3-dioxygenase and interleukin-10, suggestive of a potential tolerogenic function.
Central tolerance in the thymus is predominantly mediated by a subset of medullary epithelial cells1 that have a distinctive phenotype (cytokeratin-5+, claudin-4+, Ulex europaeus agglutinin [UEA]-1+, and major histocompatability complex [MHC]-IIhigh)2 and express autoimmune regulator (AIRE), a transcription factor that regulates the expression of peripheral tissue-specific antigens.3–5 Although most newly generated autoreactive T cells are negatively selected on interaction with AIRE+ medullary epithelial cells, a few escape central tolerance selection and reach the periphery, where peripheral tolerance induction is then required to prevent autoimmunity.6 It has been postulated that AIRE may also play a role in peripheral tolerance induction,5 but AIRE expression in human extrathymic tissues remains controversial. In particular, AIRE mRNA has been detected in various peripheral lymphoid and nonlymphoid tissues and is barely detectable in peripheral blood mononuclear cells7–9; however, expression of AIRE protein outside of thymus has been not firmly demonstrated.10–12
Recently, two independent studies reported that CD45−MHC-IIhigh cells expressing AIRE are present in murine lymph nodes (LNs).13,14 Gene expression analysis showed that these cells express a subset of tissue-specific antigens that only partially overlap with those found in medullary epithelial cells and induce deletion of autoreactive T cells.
We have performed an extensive analysis of AIRE expression in human tissues and found that, in addition to the thymus, AIRE+ cells regularly occur in peripheral lymphoid tissues. Phenotypic analysis of these cells indicates that they correspond to a subset of activated interdigitating dendritic cells expressing molecules associated with tolerogenic functions, such as indoleamine 2,3-dioxygenase and interleukin-10.
Materials and Methods
Tissues
A wide range of normal human formalin-fixed paraffin-embedded and freshly frozen samples were retrieved from the archive of the Department of Pathology, Spedali Civili of Brescia, in accordance with the protocols of the Spedali Civili of Brescia ethical board (Table 1). Fetal tissues at different gestational ages were obtained from spontaneous abortions (first trimester) and from six autopsies (second and third trimester). From all tissues at least three different samples were examined. Postnatal tissues included tonsils (20) and reactive LNs (95) from different anatomical sites (27 cervical, 25 mesenteric, 15 axillary, 12 inguinal, 8 peripancreatic, and 8 pulmonary LNs) and from patients of different ages (17, 34, and 44 LNs, respectively, from patients aged <30, 31 to 60, and >60 years) and gender (53 males and 42 females). Normal thymic biopsy samples, obtained anonymously from patients who underwent elective surgery for correction of cardiovascular defects, were used as positive controls for AIRE expression, whereas negative controls were represented by the omission of the primary antibody as well as LNs and thymic biopsy samples from patients with severe human primary immunodeficiencies lacking AIRE expression.15
Table 1.
AIRE Expression in Human Fetal and Adult Tissues
| Tissues | Results |
|---|---|
| Cardiovascular system | |
| Heart, blood vessels | Negative |
| Endocrine glands | |
| Thyroid, parathyroid, adrenal | Negative |
| Eye and annexa | |
| Retina, cornea, conjunctiva, lacrimal glands | Negative |
| Female genital system | Negative |
| Ovary and uterine tubes, vulva, cervix and uterus | Negative |
| Placenta | Negative |
| Gastrointestinal tract | |
| Tongue, lips, oral mucosae, esophagus, stomach | Negative |
| Small and large bowel | Rare positive cells in GALT (*negative) |
| Appendix | Rare positive cells in GALT (*negative) |
| Liver, bile ducts and gallbladder | Negative |
| Pancreas | Negative |
| Hemolymphopoietic system | |
| Bone marrow | Negative |
| Thymus | Positive |
| Lymph nodes, tonsils and adenoids | Positive (*negative) |
| Spleen | Negative |
| Integumentary system | |
| Skin and skin annexa | Negative |
| Male genital system | |
| Testis and epididymis, seminal vesicles, prostate | Negative |
| Mammary gland | Negative |
| Nervous system | |
| Central and peripheral nervous system | Negative |
| Respiratory system | |
| Nasal cavity, larynx, trachea, bronchi, lung, pleura | Negative |
| Salivary glands | |
| Major and minor salivary glands | Negative |
| Soft tissues | |
| Muscle, nerve, bone, cartilage | Negative |
| Urinary system | |
| Kidney, urinary tract and bladder | Negative |
When not specified, fetal and postnatal tissues show identical expression.
GALT, gut-associated lymphoid tissues, including Peyer's patches.
Different expression in fetal tissues compared to adult.
Immunohistochemical Procedures
Sections from both paraffin-embedded and frozen tissue blocks were stained using a mouse monoclonal anti-human AIRE antibody.12 Cryostat sections were fixed in acetone for 10 minutes before staining, whereas paraffin sections underwent heat-based treatment for antigen retrieval using a microwave oven or a thermostatic bath in 1.0 mmol/L EDTA buffer (pH 8.0). Single immunostains were revealed by the ChemMATE EnVision HRP system (DAKO, Glostrup, Denmark) or the Super-Sensitive Detection System (BioGenex, San Ramon, CA), followed by diaminobenzidine/hydrogen peroxide as chromogen and hematoxylin as counterstain. Double immunostains were performed for AIRE and the antigens listed in Table 2, for which appropriate biotinylated secondary antibodies were applied (DAKO), followed by streptavidin-conjugated alkaline phosphatase (DAKO); the chromogen reaction was developed by either Ferangi Blue or Vulcan Red Chromogen Kits (Biocare Medical, Concord, CA), and nuclei were counterstained with methyl green.
Table 2.
List of the Antibodies Used in Double Immunostains to Characterize AIRE-Expressing Cells in Human Lymph Nodes and Corresponding Results
| Antibodies | Species/isotype/clone/dilution/source | Results |
|---|---|---|
| Lymphoid antigens | ||
| CD3 | Rp/n.d./SP7/1:100/LV | Negative |
| CD5 | Mm/IgG1/4C7/1:50/NC | Negative |
| CD20 | Mm/IgG2a/L26/1:250/DK | Negative |
| Myeloid and macrophage antigens | ||
| CD14 | Mm/IgG2a/7/1:50/NC | Negative |
| CD33 | Mm/IgG2b/PWS44/1:100/NC | Negative |
| CD68 | Mm/IgG1/KP1/1:300/DK | Negative |
| CD163 | Mm/IgG1/10D6/1:50/TS | Negative |
| DC-associated antigens | ||
| Myeloid DCs | ||
| CD1a | Mm/IgG1/010/1:50/DK | Negative |
| CD1c/BDCA-1* | Mm/IgG2a/AD5-8E7/1:50/MC | Negative |
| CD11c | Mm/IgG2a/5D11/1:30/NC | Positive (91%)† |
| CD207/langerin | Mm/IgG2b/12D6/1:200/VL | Negative |
| CD209/DC-SIGN* | Mm/IgG2b/n.d./‡ | Negative |
| Factor XIIIa | Rp/n.d./n.d./1:100/BG | Negative |
| Fascin | Mm/IgG1/55K-2/1:50/DK | Positive (100%) |
| S-100 | Rp/n.d./n.d./1:5000/DK | Positive (78%) |
| Plasmacytoid DCs | ||
| CD123 | Mm/IgG2a/7G3/1:150/PH | Negative |
| CD303/BDCA-2 | Mm/IgG1/124B3.13/1:50/DC | Negative |
| CD2AP | Mm/IgG1/B-4/1:1000/SC | Negative |
| Activation and maturation DC-associated antigens | ||
| CCR7 | Mm/IgG2a/150503/1:50/RD | Positive (74%) |
| CD208/DC-LAMP | Mm/IgG1/104.G4/1:100/IM | Positive (83%) |
| CD40 | Mm/IgG2b/11E9/1:20/NC | Positive (100%) |
| CD83 | Mm/IgG1/HB15e/1:20/PH | Positive (42%) |
| CD86* | Gp/n.d./n.d./1:20/SC | Negative |
| HLADR | Mm/IgG1/CR3-43/1:150/DK | Positive (100%) |
| Stromal and endothelial antigens | ||
| Desmin | mm/IgG1/33/1:100/BG | Negative |
| Podoplanin | Mm/IgG1/D2-40/1:40/SG | Negative |
| Smooth muscle actin | Mm/IgG1/HHF35/1:100/DK | Negative |
| Vimentin | Mm/IgG1/V-9/1:100/BG | Negative |
| CD31 | mm/IgG2b/4C9/1:50/NC | Negative |
| Factor VIII-related antigen | Rp/n.d./n.d./1:100/NK | Negative |
| Epithelial antigens | ||
| Wide spectrum cytokeratins | Mm/IgG1/MNF116/1:100/DK | Negative |
| Cytokeratins 8/18/19 | Mm/IgG2a/CAM5.2/1:50/BD | Negative |
| Cytokeratin 5 | Rp/n.d./AF138/1:100/CV | Negative |
| Cytokeratin 8 | Rm/n.d./TROMA-1/1:200/‡ | Negative |
| Claudin-4 | Mm/IgG1/3E2C1/1:100/ZY | Negative |
| Epithelium-specific antigen | Mm/IgG1/VU-1D9/r.t.d./NC | Negative |
| UEA-1 | Lectin/n.d./n.d./1:600/VL | Negative |
| Miscellaneous | ||
| CD16 | Mm/IgG2a/2H7/1:80/NC | Negative |
| CD25 | Mm/IgG2b/4C9/1:150/NC | Negative |
| CD34 | Mm/IgG1/QBEnd-10/1:200/NK | Negative |
| CD43 | Mm/IgG1/MT1/1:40/NC | Negative |
| CD45 | Mm/IgG1/RP2-18;RP2-22/1:200/NC | Negative |
| CD45RA | Mm/IgG1/MB1/1:100/BT | Negative |
| CD45RB | Mm/IgG1/PD7-26/1:200/DK | Negative |
| CD45RC | Mm/IgG1/MT2/1:5/BT | Negative |
| CD45RO | Mm/IgG2a/UCL1/1:300/NK | Negative |
| CLUSTERIN | Mm/IgG1/7D1/1:200/NC | Negative |
| CXCL12/SDF-1 | Mm/IgG1/78018/1:50/RD | Negative |
| CXXL13/BCA1 | Gp/n.d./n.d./1:30/RD | Negative |
| IDO | Rp/n.d./n.d./1:1500/CM | Positive (92%) |
Mn, mouse monoclonal; Rm, rat monoclonal; Rp, rabbit polyclonal; Gp, goat polyclonal; n.d., non definable; r.t.d., ready to use; LV, LabVision Corporation (Fremont, CA); NC, Novocastra (Castle upon Tyne, UK); DK, DAKO (Glostrup, Denmark); TS, Thermo Fisher Scientific (Fremont, CA); MC, MACS (Gladbach, Germany); VL, Vector Laboratories (Burlingame, CA); BG, Biogenex (San Ramon, CA); PH, Pharmingen (San Diego, CA); DC, Dendritics (Lyon, France); SC, Santa Cruz Technology, Inc. (Santa Cruz, CA); RD, R&D Systems (Minneapolis, MN); IM, Immunotech (Praha, Czech Republic); SG, Signet (Dedham, MA); NK, Neomarkers (Fremont, CA); BD, Becton Dickinson (San Jose, CA); CV, Covance (Princeton, NJ); ZY, Zymed (San Francisco CA); BT, Biotest (Dreieich, Germany); CM, Chemicon (Hofheim, Germany).
Antibodies were applied on frozen sections.
Percentages of double-positive cells/total AIRE+ cells.
Anti-DC-SIGN was kindly provided by M. Cella (Washington University, St. Louis, MO) and anti-cytokeratin 8 by U.H. von Andrian (Harvard Medical School, Boston, MA).
Flow Cytometry Analysis (Fluorescence-Activated Cell Sorting) and Immunomagnetic Cell Depletion
Cell suspensions were obtained by mechanical disruption in RPMI 1640 medium supplemented with 10% fetal calf serum from mesenteric LNs draining inflammatory intestinal diseases (eg, diverticulitis or intestinal occlusion). After red blood cell lysis, cells were resuspended in EDTA/fluorescence-activated cell sorting (FACS) buffer (2% fetal calf serum, 0.02% NaN3, and 5 mmol/L EDTA in PBS) and passed through a 100-μm filter. FACS analysis was performed either on cell suspension from whole LNs or after immunomagnetic cell depletion using CD45-conjugated microbeads according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). For superficial staining at least 106 cells in 150 μl of EDTA/FACS buffer were incubated with purified mouse anti-human CD40 followed by a goat anti-mouse Alexa 488-conjugated secondary antibody and after staining with CD45-allophycocyanin, human leukocyte antigen-DR (HLADR)-PerCp CD83-phycoerythrin-conjugated antibodies (BD Pharmingen, San Diego CA). At least 500,000 events were acquired by FACSCanto II and analyzed with BD FACSDiva software (version 5.0.1).
Isolation of an AIRE-Enriched Cell Fraction
Cell suspensions from mesenteric LNs have been highly enriched for the AIRE-expressing cell population by combining low-density (OptiPrep) cell separation for dendritic cells followed by immunomagnetic cell sorting using AutoMACS. In brief, cell suspensions from LNs were obtained by mechanical disruption and digestion in a PBS buffer containing 2.5 ml of collagenase/dispase (0.5 mg/ml; Roche Diagnostics, Indianapolis, IN) and DNase (1.5 μg/ml). Cells were then resuspended in RPMI 1640 and filtered through a 100-μm cell strainer before isolation by low-density fraction using the OptiPrep protocol C21 (low-density fraction contains 25 × 106 cells). Cells were then washed in MACS buffer before immunomagnetic cell depletion with CD14-, CD19-, and DC-SIGN-conjugated microbeads, according to the manufacturer's instructions (Miltenyi Biotec) (cell fraction contains 7 × 106 cells). The negative fraction was further enriched by positive selection of the HLADR-positive cells using HLADR-conjugated microbeads.
mRNA Extraction and Real-Time PCR
Total mRNAs were extracted from fresh frozen tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. AIRE and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression were measured using “assay on demand” real-time PCR kits (AIRE: Hs00230833_m1; GAPDH: Hs99999905_m1; Applied Biosystems). cDNA was combined with 10 μl of TaqMan Universal PCR Master Mix and 2 μl of primers/probe in the thermal cycling conditions as follows: 50°C for 2 minutes, 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute. Samples were analyzed using the MJ Research PTC-200 Peltier Thermal Cycler and MJ Opticon Monitor analysis software 3.1 (Bio-Rad Laboratories, Hercules, CA). The expression level of the target gene was achieved by using the comparative cycle of threshold (Ct) method, normalized to the housekeeping gene GAPDH and represented as arbitrary units. mRNA from AIRE-enriched cell fractions was extracted as described. Total mRNA was reverse-transcribed to cDNA using SuperScript III (Invitrogen), 10 mmol/L dNTP mix, oligo(dT)18 primer, and RiboLock RNase inhibitor (Fermentas, Vilnius, Lithuania), and cDNA was run using an ABI Prism 7900 sequence Detection System (Applied Biosystems) and quantitative PCR SYBR Green Core Kit (Eurogentec, Seraing, Belgium) according to the manufacturer's instructions. SYBR Green fluorescence was measured after each extension step, and specificity of amplification was evaluated by melting curve analysis. Relative gene expression levels were calculated using the comparative Ct method. Every sample was run in three parallel reactions. Primers for all target genes and housekeeping β-actin (ACTb) gene are listed in Table 3.
Table 3.
Primer Sequences Used for Real Time RT-PCR Analysis
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| ACTb | 5′-CTGGAACGGTGAAGGTGACA-3′ | 5′-CGGCCACATTGTGAACTTTG-3′ |
| AIRE | 5′-GAGAGTGCTGAGAAGGACA-3′ | 5′-GTTTAATTTCCAGGCACATGA-3′ |
| IL10 | 5′-TTCCCAGGCAACCTGCCTAA-3′ | 5′-TGTCCAGCTGATCCTTCATTTGA-3′ |
| IDO | 5′-CCAAAGCAGCGTCTTTCAGTG-3′ | 5′-GGTGGCATATATCTTCTCATGTCC-3′ |
| CYP17A1 | 5′-GTAACCGTCTCCTCCTGCTG-3′ | 5′-ACTTCTGTGCCCTTGTCCAC-3′ |
| CYP21A2 | 5′-AGAGGGTGTTTGCTGTGGTC-3′ | 5′-GTGGAGGGACATGATGGACTAC-3′ |
| INSULIN | 5′-GCAGCCTTTGTGAACCAACA-3′ | 5′-GTGTGTAGAAGAAGCCTCGTTCC-3′ |
Image Elaboration and Cell Counting
Microscopic images were acquired with an Olympus DP70 digital camera mounted on an Olympus Bx60 microscope using CellF imaging software (Soft Imaging System GmbH, Münster, Germany). AIRE+ cells were counted on 10 different high-power fields (corresponding to 8 mm2 of tissue) for each section, selecting areas with the highest number of positive cells; values are expressed as number of cells/mm2 ± SD. Likewise, the number of AIRE+ cells coexpressing CD11c, S-100, fascin, HLADR, CD40, CD83, CD208/DC-LAMP, CCR7, and indoleamine 2,3-dioxygenase (IDO) were evaluated in 10 high-power fields, and positive cells were expressed as a percentage of double-positive cells/total AIRE+ cells.
Statistical Analysis
Statistical analysis was performed using the Mann-Whitney test for unpaired nonparametric data. Differences were considered significant when P < 0.05.
Results
Extrathymic Expression of AIRE Is Restricted to Peripheral Lymphoid Tissues
Using immunohistochemistry we investigated the expression of AIRE in a large series of human adult and fetal tissues (Table 1). In adult samples AIRE-expressing cells were identified in medullary epithelial cells of the thymus and in peripheral lymphoid tissues including LNs, tonsils, and gut-associated lymphoid tissue (GALT), with the notable exception of the spleen (Figure 1, A and B). Extrathymic AIRE+ cells were regularly found in the LNs, albeit at lower numbers than in the thymus (5.43 ± 1.27 versus 30.29 ± 4.79 AIRE+ cells/mm2); only occasional AIRE+ cells were detected in the tonsils and GALT (1.8 ± 0.6 and 1.1 ± 0.2 AIRE+ cells/mm2, respectively). Immunohistochemical data were confirmed by quantitative RT-PCR analysis (Figure 1A). In fetal tissues AIRE+ cells were detectable only in the thymus at all gestational ages (first, second, and third trimesters) (Figure 1D) and were more numerous in full-term fetuses (data not shown).
Figure 1.
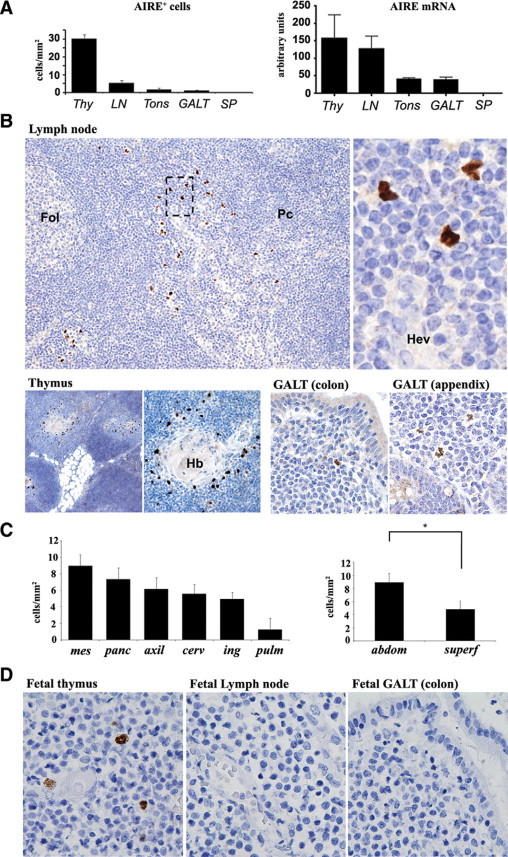
Expression of AIRE in human thymus and peripheral lymphoid tissues. A: The number of AIRE+ cells/mm2 evaluated by immunohistochemistry (left plot) and AIRE mRNA levels analyzed by quantitative RT-PCR (right plot) show that extrathymic AIRE expression occurs particularly in LNs, albeit at lower level than in the thymus (Thy), tonsils (Tons), and GALT but not in the spleen (SP). B: In LNs (top panel) AIRE+ cells are distributed as scattered cells in the interfollicular area/outer paracortex (Fol, follicle; Pc, paracortex), in the vicinity of high endothelial venules (Hev), as indicated in the higher magnification from the dotted box area (original magnification: ×10 and ×80). In the thymus (bottom panel, left), AIRE+ cells occur in the medulla and correspond to medullary epithelial cells around Hassall's bodies (Hb) (original magnification: ×4 and ×10). Rare cells are present in the colon, appendix, and GALT (bottom panel, right) (original magnification: ×20). C: The number of nodal AIRE+ cells differs in anatomical regions (left plot). The difference between AIRE+ cells in abdominal (mesenteric plus peripancreatic) compared with superficial (axillary, inguinal, and cervical) LNs is statistically significant (right plot; *P < 0.001). mes, mesenteric; panc, peripancreatic; axil, axillary; cerv, cervical; ing, inguinal; pulm, pulmonary; abdom, abdominal; superf, superficial. D: In the fetus, only the thymus contains AIRE+ cells in the medulla, whereas no positive cells occur in LNs and GALT (all panels, original magnification: ×40). All immunostains for AIRE were performed using diaminobenzidine/hydrogen peroxide as the chromogen and hematoxylin as the nuclear counterstain. Bar graphs show averages and SEs of at least three independent measurements.
In the LNs AIRE+ cells showed irregularly shaped nuclei and were located in the outer paracortex/interfollicular area, close to high endothelial venules (Figure 1B). Their distribution differed in various anatomical sites, with the highest number occurring in mesenteric (8.9 ± 1.25 AIRE+ cells/mm2) and peripancreatic (7.3 ± 1.22 AIRE+ cells/mm2) LNs compared with axillary (6.4 ± 1.17 AIRE+ cells/mm2), cervical (5.6 ± 1.42 AIRE+ cells/mm2), inguinal (4.9 ± 1.16 AIRE+ cells/mm2), and pulmonary (1.2 ± 0.83 AIRE+ cells/mm2) LNs. The difference between the number of AIRE+ cells found in abdominal versus superficial LNs was statistically significant (6.70 ± 2.2 vs. 4.5 ± 3.0 AIRE+ cells/mm2; P < 0.001) (Figure 1C). Interestingly, the number of AIRE+ cells increased during adult age, peaked between 31 and 60 years, and lowered in elderly individuals (<30 years: 4.1 ± 4.3 AIRE+ cells/mm2; 30–60 years: 5.5 ± 2.74 AIRE+ cells/mm2; >60 years: 4.7 ± 3.01 AIRE+ cells/mm2), but the differences did not reach statistical significance. AIRE+ cells were similarly distributed between genders (data not shown).
AIRE-Expressing Cells Show a Myeloid Dendritic Cell Phenotype
With the aim of characterizing the nature of the AIRE+ cells occurring in LNs, we performed a series of double immunostains (Table 2, Figure 2A), which showed that AIRE+ cells consistently coexpressed HLADR and fascin and were frequently positive for the myeloid dendritic cell markers CD11c and S100 (91.2 ± 1.6 and 78.9 ± 1.4% of total AIRE+ cells, respectively). In addition AIRE+ cells strongly expressed antigens typically found on activated or mature dendritic cells, such as CD40, CD208/DC-LAMP, CCR7, and CD83 (respectively, 90.4 ± 1.35, 83.2 ± 1.28, 74.3 ± 1.72, and 42.8 ± 1.86% of total AIRE+ cells). Interestingly, all double-positive immunostains showed that the AIRE+ cells displayed an obvious dendritic morphology and were admixed with AIRE− cells showing similar features and phenotype. Notably, AIRE+ cells were negative for CD45 common epitopes and isoforms (RA, RB, RC, and RO). It should be noted that lack of CD45 antigens is not in contrast with the dendritic cell nature of the AIRE+ cells, because we found that interdigitating dendritic cells occurring in dermatopathic lymphadenitis were largely negative for CD45 (Figure 2A). The immunohistochemical data have been confirmed on cell suspensions obtained by low-density gradient enrichment from mesenteric LNs, in which the CD45lowHLADRhigh cell fraction showed high levels of CD40 and a light increase in CD83 density (Figure 2B). Because data obtained in mice showed that nodal AIRE+ cells probably represent fibroblastic reticulum cells, we used a series of antibodies identifying this cell population of the nodal stroma (such as smooth muscle actin, vimentin, desmin, UEA-I, and subsets of cytokeratins), all showing negative results on AIRE-expressing cells (Figure 2A). Taken together, these data indicate that nodal AIRE+ cells represent a subset of interdigitating dendritic cells with a mature/activated phenotype.
Figure 2.
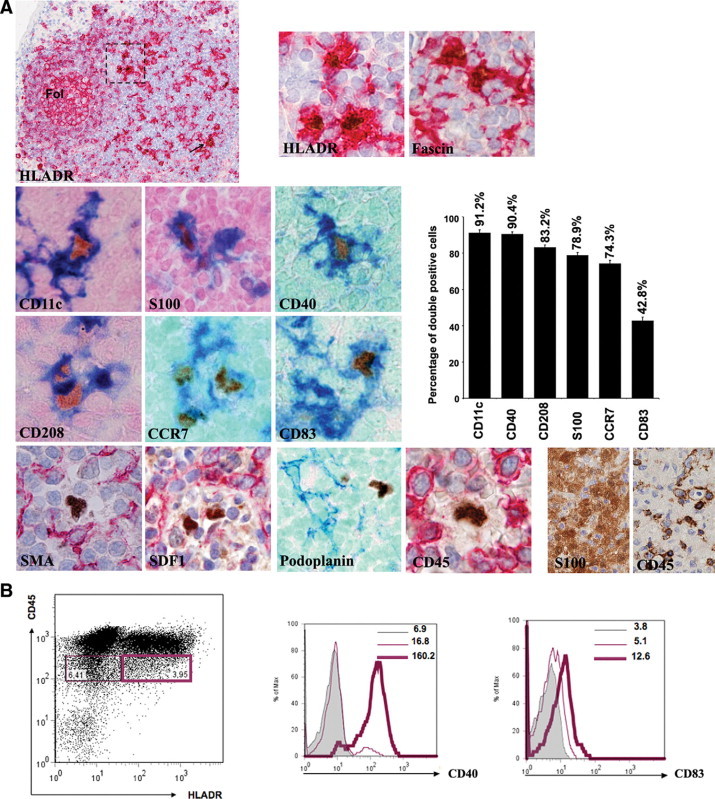
A: Characterization of nodal AIRE-expressing cells by double immunostains. AIRE+ cells consistently express HLADR and are distributed in the outer paracortex and in the interfollicular areas (arrow and dotted square), admixed with HLADR+AIRE− cells corresponding to IDCs. They coexpress other molecules normally found on IDCs, such as fascin, CD11c, and S100, as well as the DC activation and maturation antigens CD40, CD208/DC-LAMP, CCR7, and CD83. The plot shows the percentages of double-positive/total AIRE+ cells. In all figures the dendritic morphology of the AIRE+ cells is obvious. In the bottom row examples of molecules normally expressed by fibroblastic reticulum cells (smooth muscle actin [SMA], stromal cell-derived factor-1 [SDF1], and podoplanin) and lacking on the AIRE+ cells are shown. AIRE+ cells are also negative for CD45; this finding is not in contrast with their dendritic cell nature, because IDCs in dermatopathic lymphadenitis are composed of S100+ IDCs that largely lack CD45, as shown in the bottom right serial sections. In double stains AIRE is developed in brown with diaminobenzidine/hydrogen peroxide, whereas the other antigen is developed either in blue or red, and nuclear counterstain with methyl green or hematoxylin, respectively. All figures, original magnification: ×40, except for the upper left figure (×4) and the lower right two figures (×20). B: Dot plot of cell suspension (left) obtained from mesenteric LNs after low-density gradient enrichment shows distribution of the CD45lowHLADRhigh (purple heavy line) in comparison with CD45lowHLADR− (purple normal line). The CD45lowHLADRhigh cell fraction displays higher level of CD40 (center) and a light increase of CD83 (right). Gray histogram represents the isotype control. Numbers indicate the mean fluorescence intensity for each population. Representative profiles of three independent experiments are shown.
AIRE-Expressing Dendritic Cells Show a Tolerogenic Phenotype
In keeping with data obtained in mice,13,14 flow cytometry showed that the CD45low/−/HLADRhigh cells represent a minor fraction (0.2 to 0.4%) of the total LN cells (data not shown). This observation, along with the lack of a specific surface cell marker for the AIRE+ cells, made cell sorting of this cell population virtually impossible. We therefore investigated the phenotype and potential biological role of the AIRE+ cells, performing in vitro studies on cell suspensions highly enriched for AIRE-expressing cells obtained from human mesenteric LNs, by using a low-density gradient followed by negative selection of monocytes (CD14), B-cells (CD19), and immature dendritic cells (DC-SIGN/CD209), and positive selection for HLADR. Notably, only the enriched HLADR+CD14−CD19−CD209− cell fraction but not the HLADR−CD14−CD19−CD209− cell fraction showed expression of AIRE mRNA and the AIRE-dependent tissue-restricted antigens insulin, CYP17A1, and CYP21A2. AIRE and tissue antigen mRNA levels from LN sorted cells were lower than those obtained from CD45-depleted thymic stromal cells (Figure 3).
Figure 3.
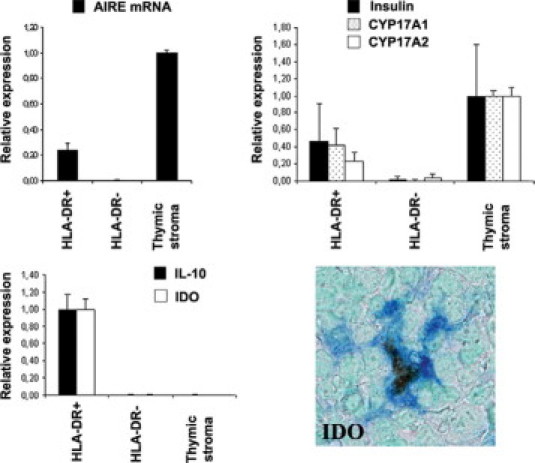
Nodal AIRE+ cells express tissue-restricted antigens and show a tolerogenic phenotype. Quantitative RT-PCR analysis on cell suspensions from mesenteric LNs previously enriched for AIRE expressing DCs (top left): the enriched HLADR+ but not the HLADR− cell fraction expresses AIRE mRNA and the AIRE-dependent tissue-restricted antigens insulin, CYP17A1, and CYP21A2 (top right). Quantitative RT-PCR shows that the HLADR+AIRE+-enriched cell fraction has an increased expression of IDO and IL-10 transcripts (bottom left), and double immunostain confirms the coexpression of IDO by the AIRE+ cells, suggesting a tolerogenic phenotype (bottom right). mRNA is expressed as relative gene expression levels. Averages and SEs (bars) of three independent experiments are shown. For double stain, AIRE is developed in brown with diaminobenzidine/hydrogen peroxide; IDO is developed in blue; nuclear counterstain is with methyl green. Original magnification, ×40.
To evaluate the hypothesis that AIRE+ dendritic cells of human LNs may express molecules related to a tolerogenic phenotype, using the same cell enrichment procedure, we analyzed by real-time PCR mRNA levels of IDO and interleukin (IL-10) and found that they were selectively expressed in the HLADR+CD14−CD19− CD209− cell fraction, whereas they were absent in the HLADR−CD14−CD19−CD209− nodal cell fraction, as well as in CD45-depleted thymic stromal cells. Double immunohistochemical analysis confirmed that the vast majority of AIRE+ cells express IDO (92.5 ± 3.8% IDO+ cells of total AIRE+ cells) (Figure 3).
Discussion
We investigated the expression of AIRE in a large series of extrathymic human fetal and postnatal tissues and found that rare cells exclusively occurring in postnatal peripheral lymphoid tissues (with the notable exception of the spleen) contain AIRE protein and mRNA. Nodal AIRE+ cells were represented by scattered cells located in the outer paracortex/interfollicular area, close to high endothelial venules, a crucial area for homing and cellular traffic of immune cells.16,17 Interestingly, AIRE+ cells were more numerous in abdominal and particularly in mesenteric LNs, anatomical sites highly exposed to antigens. In human fetuses AIRE+ cells were detectable only in the thymus, whereas they were not found in LNs and GALT. These observations indicate that AIRE expression is differently regulated in the thymus and in peripheral lymphoid tissues and that development of AIRE+ cells in the periphery may depend on instructive signals from the microenvironment.
Recently, two studies showed that murine LNs contain AIRE+ stromal cells that express MHC-II antigens. The non-hemopoietic, stromal nature of these AIRE+ cells was mainly supported by negativity for CD45; because of divergent data on the phenotype, these studies showed different conclusions on the origin of these cells, which were considered to be either a subset of fibroblastic reticulum cells (podoplanin/gp38+, VCAM-1+, ER-TR7+, and UEA-I+)13 or a subset of LN stromal cells (gp38− and ER-TR7−), sharing some features with medullary thymic epithelial cells (EpCAM+ and PD-L1+).14
Using double immunostains we have shown that the AIRE-expressing cells in human LNs are also negative for CD45 common epitopes and isoforms (RA, RB, RC, and RO) and consistently positive for HLADR; in addition, they expressed fascin, but lacked typical markers of LN stromal cells and fibroblastic reticulum cells, such as vimentin, smooth muscle actin, desmin, CXCL12/stromal cell-derived factor-1, and podoplanin/gp38.17,18 Fascin, an actin-bundling protein that regulates cytoskeleton rearrangement and development of dendrites in various cell types, is expressed at high levels in dendritic cells (DCs) and mature interdigitating dendritic cells (IDCs).19 Because AIRE+ nodal cells displayed an obvious dendritic morphology indistinguishable from that of IDCs, we hypothesized that they could represent a special subset of DCs. Remarkably, negativity for CD45 molecules does not contradict the DC nature of these AIRE+ cells,20 and we showed that IDCs in dermatopathic lymphadenitis are largely negative for CD45 as well. Moreover, normal development of DCs has been reported in CD45null mice and CD45−/− DCs show higher levels of expression of costimulatory molecules (CD40, CD80, and CD86) and MHC-II.21 Double immunostains for myeloid and plasmacytoid DC-associated antigens demonstrated that a high percentage of AIRE+ cells coexpressed CD11c and S100, as well as CD40, CD208/DC-LAMP, CCR7, and CD83. In keeping with these observations, high levels of CD40 and a light increase in CD83 density were detected among CD45lowMHC-IIhigh LN cells after low-density gradient enrichment. Taken together, these data strongly support the notion that AIRE+ cells in human LNs represent a subset of myeloid DCs related to IDCs, with a mature/activated phenotype. RNA transcripts encoding AIRE have been detected in DC subtypes obtained ex vivo and derived from culture, both in mice and humans,11,22,23 but a functional role of the protein in DCs has been questioned.5 Interestingly, low levels of AIRE expression have been shown in human monocyte and monocyte-derived DCs,11 suggesting the possibility of the existence of circulating precursors of extrathymic AIRE+ DCs.3,24 However, in keeping with data from Klamp et al8 using mRNA analysis, in our study we failed to detect AIRE+ cells in the spleen, a secondary lymphoid organ devoid of afferent lymphatics,16,17 thus suggesting that AIRE+ cells most likely migrate to lymph nodes along lymph vessels.
The murine LN stromal AIRE+ cells express tissue-specific antigens only partially overlapping with those expressed by thymic epithelial cells and are capable of inducing tolerance in autoreactive T cells.13,14 To further analyze the functional properties of human AIRE+ DCs, we separated mesenteric LNs using a low-density gradient, followed by negative selection of monocytes, B-cells, and immature DCs and positive selection for HLADR. By this approach, we enriched for a cell population that express AIRE mRNA. Notably, only the AIRE-enriched HLADR+ cell fraction was found to express the AIRE-dependent antigens insulin, CYP17A1, and CYP21A2, albeit at lower levels than those observed in CD45-depleted thymic epithelial cells.
There is increasing evidence that DCs play a key role in the establishment and maintenance of immunological tolerance.25 Thymic DCs contribute to the deletion of self-reactive T cells and to the generation of natural T-regulatory cells,26 whereas peripheral tolerogenic DCs have been suggested to mediate deletion of self-reacting T lymphocytes and/or favor expansion of induced T-regulatory cells.27 It has been shown previously that peripheral tolerogenic DCs express IDO and IL-10.28,29 We hypothesized that AIRE+ DCs could represent a peculiar subset of DCs involved in the induction of peripheral tolerance. By immunohistochemical analysis, we demonstrated that the majority (92.5%) of AIRE+ DCs express IDO. Furthermore, increased expression of IDO and IL-10 transcripts was demonstrated by real-time PCR in the HLADR+CD14−CD19−CD209− nodal cell fraction.
In conclusion, we found that AIRE+ DCs are regularly located in peripheral lymphoid tissues in the area that represents the site of entry of lymph-derived antigens and dendritic cells and of blood-derived lymphocytes through high endothelial venules. This compartment has been considered to play an important role in the initiation of immune responses,16,17 and the AIRE+ DCs might contribute to interaction with immigrating and resident cells in immune homeostasis. The demonstration that AIRE+ DCs in peripheral human lymph nodes express tissue-specific antigens and tolerogenic molecules suggests a potential role in preventing autoimmune diseases and mediating tolerance to tumor antigens.
Acknowledgements
We thank Ms. Maire Philap and Barbara Cassani for excellent technical assistance.
Footnotes
Supported in part by grants from Fondazione Cariplo (Nobel Project) to F.F., L.D.N., and A.V., Fondazione Cariplo to P.L.P., F.F., and A.V., Italian Telethon Foundation to A.V., and the Manton Foundation to L.D.N. K.K. and P.P. were supported by the European Regional Development Fund, The Wellcome Trust, and the Estonian Science Foundation (6663 and 7197).
References
- 1.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 2.Hamazaki Y, Fujita H, Kobayashi T, Choi Y, Scott HS, Matsumoto M, Minato N. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol. 2007;8:304–311. doi: 10.1038/ni1438. [DOI] [PubMed] [Google Scholar]
- 3.Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat Rev Immunol. 2008;8:948–957. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 6.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 7.Adamson KA, Pearce SH, Lamb JR, Seckl JR, Howie SE. A comparative study of mRNA and protein expression of the autoimmune regulator gene (Aire) in embryonic and adult murine tissues. J Pathol. 2004;202:180–187. doi: 10.1002/path.1493. [DOI] [PubMed] [Google Scholar]
- 8.Klamp T, Sahin U, Kyewski B, Schwendemann J, Dhaene K, Tureci O. Expression profiling of autoimmune regulator AIRE mRNA in a comprehensive set of human normal and neoplastic tissues. Immunol Lett. 2006;106:172–179. doi: 10.1016/j.imlet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki E, Kobayashi Y, Kawano O, Endo K, Haneda H, Yukiue H, Sasaki H, Yano M, Maeda M, Fujii Y. Expression of AIRE in thymocytes and peripheral lymphocytes. Autoimmunity. 2008;41:133–139. doi: 10.1080/08916930701773941. [DOI] [PubMed] [Google Scholar]
- 10.Hubert FX, Kinkel SA, Webster KE, Cannon P, Crewther PE, Proeitto AI, Wu L, Heath WR, Scott HS. A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 11.Kogawa K, Nagafuchi S, Katsuta H, Kudoh J, Tamiya S, Sakai Y, Shimizu N, Harada M. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80:195–198. doi: 10.1016/s0165-2478(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 12.Heino M, Peterson P, Kudoh J, Nagamine K, Lagerstedt A, Ovod V, Ranki A, Rantala I, Nieminen M, Tuukkanen J, Scott HS, Antonarakis SE, Shimizu N, Krohn K. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–825. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 13.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 14.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poliani PL, Facchetti F, Ravanini M, Gennery AR, Villa A, Roifman CM, Notarangelo LD. Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood. 2009;114:105–108. doi: 10.1182/blood-2009-03-211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 17.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynoso ED, Lee JW, Turley SJ. Peripheral tolerance induction by lymph node stroma. Adv Exp Med Biol. 2009;633:113–127. doi: 10.1007/978-0-387-79311-5_10. [DOI] [PubMed] [Google Scholar]
- 19.Al-Alwan MM, Rowden G, Lee TD, West KA. Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol. 2001;166:338–345. doi: 10.4049/jimmunol.166.1.338. [DOI] [PubMed] [Google Scholar]
- 20.Wood GS, Freudenthal PS, Edinger A, Steinman RM, Warnke RA. CD45 epitope mapping of human CD1a+ dendritic cells and peripheral blood dendritic cells. Am J Pathol. 1991;138:1451–1459. [PMC free article] [PubMed] [Google Scholar]
- 21.Cross JL, Kott K, Miletic T, Johnson P. CD45 regulates TLR-induced proinflammatory cytokine and IFN-β secretion in dendritic cells. J Immunol. 2008;180:8020–8029. doi: 10.4049/jimmunol.180.12.8020. [DOI] [PubMed] [Google Scholar]
- 22.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 23.Heino M, Peterson P, Sillanpaa N, Guerin S, Wu L, Anderson G, Scott HS, Antonarakis SE, Kudoh J, Shimizu N, Jenkinson EJ, Naquet P, Krohn KJ. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal. RelB-deficient and in NOD mouse. Eur J Immunol. 2000;30:1884–1893. doi: 10.1002/1521-4141(200007)30:7<1884::AID-IMMU1884>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 27.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann NY Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 28.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Horuzsko A. Expression and function of immunoglobulin-like transcripts on tolerogenic dendritic cells. Hum Immunol. 2009;70:353–356. doi: 10.1016/j.humimm.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


