Abstract
Background
Motilin, an endogenous gastrointestinal (GI) hormone, increases upper gastrointestinal tract motility and is associated with phase III of the gastric migrating motor complex. The motilin receptor agonist, atilmotin, at doses of 6, 30 or 60 µg intravenously (IV), increases the early phase of gastric emptying. Prior studies at higher doses of 100–450 µg IV demonstrated that some subjects developed noncardiac chest pain.
Aims
The aim of this study is to determine the effects of atilmotin on esophageal, lower esophageal sphincter (LES), and gastric contractility and the development of esophageal-related symptoms.
Methods
Ten healthy volunteers underwent esophageal manometry to study the effects of atilmotin on upper GI motility. Five subjects were studied on three separate days following administration of saline placebo and subsequent IV bolus dose of atilmotin (6, 30 or 150 µg). Another five subjects were studied at the highest dose (150 µg).
Results
Atilmotin at 150 µg increased proximal gastric pressure by 6.5 mmHg (P = 0.001 compared with placebo). Atilmotin increased LES pressure at all studied doses; LES pressure increased from 24 ± 2 mmHg following placebo injection to 34 ± 4 mmHg following a 30 µg dose of atilmotin (P = 0.007). In the esophagus, atilmotin increased the percentage of failed swallows at the highest dose studied. Failed swallows increased from 17 ± 7% following placebo injection to 36 ± 7% following a 150 µg dose of atilmotin (P = 0.016). Atilmotin decreased distal esophageal contractile amplitude only at the highest dose studied, from 69 ± 8 mmHg (placebo) to 50 ± 5 mmHg following 150 µg atilmotin (P = 0.018). There were no serious adverse effects or episodes of chest pain with atilmotin.
Conclusions
Atilmotin affects esophageal, LES, and gastric motility. LES and gastric pressures were increased, whereas there was disruption of esophageal peristalsis characterized by lower amplitude and failed contractions.
Keywords: Lower esophageal sphincter, Esophageal manometry, Motilin
Introduction
Motilin is an endogenous gastrointestinal (GI) hormone that increases upper GI tract motility. Motilin is secreted by mucosal endocrine cells of the GI tract in a periodic fashion during the interdigestive period approximately every 90 min and is associated with the initiation of phase III contractions of the migrating motor complex (MMC) [1]. This phase III MMC starts in the stomach and propagates distally in the GI tract; its function is to move undigested intraluminal contents from the stomach distally. Thus, motilin is considered an endogenous prokinetic hormone. Administration of exogenous motilin induces a phase III migrating motor complex in the stomach [1].
Atilmotin, [N+-α-(CH3)3Phe1,D-Arg12,Leu13,Lys14] motilin 1–14 amide, is a synthetic peptide analog of motilin that is under development for disorders of gastrointestinal motility, particularly postoperative ileus. Preclinical pharmacological evaluation of atilmotin has shown it is a potent agonist at motilin receptors in vitro and in vivo [2]. Unlike other motilin agonists, including erythromycin-like agents, atilmotin is short acting, with t1/2 less than 10 min. Atilmotin, at doses of 6, 30 or 60 µg intravenously, has been shown to increase gastric emptying within the first 30 min after administration [3]. Thus, atilmotin might have clinical utility for GI motility disorders.
Chest or throat tightness or discomfort have been seen in some patients at higher doses in fasting subjects and have shown no evidence of cardiac origin by cardiac enzymes, Holter monitoring or electrocardiogram (ECG) and had been assessed by investigators as noncardiac. In an early safety study, atilmotin produced abdominal pain and/or chest discomfort at high doses of 150, 300, and 450 µg [4]. A subsequent safety study showed that atilmotin at doses of 50, 100, and 150 µg was associated with chest discomfort [4]. Since there were no electrocardiographic changes or rhythm changes with any instances of chest pain, it was felt that the chest pain was noncardiac, and this adverse effect was postulated to be related to esophageal spasm or to an increase in esophageal and/or LES pressure. Indeed, early studies had shown that administration of exogenous motilin in humans increases lower esophageal sphincter (LES) pressure [5].
The purpose of this study was to explore an esophageal etiology of the chest pain and throat tightness reported in the early safety studies when atilmotin was given at high doses, well above those considered likely to be required for therapeutic effect. The specific aim of this study was to determine the dose-related effects of atilmotin on esophageal, LES, and gastric contractility. The hypothesis was that atilmotin increases esophageal and LES pressure in humans.
Methods
Overall Study Design
This was a single-center, two-part, single-blind, placebo-controlled, dose-escalation study evaluating the effects of three intravenous bolus doses of atilmotin and placebo on esophageal, LES, and gastric pressures and on the development of any esophageal-related symptoms, such as chest pain.
Subjects
Ten normal, healthy volunteers participated in this study. Male and female volunteers aged 18–45 years were studied. History, physical examination, blood work up (complete blood count, complete metabolic panel, thyroid function tests), and electrocardiogram were performed at baseline to ensure that the subjects were normal.
Study Protocol
Initially, five healthy volunteers were studied with esophageal manometry on three separate days at escalating doses of atilmotin (6, 30, and 150 µg IV bolus administration, as 30 µg/mL solution, followed by 10 mL saline flush, over 1 min). Swallowing function in each subject was studied with 5 ml water boluses every 30 s: 10 baseline swallows, 30 swallows over 15 min after IV saline (placebo), and 30 swallows over 15 min after IV atilmotin. The initial infusion to obtain the baseline response was saline (placebo) and the second infusion was either placebo or atilmotin. In this single-blind study, the subjects were blinded to what they were receiving; however, the investigator and coordinator were not blinded at the time of infusion. Following the dose-escalation phase of the study, during which no dose-limiting adverse event was noted, an additional five subjects were studied at only the highest dose of 150 µg.
Esophageal manometry was performed using a manometry catheter with 36 solid-state circumferential pressure sensors spaced 1 cm apart (Sierra Scientific Instruments, Inc.; Los Angeles, CA) and ManoScan equipment and software (Sierra Scientific Instruments). Cetacaine spray was used to anesthetize the oropharynx. The catheter was inserted by nasal intubation into the stomach and placed to span the pharynx, esophagus, LES, and proximal stomach. After proper placement of the catheter, the catheter was taped at the nares so that it would not move.
During the esophageal manometry study, pulse and blood pressure were monitored and ECG was recorded at baseline, and after each study drug administration. Symptoms were monitored throughout the esophageal manometry.
Analysis
Pressures were measured relative to atmospheric pressure using Sierra ManoScan equipment and software (Sierra Scientific Instruments, Inc.) as previously reported [6–8]. The important variables analyzed were: (1) proximal gastric pressure, (2) resting LES pressure, (3) nadir LES pressure measured after a swallow, (4) distal esophageal contraction amplitude with swallowing, and (5) esophageal peristaltic velocity.
LES pressure was measured as end-expiratory pressure relative to atmospheric pressure. For each esophageal contraction, the amplitude at each level and the velocity of peristalsis were calculated. An esophageal contraction was considered peristaltic if the onset of the pressure wave at adjacent ports was progressive in an aborad direction. The amplitude of esophageal contractions was measured from the esophageal baseline pressure to the peak contraction pressure. Simultaneous contractions were defined as contractions that occurred simultaneously at different levels of the esophagus. Repetitive contractions were defined as the presence of at least three peaks larger than 10 mmHg with peaks separated by at least 1 s. Spontaneous contractions were defined as contractions >30 mmHg not generated by a swallow. Failed swallows were defined as swallows that were not followed by a normal-amplitude esophageal peristaltic wave. Proximal gastric pressure was taken as the average of the proximal gastric pressure after the swallow over a 30-s period.
Results are expressed as mean ± standard error of the mean (SEM). The hypothesis tested was that atilmotin increases esophageal and LES pressures in humans. Primary comparisons were the differences in the key variables between placebo and atilmotin treatment at specific group using analysis of variance (ANOVA) followed by Student’s t test with Bonferroni correction. P values of <0.05 were considered statistically significant.
Results
General
Atilmotin affected gastric, LES, and esophageal pressures. Figure 1 depicts the changes seen manometrically (Fig. 1). The effects of atilmotin were dependent on dose and the time after administration. Figure 2 shows the temporal changes in LES pressure after intravenous administration of atilmotin. The following data and those reported in Table 1 were measured for the first five swallows after IV administration of either placebo or atilmotin.
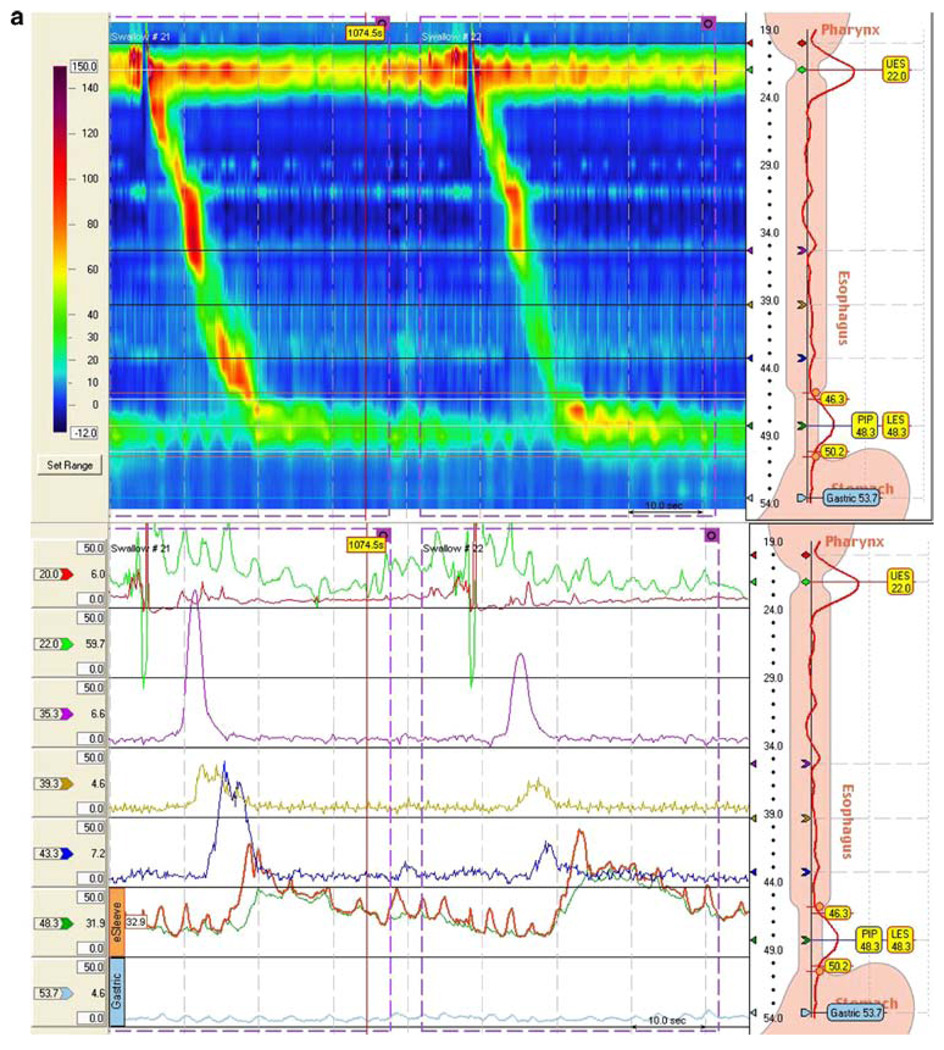
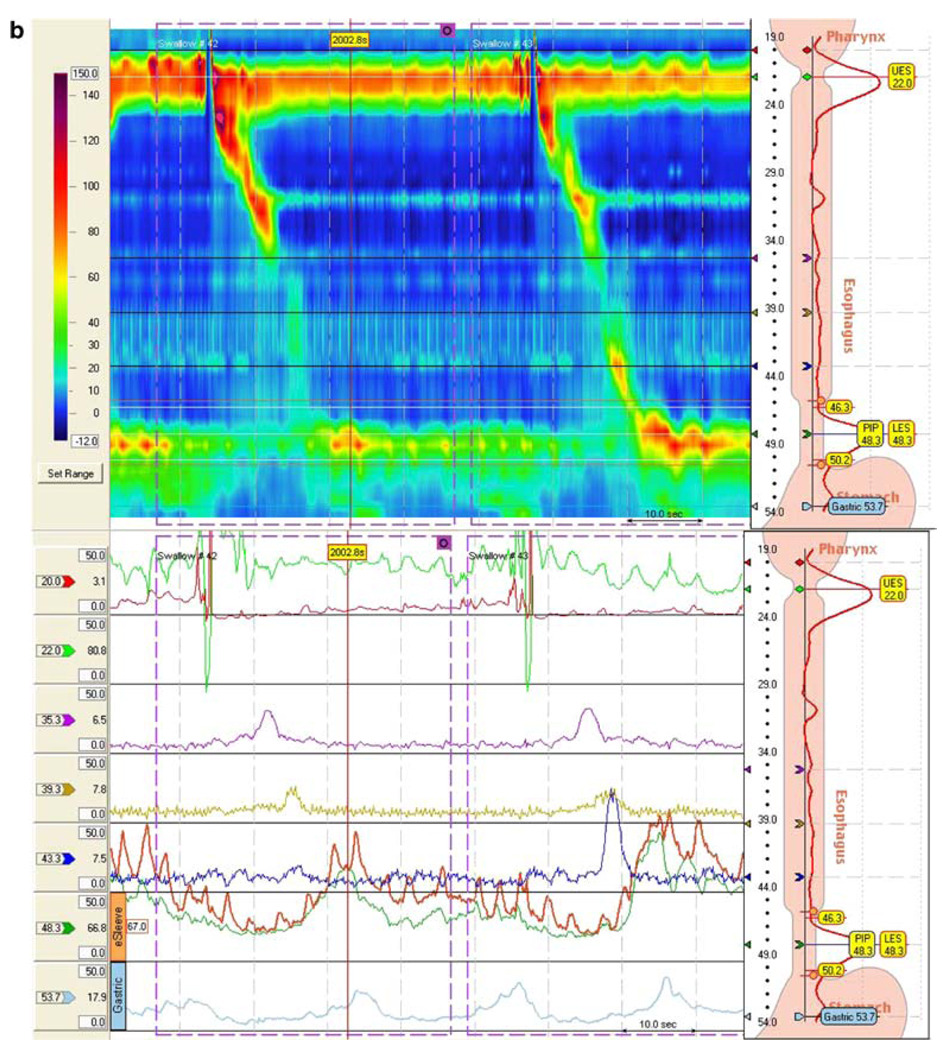
Fig. 1.
Esophageal manometry tracings illustrating the effect of atilmotin: a after placebo (saline infusion) and b after atilmotin. In each figure, the top tracing is the high-resolution contour mapping and the bottom tracing is the conventional esophageal manometry tracing. In the high-resolution contour mapping, the catheter traverses the entire esophagus from the upper esophageal sphincter at top to the lower esophageal sphincter at the bottom. The amplitude scale is shown at the left. Esophageal peristalsis after swallowing is indicated by the contour peak traversing down and to the right. a Normal subject after saline injection, b normal subject after atilmotin IV
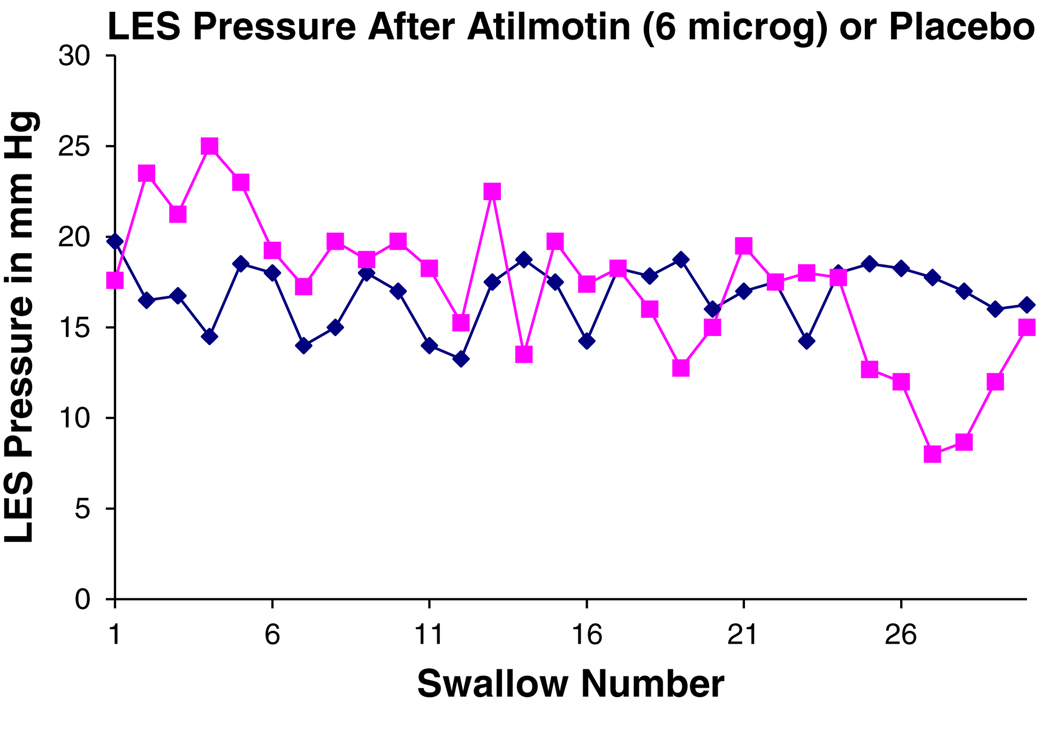
Fig. 2.
Change in LES pressure over time after atilmotin infusion. Shown is the LES pressure in mmHg (y axis) and the swallow number after 6 µg atilmotin intravenously (squares) and after saline injection (diamonds). There was an increase in LES pressure seen after the first five swallows after atilmotin infusion; thereafter there were similar values to placebo. Shown are the mean values of LES pressure relative to gastric pressure for five subjects
Table 1.
Effect of atilmotin on gastric, LES, and esophageal pressure, and esophageal peristalsis
| Placebo, N = 20 | Atilmotin 6 µg, N = 5 | Atilmotin 30 µg, N = 5 | Atilmotin 150 µg, N = 10 | |
|---|---|---|---|---|
| Proximal gastric pressure (mmHg) | 2.0 ± 0.7 | 6.8 ± 1.4 | 4.2 ± 1.7 | 8.5 ± 0.7 |
| P value versus placebo | 0.074 | 0.099 | 0.0006 | |
| Resting LES pressure (mmHg) | 24.4 ± 1.8 | 33.5 ± 2.4 | 34.2 ± 3.6 | 33.1 ± 2.2 |
| P value versus placebo | 0.018 | 0.007 | 0.0005 | |
| Residual LES pressure | ||||
| During swallowing (mmHg) | 10.7 ± 1.0 | 11.1 ± 1.3 | 11.5 ± 1.3 | 9.0 ± 1.4 |
| P value versus placebo | 0.595 | 0.187 | 0.098 | |
| Distal esophageal pressure (amplitude) | ||||
| During swallowing (mmHg) | 69.4 ± 8.3 | 69.9 ± 12.9 | 57.3 ± 7.1 | 49.6 ± 5.2 |
| P value versus placebo | 0.58 | 0.54 | 0.018 | |
| Velocity of esophageal contractions | ||||
| 5–15 cm above LES (cm/s) | 4.1 ± 1.0 | 3.2 ± 0.4 | 3.3 ± 0.3 | 3.1 ± 0.9 |
| P value versus placebo | 0.29 | 0.33 | 0.21 | |
| Failed swallows (%) | 16.6 ± 7.0 | 12.0 ± 6.6 | 8.0 ± 6.5 | 36.0 ± 6.8 |
| P value versus placebo | 0.621 | 0.374 | 0.016 |
Results are expressed as mean ± SEM relative to atmospheric pressure. Data were measured for the first five swallows after IV administration of either placebo or atilmotin
Gastric Pressure
Atilmotin at a dose of 150 µg significantly increased proximal gastric pressure. At this dose of 150 µg, proximal gastric pressure increased by 6.5 mmHg from 2.0 ± 0.7 mmHg for placebo to 8.5 ± 0.7 mmHg for atilmotin (P = 0.0006) (Table 1). Increase in gastric pressure was also noted at doses of 6 and 30 µg, but did not reach statistical significance.
LES Pressure
Atilmotin increased resting LES pressure at all doses, most prominently at 30 µg from 24.4 ± 1.8 mmHg for placebo to 34.2 ± 3.6 mmHg for atilmotin (P = 0.007). In three patients LES pressure increased to above the normal range of 8–40 mmHg: none of five in the 6 µg atilmotin group, one of five of the 30 µg atilmotin group, and two of ten in the 150 µg atilmotin group. Atilmotin did not significantly change residual LES pressure after swallowing.
Esophageal Pressure and Swallowing
Atilmotin at the dose of 150 µg increased the percentage of failed swallows (16.6 ± 7.0% with placebo to 36.0 ± 6.8%; P = 0.016), whereas no effect on the percentage of failed swallows was seen at doses of 6 or 30 µg. At 5 cm above the LES, atilmotin decreased the amplitude of distal esophageal peristaltic waves, significantly after the highest dose, 150 µg (69.4 ± 8.3 mmHg for placebo and 49.6 ± 5.2 mmHg for atilmotin; P = 0.018). The velocity of esophageal peristaltic pressure waves decreased after atilmotin (Table 1), but this effect did not reach statistical significance compared with placebo.
Adverse Events
There were no serious adverse effects or episodes of chest pain with any dose of atilmotin or placebo. One episode of abdominal discomfort occurred at the highest dose of atilmotin.
Discussion
This study demonstrates that atilmotin, a motilin receptor agonist, affects esophageal, LES, and proximal gastric motility in normal subjects. At the highest dose tested in this study, atilmotin caused an increase in LES and gastric pressures, and there was a disruption of esophageal contractions characterized by low-amplitude contractions, and at times failed contractions. The peak effect of atilmotin on all measured parameters was within the first several minutes after IV administration, consistent with its known short half-life when injected intravenously [4].
The study design for this study using intravenous injection of atilmotin was based on prior phase I studies using similar methodology [3, 4]. Acute changes in esophageal manometric pressures were measured in response to intravenous administration of atilmotin. Other studies using intravenous 13-Nle-motilin showed that peak values of LES pressure were achieved within 1–2 min, followed by a steady decrease in LES pressure [5]. Similar temporal changes in LES and gastric pressure were seen with intravenous atilmotin (Fig. 2). Because of the short duration of these effects of bolus IV administration of atilmotin, data analysis for this study was performed on the first five swallows measured following administration of atilmotin or placebo.
Atilmotin was shown to increase proximal gastric pressure. The increase in gastric pressure was seen most prominently, and reached statistical significance, with the highest dose of motilin studied (150 µg by intravenous bolus injection). The manometry catheter did not span into the distal stomach to determine whether antral contractility was affected. Other studies have shown that motilin and atilmotin can increase antral contractility and are associated with the phase III portion of the gastric MMC [2].
An increase in gastric pressure might lead to accelerated gastric emptying—an effect which was shown in another study [3]. Park et al. reported that atilmotin at doses of 6, 30, and 60 µg intravenously, 2 min after consumption of a standardized radiolabeled meal, accelerated gastric emptying of liquids (as measured by percentage emptied at 30 min) at all atilmotin doses and of solids for the 30 and 60 µg doses only when compared with placebo. The half-life of gastric emptying of liquids was significantly shorter for all atilmotin doses when compared with placebo; the half-life of gastric emptying for solids only showed a trend for the 6 and 30 µg doses. This increase in proximal gastric pressure and gastric emptying with atilmotin suggests a possible indication for gastroparesis. However, the effect of atilmotin on gastric emptying was short lived. Other motilin receptor agonists such as erythromycin, ABT-229, and GM-611 (mitemcinal) cause significant acceleration of gastric emptying [9–11].
An increase in resting LES pressure following atilmotin administration was seen in this study. An increase in LES pressure with motilin was reported nearly two decades ago by Lux et al. [5] following administration of intravenous 13-Nle-motilin. That increase in LES pressure occurred acutely (within 1–2 min); a similar result was seen in the present study. Other studies have shown that the increase in LES pressure due to motilin receptor agonists is cholinergically mediated [12, 13].
Atilmotin was associated with a disruption of esophageal contractions, with decreased amplitude of contractions and increased number of failed or disrupted esophageal contractions (compared with placebo) at the highest dose. There were decreases in the number of failed contractions at the lower doses, although these were not statistically significant. Other studies of the effect of motilin receptor agonists on esophageal function have yielded differing results [14–18]. In normal subjects and in patients with gastroesophageal reflux disease, erythromycin increases LES pressure and esophageal contraction amplitude [14–16]. However, ABT-229, a motilin receptor agonist, had little effect [17, 18].
In this study, there were no significant side-effects after administration of atilmotin. One subject developed transient abdominal pain. Importantly, there were no instances of chest pain seen in this study with atilmotin. Chest pain was noted in an earlier phase I study with atilmotin, but the lack of any concomitant change in ECG on Holter monitoring suggested a noncardiac origin (possibly esophageal or upper gastric spasm). The failure to reproduce this chest pain effect in the current study leaves the question of the etiology of such discomfort unresolved. Atilmotin caused a disruption of esophageal peristalsis with low amplitude, and at times failed contractions. Thus, it did not cause esophageal spasm. It should be noted that the prior instances of chest pain in the early safety studies occurred at doses higher than those required for atilmotin’s prokinetic effect in the study by Park et al., which demonstrated significant acceleration of early gastric emptying at a dose of 6 µg or greater [3].
In conclusion, this study demonstrates that the motilin receptor agonist, atilmotin, given by IV bolus administration, affects esophageal, LES, and gastric motility. LES and gastric pressures were increased and, at the highest dose studied (150 µg), there was a disruption of esophageal peristaltic pressure waves characterized by low amplitude and, at times, failed contractions. None of these effects was accompanied by the chest pain observed in previous studies, leaving the primary question of etiology of this effect unresolved. The increase in LES pressure occurred with a concomitant increase in proximal gastric pressure. Whether atilmotin might be useful to treat disorders of upper GI motility needs further study.
Acknowledgments
This study was supported by Baxter Healthcare Corporation.
Footnotes
Preliminary results of this study were presented at the Digestive Disease Week 2007 meeting in Washington, DC and appeared as an abstract in Gastroenterology 2007;132(4 Suppl 2):A-596.
Contributor Information
Annapurna Korimilli, Division of Gastroenterology, Department of Medicine, Temple University School of Medicine, Philadelphia, PA, USA.
Henry P. Parkman, Division of Gastroenterology, Department of Medicine, Temple University School of Medicine, Philadelphia, PA, USA Gastroenterology Section, Temple University Hospital, Parkinson Pavilion, 8th Floor, 3401 North Broad Street, Philadelphia, PA 19140, USA henry.parkman@temple.edu.
References
- 1.Itoh Z, Nakaya M, Suzuki T, Arai H, Wakabayashi K. Erythromycin mimics exogenous motilin in gastrointestinal contractile activity in the dog. Am J Physiol. 1984;247(6 Pt 1):G688–G694. doi: 10.1152/ajpgi.1984.247.6.G688. [DOI] [PubMed] [Google Scholar]
- 2.Macielag MJ, Peeters TL, Konteatis ZD, et al. Synthesis and in vitro evaluation of [Leu13] porcine motilin fragments. Peptides. 1992;13(3):565–569. doi: 10.1016/0196-9781(92)90090-p. [DOI] [PubMed] [Google Scholar]
- 3.Park M-I, Ferber I, Camilleri M, et al. Effect of atilmotin on gastrointestinal transit in healthy subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:28–36. doi: 10.1111/j.1365-2982.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 4.Baxter Healthcare Corporation. BAX-ACC-1638 Injection Clinical Investigator’s Brochure. (Ed1) 2003 Vers2. [Google Scholar]
- 5.Lux G, Rosch W, Domschke S, et al. Intravenous 13-Nle-motilin increases the human lower esophageal sphincter pressure. Scand J Gastroent. 1976;11 Suppl 39:75–79. [PubMed] [Google Scholar]
- 6.Parkman HP, Miller DL, et al. Optimal evaluation of esophageal dysphagia: esophageal manometry, esophageal transit scintigraphy or videoesophagography. Dig Dis Sci. 1996;41(7):1355–1368. doi: 10.1007/BF02088560. [DOI] [PubMed] [Google Scholar]
- 7.Hirano I, Pandolfino J. New technologies for the evaluation of esophageal motility disorders: impedance, high-resolution manometry, and intraluminal ultrasound. Gastroenterol Clin North Am. 2007;36(3):531–551. doi: 10.1016/j.gtc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 9.Janssens J, Peeters TL, VanTrappen J, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. NEJM. 1990;322(15):1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- 10.McCallum RW, Cynshi O Investigative team. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis—a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26(8):1121–1130. doi: 10.1111/j.1365-2036.2007.03461.x. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Verlinden M, Snape W, et al. Failure of a motilin receptor agonist (ABT-229) to relieve the symptoms of functional dyspepsia in patients with and without delayed gastric emptying: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. 2000;14(12):1653–1661. doi: 10.1046/j.1365-2036.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaussade S, Michopoulos S, Sogni P, Guerre J, Couturier D. Motilin agonist erythromycin increases human lower esophageal sphincter pressure by stimulation of cholinergic nerves. Dig Dis Sci. 1994;39:381–384. doi: 10.1007/BF02090212. [DOI] [PubMed] [Google Scholar]
- 13.Meissner AJ, Bowes KL, Zwick R, Daniel EE. Effect of motilin on the lower oesophageal sphincter. Gut. 1976;17:925–932. doi: 10.1136/gut.17.12.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzovaras G, Xynos E, Chrysos E, Mantides A, Vassilakis JS. The effect of intravenous erythromycin on esophageal motility in healthy subjects. Am J Surg. 1996;171:316–319. doi: 10.1016/S0002-9610(97)89633-X. [DOI] [PubMed] [Google Scholar]
- 15.Chrysos E, Tzovaras G, Epanomeritakis E, et al. Erythromycin enhances oesophageal motility in patients with gastro-oesophageal reflux. ANZ J Surg. 2001;71(2):98–102. doi: 10.1046/j.1440-1622.2001.02005.x. [DOI] [PubMed] [Google Scholar]
- 16.Pennathur A, Tran A, Cioppi M, Fayad J, Sieren GL, Little AG. Erythromycin strengthens the defective lower esophageal sphincter in patients with gastroesophageal reflux disease. Am J Surg. 1994;167:169–172. doi: 10.1016/0002-9610(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 17.Netzer P, Schmitt B, Inauen W. Effects of ABT-229, a motilin agonist, on acid reflux, oesophageal motility and gastric emptying in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:1481–1490. doi: 10.1046/j.1365-2036.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 18.van Herwaarden MA, Samsom M, van Nispen CH, Verlinden M, Smout AJ. The effect of motiin agonist ABT-229 on gastro-oesophageal reflux, oesophageal motility and lower oesophageal sphincter characteristics in GERD patients. Aliment Pharmacol Ther. 2000;14:453–462. doi: 10.1046/j.1365-2036.2000.00712.x. [DOI] [PubMed] [Google Scholar]