Abstract
Mice lacking the transcriptional repressor oncoprotein Gfi1 are unexpectedly neutropenic1,2. We therefore screened GFI1 as a candidate for association with neutropenia in affected individuals without mutations in ELA2 (encoding neutrophil elastase), the most common cause of severe congenital neutropenia (SCN; ref. 3). We found dominant negative zinc finger mutations that disable transcriptional repressor activity. The phenotype also includes immunodeficient lymphocytes and production of a circulating population of myeloid cells that appear immature. We show by chromatin immunoprecipitation, gel shift, reporter assays and elevated expression of ELA2 in vivo in neutropenic individuals that GFI1 represses ELA2, linking these two genes in a common pathway involved in myeloid differentiation.
Low neutrophil numbers lead to opportunistic infections. There are two hereditary human neutropenia syndromes: cyclic hematopoiesis4, comprising three-week oscillations of blood cells, and SCN3, consisting of statically low neutrophil counts progressing to leukemia. Heterozygous mutations of ELA2 cause cyclic hematopoiesis and about two-thirds of SCN cases. Mutations in WAS (different from those that cause Wiskott–Aldrich thrombocytopenia) also cause SCN5. Owing to its severity, SCN usually arises from new mutations, and additional genes associated with neutropenia have not yet been identified.
Gfi1 encodes a zinc finger transcriptional repressor oncoprotein identified in a retroviral insertion mutagenesis screen for tumor progression to interleukin-2-independent growth6. Gene targeting showed that Gfi1-deficient mice were unexpectedly neutropenic1,2. We therefore screened 105 unrelated neutropenic probands who did not have mutations in ELA2: 49 individuals with SCN and 56 with nonimmune chronic idiopathic neutropenia of adults (NI-CINA; ref. 7), incorporating cases of milder neutropenia diagnosed as an adult but also predisposing to leukemia.
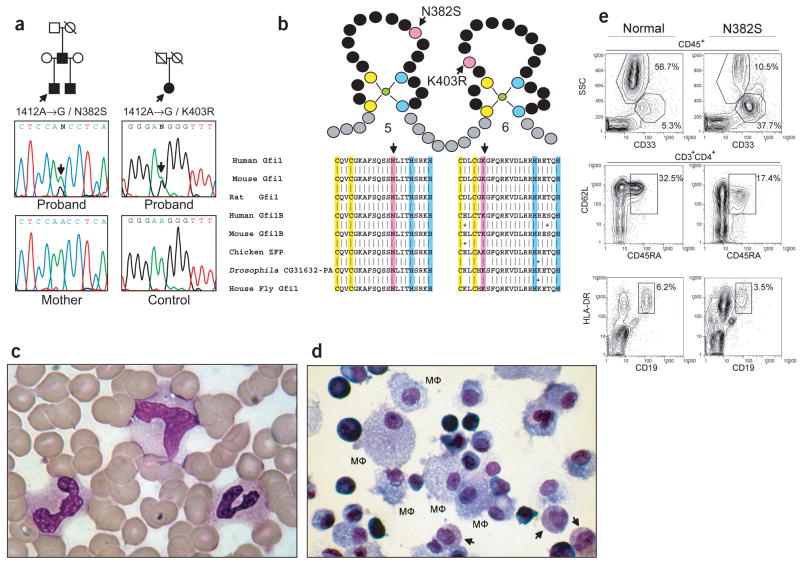
Sequencing of genomic DNA identified two probands with alterations in the GFI1 coding sequence. We identified a heterozygous 1412A→G transition (Fig. 1a) causing a N382S substitution in the fifth zinc finger (Fig. 1b) in a four-month-old boy with SCN who had a neutrophil count of zero and marked monocytosis (2,756 μl−1). The mutation segregated with his three-year-old paternal half-brother, who is identically affected, and with their father, who had recurrent pneumonia and pyogenic abscesses abating during childhood. The father’s childhood blood counts are not accessible, but at age 27, his neutrophils number 900 μl−1 and monocytes 1,200 μl−1 (by automated counting). His peripheral blood shows a population of myeloid cells that appear immature (Fig. 1c). The myeloid, but not erythroid, colony formation potential of his cultured peripheral blood is lower than normal; non-erythroid colonies had intact differentiation to monocytes or macrophages but had an excess of myeloid precursors with no mature neutrophils (Fig. 1d). Flow cytometric analysis of his peripheral blood confirmed there were fewer neutrophils and more CD45+CD33+CD11b+ cells than in a normal adult (Fig. 1e). We observed a reduction in absolute cell numbers of CD4 T lymphocytes (from 1,186 to 624 T cells μl−1) and especially naive CD3+CD4+ T lymphocytes (from 379 to 78 naive CD4 T cells μl−1). CD62L+CD45RA+ stains identified fewer naive CD4 T cells (Fig. 1e; from 32.5% to 17.4%) with confirmation by CD27+CCR7+ staining (data not shown). Moreover, B lymphocytes were also reduced (Fig. 1e; from 6.2% to 3.5%). Peripheral blood lymphocytes from both the father and the older son showed similar trends, and both had poor 3H-thymidine uptake after stimulation with phytohemagglutinin, alloantigen and Candida albicans compared with normal subjects. The child, however, had adequate circulating titers to immunizations, and all immunoglobulin isotypes were present in his serum (data not shown), indicating that, though reduced in number and activation potential, the T and B lymphocyte populations were functional.
Figure 1.
GFI1 mutations and hematopoietic phenotype. (a) Pedigrees of families with mutations in GFI1 and electropherograms of genomic DNA. Arrows indicate probands. (b) Mutations (pink) relative to zinc fingers, coordinating C (yellow) and H (blue), with BLAST alignment. (c) Peripheral blood film showing replacement of mature neutrophils with indistinct myeloid cells (Wright–Giemsa stain). (d) Cytospin of pooled GM, M colonies from peripheral blood cultures showing differentiation to macrophages (MΦ), absence of mature neutrophils and immature granular myeloid cells (arrows; Wright–Giemsa stain). (e) Flow cytometric analysis of peripheral blood from a normal adult (left) and the father in the pedigree with the N382S substitution (right) shows markedly fewer neutrophils (top; 56.7% to 10.5%), more CD33+ monocytic-like cells (top; 5.3% to 37.7%) and fewer naive CD4 T lymphocytes (middle; 32.5% to 17.4%) and B lymphocytes (bottom; 6.2% to 3.5%).
We identified a second mutation in a 66-year-old woman with NI-CINA, who was heterozygous with respect to a 1475A→G transition in GFI1 (Fig. 1a) causing the amino acid substitution K403R in the sixth zinc finger (Fig. 1b). She was found to be neutropenic ten years earlier, and since then she has had persistently low neutrophil number (<1,700 μl−1) and elevated monocytes (>600 μl−1). Analysis for acquired causes has proven negative, including drug exposures and autoimmune serologies (antibodies against nuclear antigens, against double-stranded DNA, against polymorphonuclear leukocytes and against rheumatoid factors). She is not known to have ever had a normal blood count. There is no known history of infectious complications. She lacks other living family.
These amino acid substitutions are probably important, given that Asn382 and Lys403 are identical in Gfi1 homologs in divergent species and are conserved in the paralog Gfi1b (Fig. 1b). No GFI1 coding sequence alterations were indentified in 76 control chromosomes; further sequencing of the final exon, containing both 1412A→G and 1475A→G, found no other sequence changes among 484 control chromosomes.
The phenotypes of individuals with mutations in GFI1 and of Gfi1-knockout mice are similar with respect to responsiveness to granulocyte colony-stimulating factor (G-CSF), monocytosis, lymphopenia and appearance of abnormal blood cells with features of both immature neutrophils and monocytes. Complete deletion of Gfi1 results in runting and inner ear defects in the mouse model8, but humans with amino acid substitutions show no evidence of these or other developmental problems. The combined neutropenia and immunodeficiency resembles the WHIM syndrome of myelokathexis9, but the undifferentiated appearance of the neutrophils differs from the hypermature appearance of neutrophils in WHIM syndrome.
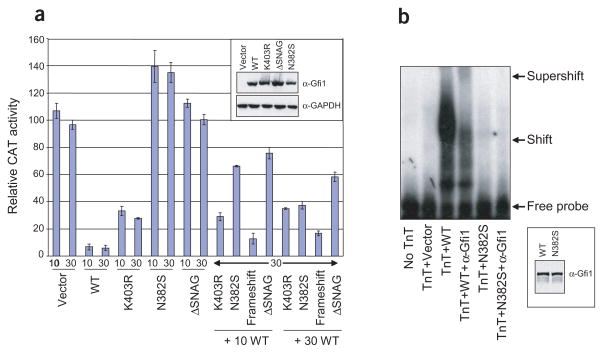
In contrast to gene-targeted mice, in which deletion of one copy of Gfi1 produces a normal phenotype, heterozygous mutations of GFI1 appear to cause disease in humans. We therefore measured the transcriptional consequences of the mutations in transient transfection of NIH3T3 cells, which do not express GFI1. Compared to cDNA expression of wild-type protein and a negative control peptide with a deletion of the essential N-terminal SNAG domain10, N382S abolished and K403R diminished repressor activity on a synthetic dimerized Gfi1-binding site fused to a herpes simplex virus (HSV) thymidine kinase (TK) promoter driving a chloramphenicol acetyl transferase (CAT) reporter (Fig. 2a). Both mutations acted in a dominant negative manner when expressed competitively with the wild-type protein. HEK293T cells offer confirmatory results using a cytomegalovirus immediate early promoter (data not shown), which is also responsive to Gfi1 repression11.
Figure 2.
Transcriptional and DNA-binding consequences of mutations in GFI1. (a) Transient transfection assays in NIH3T3 cells testing repression by the indicated GFI1 cDNA on a TK–CAT reporter fused to dimerized Gfi1 consensus binding site. Data shown are the mean ± s.e. of three trials. Numbers indicate vector quantity (ng). Immunoblot of transfected cell extracts detected with antibodies to Gfi1 (α-Gfi1) and GAPDH (α-GAPDH) shows consistent protein expression (inset). WT, wild-type. (b) EMSA using in vitro synthesized (TnT system) proteins with 5′-32P-labeled double-stranded oligonucleotide containing Gfi1 consensus binding site supershifted with antibody to Gfi1 (α-Gfi1). Immunoblot of TnT-synthesized proteins shows consistent protein expression (inset).
The fifth zinc finger residue Asn382 contacts an adenine nucleotide through the minor groove of the Gfi1 DNA-binding site11. The sixth zinc finger, containing Lys403, does not contribute to sequence-specific DNA binding10. In an electrophoretic mobility shift assay (EMSA) using in vitro synthesized proteins, the N382S mutation abolished recognition of a consensus binding site (Fig. 2b), but the K403R mutation had no effect (data not shown). N382S probably acts in a dominant negative manner by preserving interaction with other components of the transcriptional machinery but losing DNA-binding ability. The phenotypically milder K403R mutation could achieve a dominant negative effect by maintaining DNA binding but failing to associate with another factor, similar to the mechanism by which mutations of GATA1 cause thrombocytopenia, in which disruption of a zinc finger not involved in DNA binding blocks association with co-activator FOG12. Alternatively, our preliminary data (R.E.P. and M.H., unpublished data) suggest that Lys403 may be a site of addition of the small ubiquitin-related modifier SUMO.
The variability of neutropenia between the two pedigrees probably results from the severity of the effect of the two different mutations on Gfi1 transcriptional repressor activity. The variability of phenotype between the father and his two affected sons in the family with the N382S substitution may arise, in part, from an increase in the number of indistinctly differentiated myeloid cells that (at least with the deficient mice) occurs with aging. Alternatively, the father could be a statistical outlier representing an ascertainment bias in which only more mildly affected individuals will live to a reproductive age. We were able to exclude two other possible explanations. The father’s milder phenotype is not the result of mosaicism, as DNA from purified neutrophils or monocytes and from cultured erythroid colonies showed that all subclones possessed the mutation (data not shown). He has also not acquired mutations in the gene encoding the G-CSF receptor (data not shown), which is not uncommon in SCN13.
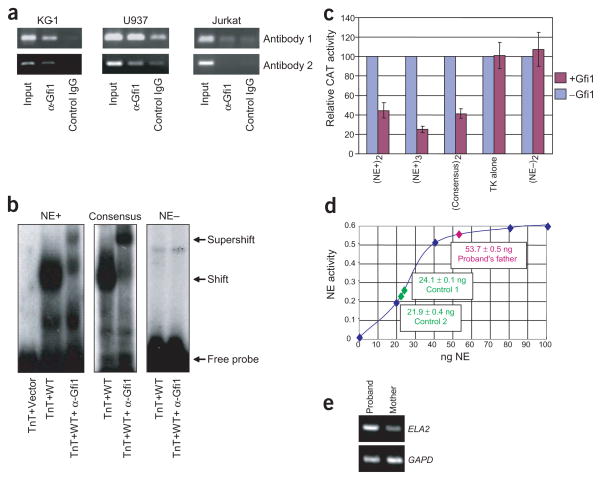
We hypothesized that GFI1 represses ELA2, thus linking mutations in the two genes to a common phenotype. As a test, we carried out chromatin immunoprecipitation (ChIP) analysis. ELA2 is expressed in promyelocytes but not in differentiated granulocytes. We cross-linked DNA-bound protein in vivo in human KG-1 and U937 promyelocytes and, as a negative control, Jurkat T cells (all three cells express GFI1, but only the first two express ELA2 (ref. 14), as the ELA2 chromatin is closed in lymphocytes). Two different antibodies immunoprecipitate GFI1-associated genes from chromatin. ELA2-specific PCR of a 3-kb upstream fragment confirmed Gfi1 binding in KG-1 and U937 cells but not in Jurkat cells (Fig. 3a). We identified nine potential Gfi1-binding sites in this 3-kb upstream ELA2 promoter region. We tested each one and confirmed that two, −2,714 to −2,740 (Fig. 3b) and −336 to −362 (data not shown), are recognized by Gfi1 in EMSA, whereas an A-T rich element in ELA2 at −300 to −326 serves as a negative control (Fig. 3b). Both the −2,714 to −2,740 (Fig. 3c) and the −336 to −362 (data not shown) binding sites, but not the −300 to −326 negative control (data not shown), functioned as repressor elements when fused to a TK promoter driving a CAT reporter in a transient transfection assay of Gfi1 in NIH3T3 cells. Concordantly, ELA2 expression is upregulated in the bone marrow of gene-targeted mice2.
Figure 3.
ChIP assay showing Gfi1 targeting of ELA2. (a) PCR of ELA2 promoter in ChIP assay on indicated cell lines showing chromatin immunoprecipitation with two different specific or control antibodies. (b) EMSA with TnT-synthesized Gfi1 of oligonucleotide containing Gfi1 binding site identified in ELA2 at −2,714 to −2,740 (NE+), consensus Gfi1 binding site as a positive control and inactive A-T rich element in ELA2 at −300 to −326 (NE–) as a negative control. (c) Transient transfection assays in NIH3T3 cells testing repression of the indicated binding site (from b) fused to a TK–CAT reporter, in the presence or absence of expression of Gfi1. Data shown are the mean ± s.e. of three trials. Subscripts indicate the number of times the binding site is multimerized in the reporter construct. Each construct has a different basal level of expression, so activity in the presence of Gfi1 (red) is normalized to activity in its absence (blue). (d) Neutrophil elastase (NE) activity in peripheral blood from the father of the pedigree with the N382S substitution (pink) compared with that of two normal adult controls (green) superimposed on a standard curve using indicated quantity of purified enzyme (blue). Data shown are the mean ± s.e. of two measurements from 105 lysed mononuclear cells. (e) Quantitative RT–PCR of ELA2 expression, compared to GAPD control, in non-erythroid colonies formed from the peripheral blood of the three-year-old son in the pedigree with the N382S substitution versus his mother. Representative results with oligo(dT)-primed RNA are shown.
To determine if ELA2 expression is similarly increased in vivo in humans with mutations in GFI1, we carried out two experiments. First, we measured neutrophil elastase enzymatic activity in extracts of peripheral mononuclear cells. To avoid confounding effects of treatment with G-CSF, we measured the activity in the cells of the father in the pedigree with the N382S substitution, who is not receiving G-CSF therapy. Neutrophil elastase activity in his cells was 2.33 times higher than the mean activity in two normal controls. Second, we measured ELA2 expression by RT–PCR. To avoid both confounding effects of G-CSF treatment and unscheduled bone marrow aspiration, we did these studies in non-erythroid colonies differentiated in vitro from peripheral blood obtained from the three-year-old son in the pedigree with the N382S substitution. Compared with his mother, and in contrast to expression of GAPD as an internal control, ELA2 transcription was higher. These results independently show that mutations in GFI1 cause overexpression of ELA2.
The identification of mutations in GFI1 that lead to SCN and other recent results place the effects of the SCN-causing ELA2 mutations in context. These ELA2 mutations do not consistently abrogate proteolytic activity, and their biochemical consequences are unclear15. Most commonly, these mutations delete the C-terminal extension of neutrophil elastase. Recent results show that mutations of AP3B1, encoding a subunit of an adapter protein complex required for post-translational trafficking from the Golgi to the lysosome, are the cause of a neutropenic variant16 of Hermansky–Pudlak syndrome17 and of canine cyclic hematopoiesis (K.F.B. and M.H., unpublished data) and that the C terminus of neutrophil elastase contains an adapter protein recognition signal required for lysosomal/granule localization. In the absence of either the adapter protein or this C-terminal signal, neutrophil elastase is mistrafficked, so that too much neutrophil elastase is delivered to particular subcellular compartments (membranes). We propose that the GFI1 mutations, by causing overexpression of neutrophil elastase, achieve a similar effect by generally increasing levels of neutrophil elastase in all subcellular locations, including those that accumulate too much enzyme as a result of mutations in normal post-translational trafficking pathways.
METHODS
Mutational analysis
We obtained informed consent from the subjects using protocols approved by the Institutional Review Boards of the University of Washington, University of Louisville and University of Crete. We amplified by PCR all exons and exon-intron junctions of GFI1 from genomic DNA extracted from peripheral blood and sequenced both strands with ABI Big Dye terminator chemistry using ABI capillary electrophoresis.
Colony formation assay
We overlaid peripheral blood on Ficoll-Hypaque (Beckman Coulter) density gradients. We removed interphase cells and cultured them at desired concentrations in methylcellulose supplemented with cytokines18 for 14 d. To evaluate morphology, we picked non-erythroid colonies through a dissecting microscope and fixed and stained cytocentrifuge preparations.
Transient transfection assay
We used LipofectAMINE PLUS (GIBCO) to transiently cotransfect reporters (1 μg) into 106 NIH3T3 cells (ATCC) with Renilla luciferase vector pRL-TK (200 ng) as an internal control and harvested cell lysates 40 h later. We measured CAT and luciferase activities with the CAT ELISA Kit (Roche) and Dual-Luciferase Assay (Promega), respectively, normalizing CAT activity to luciferase activity. We expressed Gfi1 (amplified by RT–PCR from human bone marrow) and its derivatives from pCS2+ (100 ng in Fig. 3c and as noted in Fig. 2a). We generated N382S, ΔSNAG and frameshift (+1 insertion in second codon) variants by oligonucleotide cassette mutagenesis. The TKCAT reporter contains the HSV minimal TK promoter directing expression of the CAT gene, whereas B30x2-TKCAT also contains dimerized high-affinity synthetic Gfi1 binding sites10; both were gifts of P. Tsichlis (Sackler School, Tufts University). We generated TKCAT constructs containing different Gfi1 binding sites or control sites as follows: we excised the two B30 boxes by BglII/BamHI digestion, Klenow polishing, CIP dephosphorylation and ligation of double-stranded T4-kinased oligonucleotides. We immunoblotted cell extracts using nitrocellulose electroblotting and used goat monoclonal antibody against Gfi1 N-20 (Santa Cruz) and mouse monoclonal antibody against GAPDH (Research Diagnostics) for detection with the ECL system (Amersham).
EMSA
For Gfi1 synthesis, we used the TnT coupled transcription/translation system (Promega) with the expression vectors’ SP6 promoter. We labeled double-stranded oligonucleotides with 5′-32P using T4 kinase. The binding reaction mixture (10 μl final volume) contained labeled probes (2,000 cpm) in binding buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM MgCl2, 1 mM ZnSO4, 0.5 mM dithiothreitol, 0.5 mM EDTA, 10% glycerol, 0.05% Nonidet P-40), 1 μg poly (dI-dC) and 2% of the in vitro translated protein (1 μl). The binding reactions were incubated for 20 min at room temperature. We added 200 ng antibody against Gfi1 N-20 for supershift. Probe sequences are available on request.
ChIP assay
We carried out ChIP as described19 with the following modifications. We fixed 200-ml volumes of KG-1, U937 and Jurkat (2 ×108) cells (ATCC) with 1% formaldehyde for 10 min at 37 °C and then quenched the reaction with 0.125 M glycine. Sonication of cells (Fisher Scientific Ultrasonic Dismembrator Model 500) in 1× RIPA buffer (containing proteinase inhibitors) on ice generated soluble chromatin complexes with DNA fragment length <3 kb. Each reaction contained the equivalent of ~107 cells (1.5 ml soluble chromatin) and either 15 μl antibody against Gfi1 N-20 (antibody 1) or a 1:500 dilution of rabbit antibody to amino acids 12–26 of GFI10 (custom raised by Sigma Genosys; antibody 2). About 2 ng of immunoprecipitated DNA served as template in each reaction in a total volume of 20 μl and corresponded to about 1/10,000 to 1/20,000 of the genomic DNA isolated from 1.5 ml of soluble chromatin. We did experiments with antibody 1 and antibody 2 in triplicate and duplicate, respectively, with representative results shown.
Neutrophil elastase activity assay
We measured neutrophil elastase activity in extracts of 105 mononuclear cells from freshly drawn peripheral blood purified on Ficoll-Hypaque density gradients spectrophotometrically on the specific substrate suc-Ala-Ala-Ala-pNa as described15.
RT–PCR
We isolated total RNA from 4 ×103 cells from pooled non-erythroid colonies formed from peripheral blood (as described above) from the indicated individual using the QIAamp RNA Blood Mini-kit (Qiagen). Two step RT–PCR used the TaqMan Gold RT-PCR kit (ABI). We carried out semi-quantitative expression analysis of ELA2 using three different specific human primer pairs and found consistent results with both random or oligo(dT) primed RNA. Primer sequences are available on request.
Acknowledgments
We thank P. Tsichlis for reagents and advice, J. Miller for advice, K. Williams for technical assistance and the subjects for their cooperation. M.H. thanks the late D.M. Horwitz for his encouragement and support. This work was supported by the U.S. National Institutes of Health, Doris Duke Foundation, Leukemia & Lymphoma Society and Burroughs-Wellcome grants (M.H.) and Hope Street Kids grant (H.L.G.).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Karsunky H, et al. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet. 2002;30:295–300. doi: 10.1038/ng831. [DOI] [PubMed] [Google Scholar]
- 2.Hock H, et al. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity. 2003;18:109–120. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- 3.Dale DC, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96:2317–2322. [PubMed] [Google Scholar]
- 4.Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Gen. 1999;23:433–436. doi: 10.1038/70544. [DOI] [PubMed] [Google Scholar]
- 5.Devriendt K, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001;27:313–317. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- 6.Gilks CB, Bear SE, Grimes HL, Tsichlis PN. Progression of interleukin-2 (IL-2)-dependent rat T cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol Cell Biol. 1993;13:1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadaki HA, et al. Two patients with nonimmune chronic idiopathic neutropenia of adults developing acute myeloid leukemia with aberrant phenotype and complex karyotype but no mutations in granulocyte colony-stimulating factor receptor. Ann Hematol. 2002;81:50–54. doi: 10.1007/s00277-001-0401-z. [DOI] [PubMed] [Google Scholar]
- 8.Wallis D, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- 9.Gorlin RJ, et al. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91:368–376. [PubMed] [Google Scholar]
- 10.Grimes HL, Chan TO, Zweidler-McKay PA, Tong B, Tsichlis PN. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol Cell Biol. 1996;16:6263–6272. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zweidler-Mckay PA, Grimes HL, Flubacher MM, Tsichlis PN. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol Cell Biol. 1996;16:4024–4034. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols KE, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz M, et al. Role of neutrophil elastase in bone marrow failure syndromes: molecular genetic revival of the chalone hypothesis. Curr Opin Hematol. 2003;10:49–54. doi: 10.1097/00062752-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Duan Z, Horwitz M. Targets of the transcriptional repressor oncoprotein Gfi-1. Proc Natl Acad Sci USA. 2003;100:5932–5937. doi: 10.1073/pnas.1031694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li FQ, Horwitz M. Characterization of mutant neutrophil elastase in severe congenital neutropenia. J Biol Chem. 2001;276:14230–14241. doi: 10.1074/jbc.M010279200. [DOI] [PubMed] [Google Scholar]
- 16.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky–Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 17.Anikster Y, et al. Mutation of a new gene causes a unique form of Hermansky–Pudlak syndrome in a genetic isolate of central Puerto Rico. Nat Genet. 2001;28:376–380. doi: 10.1038/ng576. [DOI] [PubMed] [Google Scholar]
- 18.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of α4β1 over β2-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 19.Duan Z, Stamatoyannopoulos G, Li Q. Role of NF-Y in in vivo regulation of the γ-globin gene. Mol Cell Biol. 2001;21:3083–3095. doi: 10.1128/MCB.21.9.3083-3095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]