Abstract
The 3α,5α- and 3α,5β-reduced derivatives of progesterone, deoxycorticosterone, dehydroepiandrosterone and testosterone enhance GABAergic neurotransmission and produce inhibitory neurobehavioral and anti-inflammatory effects. Despite substantial information on the progesterone derivative (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP, allopregnanolone), the physiological significance of the other endogenous GABAergic neuroactive steroids has remained elusive. Here, we describe the validation of a method using gas chromatography-mass spectrometry to simultaneously identify serum levels of the eight 3α,5α- and 3α,5β-reduced derivatives of progesterone, deoxycorticosterone, dehydroepiandrosterone and testosterone. The method shows specificity, sensitivity and enhanced throughput compared to other methods already available for neuroactive steroid quantification. Administration of pregnenolone to rats and progesterone to women produced selective effects on the 3α,5α- and 3α,5β-reduced neuroactive steroids, indicating differential regulation of their biosynthetic pathways. Pregnenolone administration increased serum levels of 3α,5α-THP (+1488%, p<0.001), (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC, +205%, p<0.01), (3α,5α)-3-hydroxyandrostan-17-one (3α,5α-A, +216%, p<0.001), (3α,5α,17β)-androstane-3,17-diol (3α,5α-A-diol, +190%, p<0.01). (3α,5β)-3-hydroxypregnan-20-one (3α,5β-THP) and (3α,5β)-3-hydroxyandrostan-17-one (3α,5β-A) were not altered, while (3α,5β)-3,21-dihydroxypregnan-20-one (3α,5β-THDOC) and (3α,5β,17β)-androstane-3,17-diol (3α,5β-A-diol) were increased from undetectable levels to 271 ± 100 and 2.4 ± 0.9 pg ± SEM, respectively (5/8 rats). Progesterone administration increased serum levels of 3α,5α-THP (+1806%, p<0.0001), 3α,5β-THP (+575%, p<0.001), 3α,5α-THDOC (+309%, p<0.001). 3α,5β-THDOC levels were increased by 307%, although this increase was not significant because this steroid was detected only in 3/16 control subjects. Levels of 3α,5α-A, 3α,5β-A and pregnenolone were not altered. This method can be used to investigate the physiological and pathological role of neuroactive steroids and to develop biomarkers and new therapeutics for neurological and psychiatric disorders.
Keywords: GABAergic Neuroactive Steroids, Pregnenolone, Gas Chromatography-Mass Spectrometry
1. Introduction
Neuroactive steroids are endogenous neuromodulators, synthesized de novo in the brain as well as in the adrenals and gonads. They have potent effects on neurotransmission mediated by γ-aminobutyric acid type A (GABAA) receptors[1] on which they act through specific binding sites on the α subunits[2]. The 3α,5α- and 3α,5β-reduced metabolites of progesterone, deoxycorticosterone, dehydroepiandrosterone (DHEA) and testosterone[3-5] induce potent GABAergic actions that result in anxiolytic, anticonvulsant, sedative/hypnotic, cognitive and pro-copulatory effects[6,7].
GABAergic neuroactive steroids play a crucial role in physiological states like stress[8], pregnancy[9], ovarian cycling[10,11], puberty[12,13] and aging[14]. Their concentrations are increased by administration of various psychoactive drugs, including ethanol[15,16], nicotine[17], caffeine[18], tetrahydrocannabinol[19], morphine[19,20], antidepressants[21-23] and the atypical antipsychotics clozapine and olanzapine[24-27]. GABAergic neuroactive steroid levels are altered in several mood and emotional disorders, including anxiety, depression, premenstrual dysphoric disorder, schizophrenia, epilepsy and drug addiction[5,16,22,28,29]. Furthermore, neuroactive steroids possess neuroprotective and neurotrophic effects[30-32] and their levels are altered in neurodegenerative diseases[33].
Recent evidence suggests a therapeutic potential for neuroactive steroids in traumatic brain injury[34,35], multiple sclerosis[36,37], epilepsy[38], Niemann-Pick type C disease[30], schizophrenia[29,39,40], depression[21,22], and alcoholism[16]. In fact, clinical trials using the precursors pregnenolone and progesterone or the synthetic neuroactive steroid ganaxolone are under investigation for traumatic brain injury[35], multiple sclerosis[36,37], schizophrenia[41,42], and epilepsy[38] (see also Morrow, 2007[7] for review). It is postulated that the therapeutic effects of progesterone for traumatic brain injury[43] and pregnenolone for schizophrenia[41] are due, in part, to their conversion into the potent GABAergic neuroactive steroid (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP). Indeed, pregnenolone is metabolized into several steroid hormones and their 3α,5α- and 3α,5β-reduced metabolites also possess GABAergic properties. Since progesterone, deoxycorticosterone, DHEA and testosterone are present at high concentrations in blood, the 3α,5α- and 3α,5β-reduced GABAergic metabolites of these steroids are likely to play important physiologic roles.
In most of the aforementioned studies, neuroactive steroid levels were measured using a radioimmunoassay. Radioimmunoassays can provide highly sensitive measurements of neuroactive steroids in the brain, especially after extensive clean-up or purification by HPLC. However, in serum samples neuroactive steroids are present at much lower concentrations. Furthermore, the lack of specific antibodies does not allow the simultaneous measurement of multiple neuroactive steroids. In addition, antibodies currently available do not always discriminate among the different isomers of the same compound and this limitation appears to be particularly important in the case of human studies. Humans synthesize both 3α,5α- and 3α,5β-reduced neuroactive steroids[22,44-47]. Considering the abundance of precursors and the common metabolic enzymes, it is likely that the GABAergic metabolites of progesterone, deoxycorticosterone, DHEA and testosterone are both singularly and coordinately significant physiological regulators of central nervous system excitability (see special issue on neuroactive steroids[7]).
Studies using gas chromatography and mass spectrometry (GC-MS) detection of neuroactive steroids have appeared, but are restricted to a few of the GABAergic steroids[21,22,29,47-51] (see also Purdy et al.[52] for review). GC-MS allows the simultaneous detection of low amounts of neuroactive steroids in brain, cerebrospinal fluid or serum samples. Here we describe the validation of a GC-MS method following solid phase purification to simultaneously quantify serum levels of eight GABAergic neuroactive steroids: 3α,5α-THP, (3α,5β)-3-hydroxypregnan-20-one (3α,5β-THP), (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC), (3α,5β)-3,21-dihydroxypregnan-20-one (3α,5β-THDOC), (3α,5α)-3-hydroxyandrostan-17-one (3α,5α-A), (3α,5β)-3-hydroxyandrostan-17-one (3α,5β-A), (3α,5α,17β)-androstane-3,17-diol (3α,5α-A-diol), (3α,5β,17β)-androstane-3,17-diol (3α,5β-A-diol) and their precursor pregnenolone. The biosynthetic pathway is shown in Figure 1. This method has several distinct advantages over prior procedures that require labor intensive HPLC purification, relatively large sample volumes of 1 ml, and quantify fewer neuroactive steroids (see Purdy et al.[52] for review). With this comprehensive method, we investigated the effects of pregnenolone administration to rats and progesterone administration to women on serum levels of these neuroactive steroids.
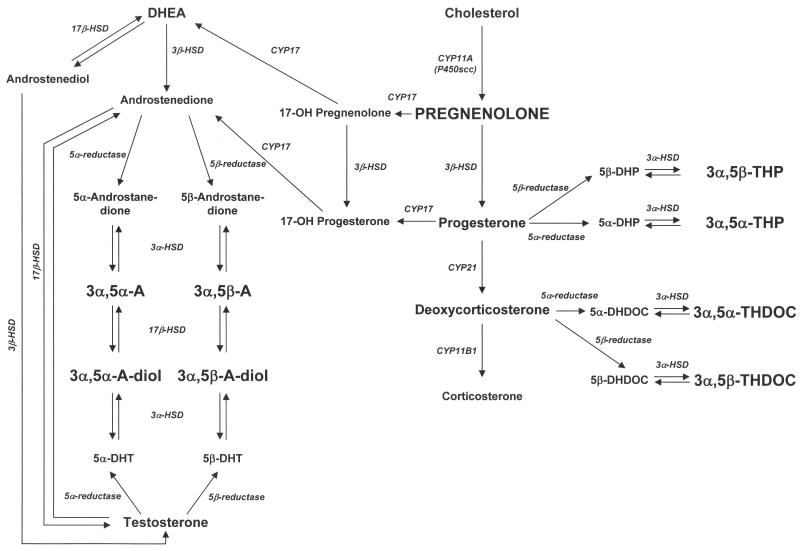
Figure 1.
Biosynthetic pathway for the GABAergic neuroactive steroids measured herein (indicated in bold capitalized letters). Abbreviations 3α,5α-THP: (3α,5α)-3-hydroxypregnan-20-one; 3α,5β-THP: (3α,5β)-3-hydroxypregnan-20-one; 3α,5α-THDOC: (3α,5α)-3,21-dihydroxypregnan-20-one; 3α,5β-THDOC: (3α,5β)-3,21-dihydroxypregnan-20-one; 3α,5α-A: (3α,5α)-3-hydroxyandrostan-17-one; 3α,5β-A: (3α,5β)-3-hydroxyandrostan-17-one; 3α,5α-A-diol: (3α,5α,17β)-androstane-3,17-diol; 3α,5β-A-diol: (3α,5β,17β)-androstane-3,17-diol; 5α-DHP: 5α-dihydroprogesterone; 5β-DHP: 5β-dihydroprogesterone; 5α-DHDOC: 5α-dihydrodeoxycorticosterone; 5β-DHDOC: 5β-dihydrodeoxycorticosterone; 5α-DHT: 5α-dihydrotestosterone; 5β-DHT: 5β-dihydrotestosterone; DHEA: dehydroepiandrosterone; HSD: hydroxysteroid dehydrogenase.
2. Materials and Methods
2.1. Materials/Chemicals
Steroid standards (>99% purity) for 3α,5α-A, 3α,5β-A, 3α,5α-A-diol, 3α,5β-A-diol, 3α,5β-THP, 3α,5β-THDOC and pregnenolone were purchased from Steraloids, Inc. (Newport, RI, USA). Deuterium-labeled standards (>95% purity) for (d4-17,21,21,21)-3α,5α-THP and (d3-17,21,21)-3α,5α-THDOC were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). (d4-2,2,4,4)-3α,5α-A was purchased from Cerilliant (Round Rock, TX, USA). 3α,5α-THP, 3α,5α-THDOC and (d4-17,21,21,21)-pregnenolone (98% purity) were synthesized by Dr. R.H. Purdy (Veterans Medical Research Foundation, San Diego, CA, USA). Derivatization reagent heptafluorobutyric acid anhydride was purchased from Pierce (Rockford, IL, USA). Monodeuterated ethanol was from Sigma-Aldrich (St. Louis, MO, USA). Organic solvents were pesticide grade from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
2.2. Steroid extraction and separation
Neuroactive steroids were purified from serum by solid phase extraction as described by Budzinski et al.[53], with some modifications. Serum samples (300 μl) were spiked with 400 pg/ml of each deuterated internal standard and applied to C18 solid phase extraction columns (RPN1910, 500 mg, GE Healthcare, UK) that had been preconditioned with 4 ml methanol and 4 ml distilled water. The column containing the sample was washed with 4 ml distilled water in order to remove high polar impurities. Columns were dried under vacuum for 30 minutes and neuroactive steroids were then eluted with 2 ml methanol. The extracts were evaporated in a speed vacuum concentrator (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The dry residue was resuspended in 2 ml of ethyl acetate/methanol (80/20, v/v) and the sample was filtered through a NH2 column (Supelclean LC-NH2, 500 mg, Supelco, Bellefonte, PA, USA) previously preconditioned with 4 ml of ethyl acetate and 4 ml of ethyl acetate/methanol (80/20, v/v). The neuroactive steroids passed unretained through the sorbent, and the eluate was collected. The NH2 column was further rinsed with 2 ml more of the solvent mixture and the combined eluates were evaporated in the speed vacuum concentrator before derivatization. Purification with the NH2 column was necessary to improve the validation and accuracy of the assay. We found that % accuracy for 3α,5α-THP, 3α,5β-THP, 3α,5α-THDOC and 3α,5β-THDOC was 72 ± 10.8, 115 ± 21.0, 129 ± 13.2 and 128 ± 14.7, respectively, after C18 column purification alone, vs. 88 ± 4.6, 101 ± 6.1, 102 ± 2.7 and 106 ± 4.6, respectively, after the combination of C18 plus NH2 column purification.
2.3. Preparation of calibration curves
Neuroactive steroid standards were dissolved in reagent grade alcohol (Thermo Fisher Scientific, Inc., Waltham, MA, USA) to obtain 10 mg/ml stocks. Stocks were made fresh every month and were diluted the day of the experiment to obtain 2, 10, 20, 50, 100, 200, 500, 1000, 2000 and 3000 pg/ml concentrations for each steroid. Calibration curves were made in 300 μl distilled water spiked with 5 μl human charcoal-stripped serum (Gemini Bio-Products, Woodland, CA, USA), with 400 pg/ml of each deuterated internal standard and with the appropriate known concentration of neuroactive steroids. This concentration of human charcoal-stripped serum was chosen since undiluted serum contained 10-40 pg/ml of six of the eight neuroactive steroids that could alter the accuracy of the assay for low concentrations of steroids. A blank standard (5 μl human charcoal-stripped serum in 300 μl distilled water) was also included. Calibration curves underwent the same extraction procedure as the samples.
2.4. Derivatization
Samples were derivatized according to Uzunova et al.[22] and Marx et al.,[29]. Dried samples after purification were resuspended in 600 μl methanol and transferred to derivatization vials (Wheaton, Millville, NJ, USA). Methanol was evaporated and samples were resuspended in 450 μl of ethyl acetate and 50 μl of heptafluorobutyric acid anhydride, followed by vortex mixing. Samples were allowed to react for two hours at room temperature and were dried under a gentle stream of nitrogen. Derivatized samples were resuspended in 10 μl of heptane and 2 μl of each sample was injected in duplicate into the GC-MS.
2.5. GC-MS analysis
Analysis was carried out on an Agilent 6890 gas chromatograph coupled to a 5973 mass selective detector (Agilent Technologies, Inc., Santa Clara, CA, USA) operated in negative chemical ionization mode. A capillary column (30 m × 0.25 mm, 0.25 μm film thickness, 5%-phenyl-methylpolysiloxane, J&W Scientific, Agilent Technologies, Inc., Santa Clara, CA, USA) was used to separate the derivatives of each neuroactive steroid. Samples (2 μl) were injected into the GC in splitless mode at 12 psi and at 250°C using a 7683 series injector (Agilent Technologies, Inc., Santa Clara, CA, USA). The carrier gas was ultrapure helium (99.9995%, National Welders, Durham, NC, USA) set at constant flow of 1.0 ml/min. Methane (99.999% National Welders, Durham, NC, USA) was the reagent gas. The initial GC oven temperature was 75°C (0.86 min hold), followed by an increase to 210°C at 35° increments (3 min hold), an increase to 235°C at 2.5° increments (9 min hold) and finally to 310°C at 25° increments (2 min hold). The transfer line temperature was maintained at 280°C. Neuroactive steroids were analyzed by single ion monitoring. The data acquisition was broken into retention windows corresponding to the elution of the different neuroactive steroid groups (Table 1). The temperatures of mass spectrometer source and quadrupole were 150°C.
Table 1.
Retention time, target ion and qualifier ion for each neuroactive steroid and deuterium labeled internal standard (IS).
| Neuroactive steroid | Retention Time | Target Ion m/z | Qualifier Ion m/z |
|---|---|---|---|
| Group 1: 13.5-18 min | |||
| 3α,5α-A-diol | 14.69 | 664.4 | 684.4 |
| 3α,5β-A-diol | 15.06 | 664.4 | 684.4 |
| d4-3α,5α-A (IS) | 15.42 | 470.4 | 450.4 |
| 3α,5α-A | 15.47 | 466.4 | 446.4 |
| 3α,5β-A | 15.85 | 446.4 | 466.4 |
| Group 2: 18-21 min | |||
| d4-3α,5α-THP (IS) | 19.09 | 497.4 | 477.4 |
| 3α,5α-THP | 19.16 | 474.4 | 494.4 |
| 3α,5β-THP | 19.47 | 474.4 | 494.4 |
| Group 3: 21-23.5 min | |||
| d4-Pregnenolone (IS) | 21.82 | 495.4 | 475.4 |
| Pregnenolone | 21.93 | 492.4 | 472.4 |
| Group 4: 23.5-31 min | |||
| d3-3α,5α-THDOC (IS) | 23.91 | 492.4 | 516.4 |
| 3α,5α-THDOC | 24.07 | 490.4 | 513.4 |
| 3α,5β-THDOC | 24.45 | 490.4 | 513.4 |
Neuroactive steroids were identified by monitoring the two most abundant ions (target ion and qualifier ion) at the corresponding retention time for each compound. The data acquisition was broken into retention windows corresponding to the elution of the different neuroactive steroid groups.
2.6. Biological application of the method
Male Sprague-Dawley rats (200-250 g) were purchased from Harlan (Indianapolis, IN, USA). After arrival at the animal facility, rats were allowed to acclimate for one week. They were housed four per cage under 12h light, 12h dark cycle (light on from 0700 to 1900 h) and at a constant temperature of 22 ± 2°C and relative humidity of 65%. They had free access to water and standard laboratory food at all times. Rats were habituated to handling and intraperitoneal (i.p.) injection every day for five days before the experiment. Pregnenolone, 50 mg/kg, was dissolved in 45% β-cyclodextrine and was administered i.p. 60 minutes before sacrifice. This interval was chosen to allow time for conversion of pregnenolone to its GABAergic metabolites. The blood was collected from the trunk into red cap Vacutainer tubes, immediately after decapitation. It was centrifuged (3000 rpm for 15 min at 4°C) and serum samples were stored in plastic minivials at -80°C until use. Adequate measures were taken to minimize pain or discomfort of the animals. Animal care and handling throughout the experimental procedures were in accordance with National Institutes of Health Guidelines under Institutional Animal Care and Use Committee approved protocols.
Human blood samples were obtained from healthy women volunteers as previously described[54]. Each woman was tested during days 2-6 of the follicular phase of menstrual cycle, when endogenous levels of progesterone are low[55-57]. Blood samples for baseline were taken 15 minutes before and 160 minutes after oral administration of 300 mg of micronized progesterone. Neuroactive steroid determination was conducted in serum. The study was conducted in compliance with the Declaration of Helsinki (http://www.wma.net/e/policy/b3.htm) and was approved by the University of North Carolina at Chapel Hill, Committee on Protection of the Rights of Human Subjects. Informed consent was obtained from participants after the study was fully explained.
2.7. Statistical analysis
Linear regression curves, Pearson's correlations and statistical analysis were performed using a commercially available statistical program (GraphPad Prism 4.0, GraphPad Software, San Diego, CA, USA). The unpaired t-test was used to compare the effects of pregnenolone administration on rat serum neuroactive steroid levels. The paired t-test was used to compare the effects of progesterone challenge on each neuroactive steroid in the human samples.
3. Results
3.1. Analytical method validation
Chromatography and mass spectrometry
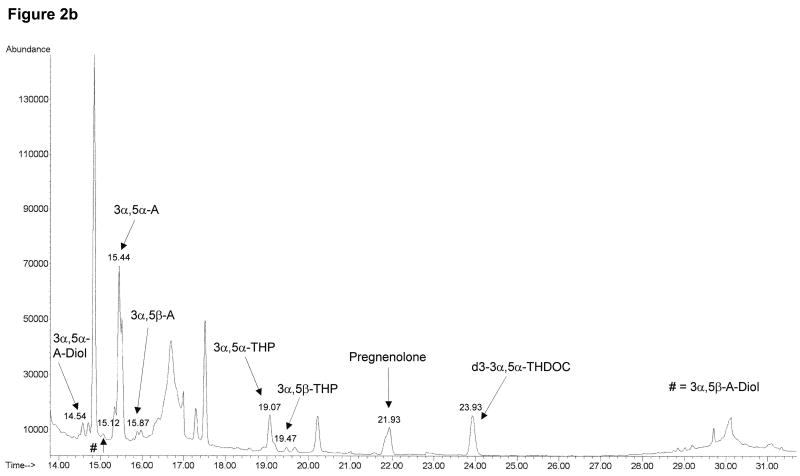
The neuroactive steroids were identified by monitoring specific ions at the corresponding retention time as shown in Table 1. The data acquisition was broken into retention windows corresponding to the elution of the different neuroactive steroid groups (Table 1). Total ion chromatograms after injection of 1000 pg/ml of neuroactive steroid standards in human charcoal-stripped serum (Figure 2a) and after injection of human serum under basal conditions (Figure 2b) show a good separation for the steroids of interest. We also obtained clear separation of the four different isomers, 3α,5α-, 3α,5β-, 3β,5α-and 3β,5β-THP (Figure 3), thus preventing any false estimates due to interference.
Figure 2.
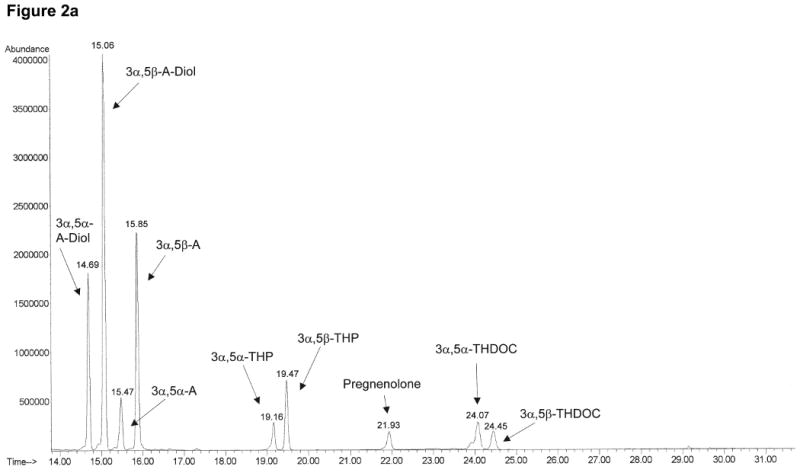
Total ion chromatograms of neuroactive steroids obtained in selected ion monitoring mode for 1000 pg/ml standard mixture in human charcoal-stripped serum (a) and human serum under basal conditions (b). The data acquisition was broken into retention windows corresponding to the elution of the different neuroactive steroid groups, as shown in Table 1. The target and qualifier ions monitored for each neuroactive steroid are also shown in Table 1.
Figure 3.
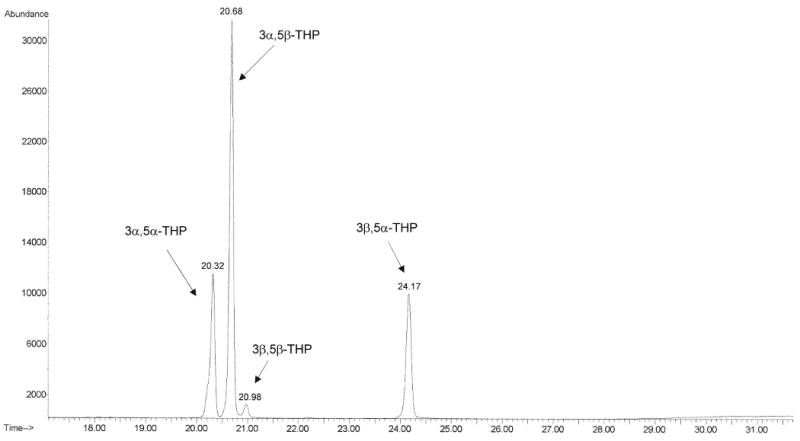
Total ion chromatogram obtained in selected ion monitoring shows good separation of a 1000 pg/ml mixture of 3α,5α-, 3α,5β-, 3β,5α- and 3β,5β-THP isomers. For each isomer, the ions 474.4 and 494.4 were monitored (as indicated in Table 1).
Calibration standard curves
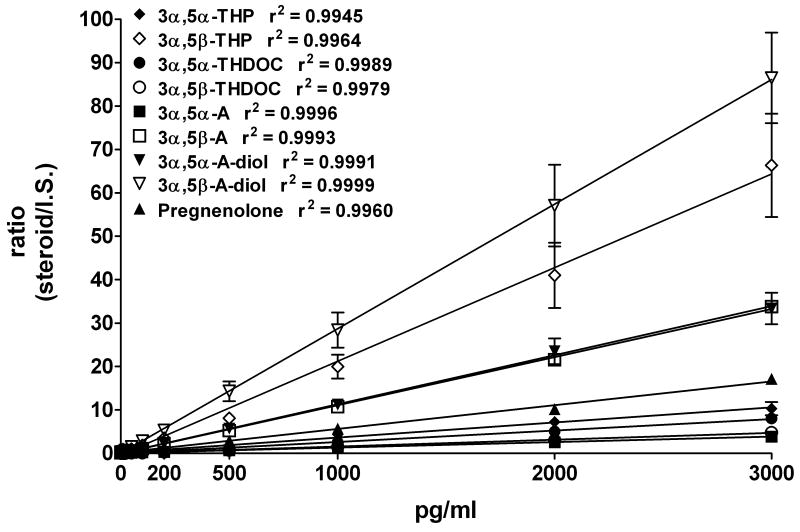
Unknown amounts of each neuroactive steroid were obtained by interpolating linear regression standard curves. The area ratio of mass to charge (m/z) for each neuroactive steroid relative to the m/z of the respective deuterated internal standard was plotted against the concentrations of neuroactive steroids (2, 10, 20, 50, 100, 200, 500, 1000, 2000 and 3000 pg/ml, Figure 4). Correlation coefficient values (r2) were between 0.995 and 0.999, as shown in Figure 4.
Figure 4.
Calibration curves for each neuroactive steroid standard. Steroid amounts (0, 2, 10, 20, 50, 100, 200, 500, 1000, 2000 and 3000 pg/ml) were plotted versus area ratios of their respective deuterium-labeled internal standards. The data is the average ± SEM of three to five experiments for each neuroactive steroid.
No detectable signal was observed for any of the neuroactive steroids in a blank injected after the highest standard concentration (data not shown), demonstrating absence of carryover from a high concentration sample to the next sample. Nonetheless, to control for possible carryover, a blank was injected following every third sample.
Method validation
To validate the method and determine accuracy, sensitivity, extraction efficiency and linearity, we spiked 300 μl of human charcoal-stripped serum with increasing concentrations of neuroactive steroids. Linear regression plots of the theoretical vs. the actual value were obtained for each neuroactive steroid (Figure 5). Pearson correlations suggest that the measured concentrations of neuroactive steroids are very close or identical to the spiked amount, demonstrating good accuracy and linearity of the method and suggesting a good estimation of recovery for each neuroactive steroid across the purification/derivatization procedures. The pregnenolone assay was also validated (Linear regression: y = 1.051× - 21.62; r2 = 0.9966; Pearson r = 0.9983; p<0.0001, figure not shown). Low concentrations of 3α,5α-THP, 3α,5β-THP, 3α,5α-THDOC, 3α,5β-THDOC, 3α,5α-A, 3α,5β-A and pregnenolone were detected in human charcoal-stripped serum and this resulted in overestimation of steroid levels at concentrations between 10 and 100 pg/ml (Figure 5). In contrast, the 3α,5α- and 3α,5β-A-diols exhibited absolute accuracy even at low concentrations, consistent with the lack of any levels detected in human charcoal-stripped serum.
Figure 5.
Analytical validation of the assay. Linear regression plots for each neuroactive steroid obtained by spiking 300 μl of charcoal-stripped human serum with increasing concentrations of neuroactive steroids (0, 10, 30, 50, 100, 300, 500 and 1000 pg/ml). Squares represent the actual amount spiked and triangles represent the amount obtained after the entire experimental procedure. The data is the average ± SEM of three to five experiments for each neuroactive steroid.
Precision and reproducibility of GC-MS
Intra-assay and inter-assay variability for each steroid are shown in Table 2. For intra-assay variability, five replicates of quality control samples (human charcoal-stripped serum spiked with 30, 300 and 3000 pg/ml) were assayed along with the calibration standards in the same assay. Inter-assay variability was obtained by comparing five different analytical runs of quality control samples (human charcoal-stripped serum spiked with 30, 300 and 3000 pg/ml).
Table 2.
Intra- and inter-assay precision of GC-MS analysis of neuroactive steroids.
| Neuroactive steroid | Intra-assay (% CV) | Inter-assay (% CV) | ||||
|---|---|---|---|---|---|---|
| 30 pg/ml | 300 pg/ml | 3000 pg/ml | 30 pg/ml | 300 pg/ml | 3000 pg/ml | |
| 3α,5α-THP | 11.10 | 5.60 | 9.68 | 17.02 | 6.47 | 5.95 |
| 3α,5β-THP | 7.45 | 5.21 | 9.86 | 4.87 | 11.21 | 5.11 |
| 3α,5α-THDOC | 6.92 | 3.98 | 6.88 | 5.88 | 5.24 | 8.52 |
| 3α,5β-THDOC | 4.79 | 4.07 | 7.35 | 3.95 | 7.66 | 7.69 |
| 3α,5α-A | 7.19 | 7.41 | 2.90 | 14.72 | 8.42 | 8.05 |
| 3α,5β-A | 8.47 | 6.12 | 5.15 | 6.54 | 6.88 | 2.50 |
| 3α,5α-A-diol | 10.29 | 5.43 | 4.52 | 10.31 | 6.30 | 3.85 |
| 3α,5β-A-diol | 12.64 | 6.47 | 5.47 | 16.10 | 7.36 | 2.53 |
| Pregnenolone | 7.69 | 2.65 | 7.48 | 10.51 | 6.41 | 8.58 |
For intra-assay variability, five replicates of quality control samples (human charcoal-stripped serum spiked with 30, 300 and 3000 pg/ml) were assayed along with the calibration standards in the same run. Inter-assay variability was obtained by comparing five different analytical runs of quality control samples (human charcoal-stripped serum spiked with 30, 300 and 3000 pg/ml). Coefficients of variation (CV) were calculated as standard deviation*100, divided by the mean.
Limit of detection of GC-MS
Only peaks with a signal to noise ratio equal to or greater than 5 were quantified. The lowest amount in the calibration standard curve that was accurately and repeatedly detected was 2 pg/ml for 3α,5β-THP, 3α,5α-THDOC, 3α,5β-THDOC, 3α,5β-A, 3α,5α-A-diol and 3α,5β-A-diol, and 10 pg/ml for 3α,5α-THP, 3α,5α-A and pregnenolone. The limit of detection did not differ for quality control samples where undiluted stripped serum (300 μl) was used as the matrix.
3.2. Biological application of the method
This GC-MS assay allows the simultaneous quantification of multiple neuroactive steroids. To test its biological application, we investigated the effects of pharmacological challenge with pregnenolone and progesterone, since these steroids have been used for treatment of human disease. Pregnenolone (50 mg/kg, i.p.) was administered to male rats (Figure 6). Basal neuroactive steroid levels (pg/ml ± SEM) in serum from male rats were: 225 ± 8.4 for 3α,5α-THP, 92 ± 2.5 for 3α,5α-THDOC, 64 ± 5.4 for 3α,5α-A, 78 ± 14 for 3α,5β-A and 118 ± 25 for 3α,5α-A-diol. Basal 3α,5β-THP (145 ± 1.0) was detected only in 4/8 rats. 3α,5β-THDOC and 3α,5β-A-diol were undetectable. Administration of pregnenolone significantly increased levels of 3α,5α-THP (+1488%, p<0.001), 3α,5α-THDOC (+205%, p<0.01), 3α,5α-A (+216%, p<0.001) and 3α,5α-A-diol (+190%, p<0.01). In contrast, the increase in 3α,5β-THP (+569%) was not statistically significant. 3α,5β-THDOC and 3α,5β-A-diol were also increased, from undetectable levels to 271 ± 101 and to 2.4 ± 0.9 (pg ± SEM; 5/8), respectively, while 3α,5β-A levels did not change. Basal pregnenolone levels were 293 ± 84 (pg ± SEM, data not shown); they were dramatically increased (greater than 1000 fold) in all rats following pregnenolone administration, but the precise measure of this increase was above the range of detection.
Figure 6.
Effect of pregnenolone administration to male rats on neuroactive steroid serum levels. Pregnenolone (50 mg/kg) or vehicle (45% β-cyclodextrine) was administered i.p. 60 minutes before sacrifice. Levels are expressed as pg/ml and are average ± SEM of 8 subjects. In the vehicle-treated group 3α,5β-THP was detected in 4/8 rats, while 3α,5β-THDOC and 3α,5β-A-diol were undetectable (n.d.). *p<0.01 and **p<0.001 vs. the respective vehicle-treated control values; unpaired t test.
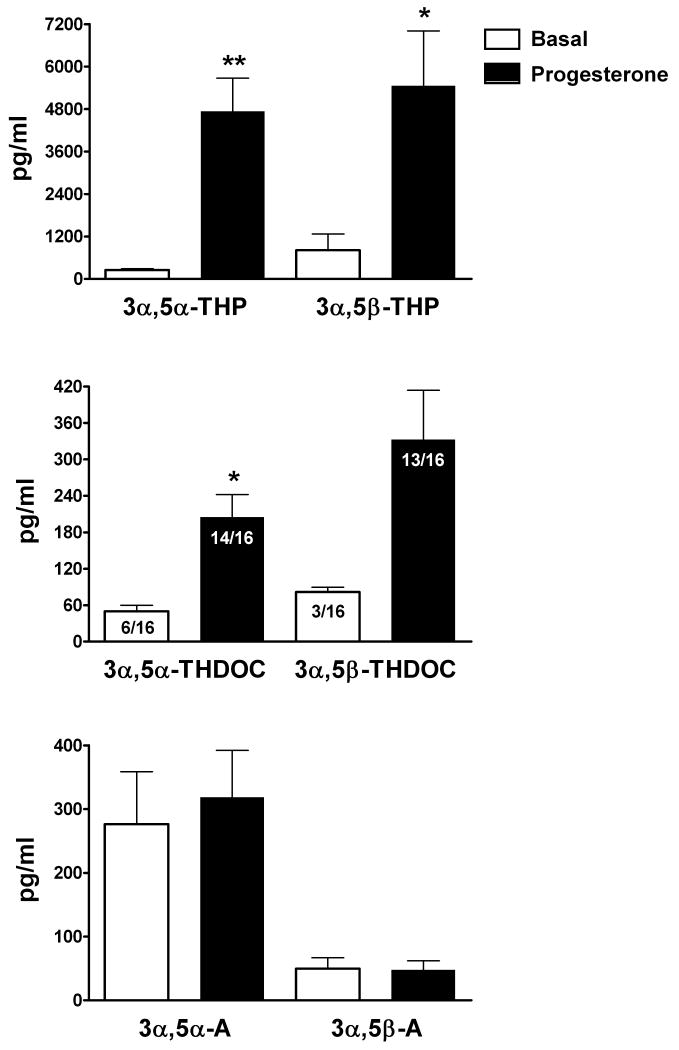
Next, we investigated the effects of pharmacological challenge with oral micronized progesterone (300 mg) in 16 healthy women (Figure 7). Basal neuroactive steroid serum levels (pg/ml ± SEM) during the follicular phase were: 249 ± 35 for 3α,5α-THP, 810 ± 458 for 3α,5β-THP, 277 ± 82 for 3α,5α-A and 50 ± 17 for 3α,5β-A. 3α,5α-THDOC was detected in 6/16 subjects and basal levels were 50 ± 9.9; 3α,5β-THDOC was detected only in 3/16 subjects with basal levels of 82 ± 9.2. As expected, administration of progesterone significantly increased levels of 3α,5α-THP (+1806%, p<0.0001), 3α,5β-THP (+575%, p<0.001) and 3α,5α-THDOC (+309%, p<0.001). 3α,5β-THDOC was also increased by 307%, however this increase did not reach statistical significance, probably due to the small number of control subjects with detectable levels (3/16). Indeed, levels of 3α,5β-THDOC were detected in 13/16 subjects after pharmacological challenge with progesterone. In contrast, administration of progesterone did not alter 3α,5α-A and 3α,5β-A levels. Pregnenolone levels were also not altered by progesterone challenge (806 ± 132 and 861 ± 113 pg/ml ± SEM for basal and after progesterone challenge, respectively; data not shown). The testosterone metabolites 3α,5α-A-diol and 3α,5β-A-diol were not measured in these subjects.
Figure 7.
Progesterone challenge to healthy women selectively alters neuroactive steroid serum levels. Blood samples were obtained 15 minutes before (basal) and 160 minutes after oral administration of 300 mg of micronized progesterone. Levels are expressed as pg/ml and are average ± SEM of 16 subjects. 3α,5α-THDOC was detected only in 6/16 subjects in the basal group and 14/16 in the progesterone group. 3α,5β-THDOC was detected only in 3/16 subjects in the basal group and 13/16 in the progesterone group. *p<0.001 and **p<0.0001 vs. the respective basal values; paired t test.
4. Discussion
We have described a highly selective and validated procedure for the simultaneous measurement of GABAergic 3α,5α- and 3α,5β-reduced metabolites of progesterone, deoxycorticosterone, DHEA and testosterone, as well as their precursor pregnenolone in serum. Although several GC-MS methods are available for measurements of neuroactive steroids[21,22,29,47-51] (see also Purdy et al.[52] for review), this is the first demonstration and validation of procedures that allow the simultaneous measurement of eight GABAergic neuroactive steroids and one precursor from the same sample. The method employs highly sensitive negative chemical ionization GC-MS and allows the identification of different isomers (both 5α- and 5β-reduced neuroactive steroids). The method has been validated using spiked human charcoal-stripped serum samples and sample preparation and purification is achieved with relatively high-throughput. This method allows a good separation of all these neuroactive steroids, including all four isomers, 3α,5α-, 3α,5β-, 3β,5α- and 3β,5β-THP, thus preventing any false estimates due to interference. The ability to measure all eight GABAergic neuroactive steroids from the same 300 μl aliquot of serum is particularly useful when only small volumes of sample can be obtained (frequently the case in clinical studies). The intra- and inter-assay variability and sensitivity are similar to previous published work for 3α,5α-THP[46,50], 3α,5β-THP[46], 3α,5α-A[47], 3α,5β-A[47], 3α,5α-A-diol[47], 3α,5β-A-diol[47] and pregnenolone[46,47,50,51] in serum. No data is available for 3α,5α-THDOC and 3α,5β-THDOC.
This assay can be used to measure neuroactive steroids in biological samples. This is important especially because clinical trials using the neuroactive steroid precursors progesterone and pregnenolone have appeared for traumatic brain injury, multiple sclerosis and schizophrenia[35-37,41,42]. Indeed, it is not yet clear if the therapeutic effects in these trials are due to progesterone or pregnenolone per se or to their neuroactive metabolites. Therefore, this improved GC-MS-based method for neuroactive steroids measurement could be used to evaluate the therapeutic role of these compounds. However, the clinical utility of this method is yet to be established and further studies are needed.
The assay has several limitations including its high cost and labor intensive procedures. While the sensitivity of the assay for 3α,5α-THP is comparable to some previous studies[46,50], other authors have reported slightly better sensitivity using HPLC purification prior to GC-MS[29] or better sensitivity to 3α,5α-THP, 3α,5α-A and 3α,5β-A using different GC-MS settings[47], but direct comparisons of solid phase purification vs. HPLC purification or the different GC-MS settings have yet to be performed and are needed to determine which approach offers more sensitivity. The limit of detection is unlikely to limit the usefulness of the assay since neuroactive steroid levels in the physiological range are reliably detected, but substantial decreases in neuroactive steroid levels may not be quantifiable.
This assay has been validated for its use in serum samples. However, the same approach could be used to measure cerebrospinal fluid levels of neuroactive steroids. There is very limited data on neuroactive steroid levels in cerebrospinal fluid. Levels of 3α,5α-THP[22,45,58,59], and pregnenolone[22,45,60,61] have been measured in human cerebrospinal fluid and are altered in depression and post-traumatic stress disorder[22,59,60]. 3α,5α-A has also been found in human cerebrospinal fluid[45]. A direct comparison of cerebrospinal fluid and serum levels has only been made for 3α,5α-THP, where levels were found to be the same in both biological fluids and were similarly decreased in patients with Parkinson's disease[58]. Furthermore, Naylor et al.,[61] showed that cerebrospinal fluid levels of both pregnenolone and DHEA are correlated with brain levels, suggesting that changes in cerebrospinal fluid neuroactive steroids levels are a good indication of changes in brain levels of these neuromodulators. These methods may be useful to expand our knowledge of GABAergic neuroactive steroids in human cerebrospinal fluid.
We tested the biological application of the assay in rats by measuring neuroactive steroid levels following acute administration of pregnenolone. Basal levels of 3α,5β-THP, 3α,5β-THDOC, 3α,5β-A, and 3α,5β-A-diol were very low or undetectable in serum from control rats, as expected, due to low 5β-reductase activity. This enzyme is expressed mainly in the liver and its activity decreases in adult rats[62]. The administration of pregnenolone to male rats induced a dramatic increase in the 3α,5α-reduced neuroactive steroids, with greater than ten-fold increases in 3α,5α-THP and approximately three-fold increases in 3α,5α-THDOC, 3α,5α-A, and 3α,5α-A-diol. These results suggest that pregnenolone selectively promotes the production of progesterone and its 3α,5α-reduced metabolites in male rats, perhaps since the other steroids require more biosynthetic steps for their production. It is possible that different ratios would be found at later time points or in specific endocrine tissues such as adrenals, testicles or brain.
We tested the application of the assay in humans by measuring neuroactive steroid levels following acute administration of progesterone. Basal 3α,5α- and 3α,5β-THDOC are not particularly abundant in humans. Levels were less than 30 pg/ml in 10/16 and 13/16 healthy women, respectively. Higher levels of 3α,5α-THDOC were measured in humans by radioimmunoassay[63] (basal values 2 ng/ml) or by GC-MS[64] (basal values 67 pg/ml), likely due to different technique or to lack of appropriate internal standards in those assays. Basal levels of the DHEA metabolites 3α,5α-A and 3α,5β-A were similar to the progesterone metabolites, however the level of 3α,5α-A was five fold higher than 3α,5β-A while 3α,5β-THP was twice the level of 3α,5α-THP. The relative ratios of different steroids may have undetermined physiological relevance. Progesterone administration elevated all four GABAergic neuroactive steroids derived from progesterone and deoxycorticosterone, however effects on 3α,5β-THDOC were not significant, probably since this steroid was undetectable prior to progesterone administration in 13/16 subjects. There was no effect of progesterone on the DHEA metabolites. Because progesterone increased both 3α,5α- and 3α,5β-THDOC levels in healthy women, these neuroactive steroids may have biological relevance, particularly during the luteal phase of the menstrual cycle, during pregnancy or after stimulation of the hypothalamic-pituitary-adrenal axis[9,10,46,55,57,65,66]. In contrast to humans, 3α,5α-THDOC is measurable in serum of male rats under control conditions. This could be attributed to a different progesterone metabolism in humans vs. rodents.
Our results show that administration of pregnenolone or progesterone selectively alters GABAergic neuroactive steroid levels. This would suggest that the therapeutic potential of pregnenolone and progesterone could be attributable to their conversion into the GABAergic neuroactive steroids. Indeed, the neuroactive steroid 3α,5α-THP is protective in animal models of traumatic brain injury[43]. In addition, 3α,5α-THP levels are altered in both traumatic brain injury[34] and schizophrenia[29], two of the disorders for which progesterone and pregnenolone, respectively, have shown a therapeutic efficacy. However, the therapeutic potential of neuroactive steroids may have even greater applicability since there is evidence of dysregulation in several neurological and psychiatric diseases[5,16,22,28-30,67]. Furthermore, 3α,5α-THP has neuroprotective and neurotrophic effects[30-32], suggesting a therapeutic potential for neurodegenerative diseases[33,68]. 3α,5α-THP reduces inflammatory cytokines after traumatic brain injury[69], suggesting a role for neuroactive steroids in the inflammation process, a field that is relatively unexplored.
Furthermore, the relevance of the different 5α- vs. 5β-isomers of the GABAergic neuroactive steroids is not well understood, likely due to the lack of a convenient method to measure them. The 5β-isomers, 3α,5β-THP and 3α,5β-THDOC, are equipotent modulators of GABAA receptors and may therefore be equally important in brain physiology. Some studies have shown they are present in human serum and modulated by pregnancy, disease and treatment. For example, 3α,5α-THP and 3α,5β-THP vary across pregnancy, but increases in 3α,5α-THP were much greater[46,65]. A decrease in the ratio of the 5α/5β isomers of THP was observed around parturition[70]. Depressed patients exhibit a decrease in peripheral 3α,5α-THP and 3α,5β-THP[44] and treatment with the antidepressant mirtazapine increased their concentrations[71]. Concentrations of 3α,5α-THP and 3α,5β-THP were also decreased during panic attacks[72]. Further studies are clearly needed to explore the role of both 5α- and 5β-isomers of all GABAergic neuroactive steroids in health and disease.
Finally, the simultaneous measurement of the 3α,5α- and 3α,5β-reduced neuroactive steroids can also be used to explore differential regulation of the biosynthetic pathways involved in the conversion of progesterone, deoxycorticosterone, DHEA and testosterone and to explore whether certain physiological or pathological states are associated with alterations in the different metabolic pathways that lead to neuroactive steroids synthesis. While most prior studies have focused on the progesterone metabolite 3α,5α-THP, the physiological significance of the deoxycorticosterone, DHEA and testosterone GABAergic metabolites are largely unknown. The simultaneous measurement of these neuroactive steroids is important not only to further explore their physiological role, but also to identify biomarkers of disease risk, treatment response and more selective therapeutic targets.
Acknowledgments
This study was supported by the National Institute of Health grants R01 AA010564, UO1 AA013515 and UO1 AA016677 to ALM and RO1 MH051246 to SSG.
The authors wish to express their appreciation to Drs. Synthia H. Mellon (University of California, San Francisco), Robert H. Purdy (The Scripps Research Institute, La Jolla, and Veterans Medical Research Foundation, San Diego, California) and Elena Romeo (Tor Vergata University, Rome, Italy) for many helpful discussions and suggestions during the course of development and validation of this assay. We also wish to thank Dr. Steven M. Paul (Ely Lilly and Company, Indianapolis, Indiana) for helpful comments on the manuscript.
List of abbreviations used for neuroactive steroids
- 3α,5α-THP
(3α,5α)-3-hydroxypregnan-20-one
- 3α,5β-THP
(3α,5β)-3-hydroxypregnan-20-one
- 3α,5α-THDOC
(3α,5α)-3,21-dihydroxypregnan-20-one
- 3α,5β-THDOC
(3α,5β)-3,21-dihydroxypregnan-20-one
- 3α,5α-A
(3α,5α)-3-hydroxyandrostan-17-one
- 3α,5β-A
(3α,5β)-3-hydroxyandrostan-17-one
- 3α,5α-A-diol
(3α,5α,17β)-androstane-3,17-diol
- 3α,5β-A-diol
(3α,5β,17β)-androstane-3,17-diol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul SM, Purdy RH. Neuroactive steroids. FASEB Journal. 1992;6:2311–22. [PubMed] [Google Scholar]
- 2.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 3.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 4.Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3α-androstanediol 1. Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996;79:109–18. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski RM, Marini H, Kim W, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–27. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggio G, Purdy RH. International Review of Neurobiology. Vol. 46. New York: Academic Press; 2001. Neurosteroids and Brain Function. [Google Scholar]
- 7.Morrow AL, Hosie AM, Wilkins ME, Smart TG, Herd MB, Belelli D, Lambert JJ, Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S, Smith SS, Shen H, Gong QH, Zhou X, Schumacher M, Guennoun R, Stein DG, De Nicola AF, Mellon SH, Girdler SS, Klatzkin RR, Biggio G, Concas A, Follesa P, Sanna E, Serra M. Neurosteroids Special Issue. In: Morrow AL, editor. Pharmacol Ther. Vol. 116. 2007. pp. 1–172. [Google Scholar]
- 8.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–9. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- 11.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 12.Grobin AC, Morrow AL. 3α-Hydroxy-5α-pregnan-20-one levels and GABAA receptor-mediated 36Cl- flux across development in rat cerebral cortex. Brain Res Dev Brain Res. 2001;131:31–9. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–77. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Morrow AL, VanDoren MJ, Devaud LL. Effects of progesterone or neuroactive steroid? Nature. 1998;395:652–3. doi: 10.1038/27106. [DOI] [PubMed] [Google Scholar]
- 16.Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–77. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–90. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- 18.Concas A, Porcu P, Sogliano C, Serra M, Purdy RH, Biggio G. Caffeine-induced increases in the brain and plasma concentrations of neuroactive steroids in the rat. Pharmacol Biochem Behav. 2000;66:39–45. doi: 10.1016/s0091-3057(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 19.Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL. Cortical 3α-hydroxy-5α-pregnan-20-one levels after acute administration of Δ9-tetrahydrocannabinol, cocaine and morphine. Psychopharmacology (Berl) 2005;179:544–50. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- 20.Concas A, Sogliano C, Porcu P, Marra C, Brundu A, Biggio G. Neurosteroids in nicotine and morphine dependence. Psychopharmacology (Berl) 2006;186:281–92. doi: 10.1007/s00213-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 21.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A. 1996;93:12599–604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisu MG, Serra M. Neurosteroids and neuroactive drugs in mental disorders. Life Sci. 2004;74:3181–97. doi: 10.1016/j.lfs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Marx CE, Duncan GE, Gilmore JH, Lieberman JA, Morrow AL. Olanzapine increases allopregnanolone in the rat cerebral cortex. Biol Psychiatry. 2000;47:1000–4. doi: 10.1016/s0006-3223(99)00305-4. [DOI] [PubMed] [Google Scholar]
- 25.Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–97. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 26.Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- 27.Marx CE, Shampine LJ, Khisti RT, Trost WT, Bradford DW, Grobin AC, Massing MW, Madison RD, Butterfield MI, Lieberman JA, Morrow AL. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav. 2006;84:609–17. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–97. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- 29.Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–63. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- 30.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–11. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 31.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–18. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 32.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–18. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. 2006;60:1287–94. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148:2505–17. doi: 10.1210/en.2006-1678. [DOI] [PubMed] [Google Scholar]
- 35.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 36.El-Etr M, Vukusic S, Gignoux L, Durand-Dubief F, Achiti I, Baulieu EE, Confavreux C. Steroid hormones in multiple sclerosis. J Neurol Sci. 2005;233:49–54. doi: 10.1016/j.jns.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Pieribone VA, Tsai J, Soufflet C, Rey E, Shaw K, Giller E, Dulac O. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48:1870–4. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- 39.Marx CE, Shampine LJ, Duncan GE, Vandoren MJ, Grobin AC, Massing MW, Madison RD, Bradford DW, Butterfield MI, Lieberman JA, Morrow AL. Clozapine markedly elevates pregnenolone in rat hippocampus, cerebral cortex, and serum: Candidate mechanism for superior efficacy? Pharmacol Biochem Behav. 2006;84:598–608. doi: 10.1016/j.pbb.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Ritsner M, Maayan R, Gibel A, Weizman A. Differences in blood pregnenolone and dehydroepiandrosterone levels between schizophrenia patients and healthy subjects. Eur Neuropsychopharmacol. 2007;17:358–65. doi: 10.1016/j.euroneuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Marx CE, Keefe RS, Leimone LA, Hamer RM, Bradford DW, Dunn L, Payne VM, Naylor JC, Savitz AJ, Strauss JL, Lieberman JA, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting neurocognitive and negative symptoms in schizophrenia. Biol Psychiatry. 2007;61:13S. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savitz AJ, Silverstein SM, McGovern KC, Schenkel L, Grant L. The neurosteroid, pregnenolone, reduces negative symptoms in patients with schizophrenia: results of a preliminary double-blind study. Schizophr Bull. 2007;33:458–9. [Google Scholar]
- 43.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–97. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–3. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 45.Kim YS, Zhang HJ, Kim HY. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Anal Biochem. 2000;277:187–95. doi: 10.1006/abio.1999.4384. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–79. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- 47.Kancheva L, Hill M, Vcelakova H, Vrbikova J, Pelikanova T, Starka L. The identification and simultaneous quantification of neuroactive androstane steroids and their polar conjugates in the serum of adult men, using gas chromatography-mass spectrometry. Steroids. 2007;72:792–801. doi: 10.1016/j.steroids.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Cheney DL, Uzunov D, Costa E, Guidotti A. Gas chromatographic-mass fragmentographic quantitation of 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci. 1995;15:4641–50. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liere P, Akwa Y, Weill-Engerer S, Eychenne B, Pianos A, Robel P, Sjövall J, Schumacher M, Baulieu EE. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;739:301–12. doi: 10.1016/s0378-4347(99)00563-0. [DOI] [PubMed] [Google Scholar]
- 50.Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–66. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- 51.Labombarda F, Pianos A, Liere P, Eychenne B, Gonzalez S, Cambourg A, De Nicola AF, Schumacher M, Guennoun R. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology. 2006;147:1847–59. doi: 10.1210/en.2005-0955. [DOI] [PubMed] [Google Scholar]
- 52.Purdy RH, Fitzgerald RL, Everhart ET, Mellon SH, Alomary AA, Parsons LH. The analysis of neuroactive steroids by mass spectrometry. In: Baker G, Dunn S, Holt A, editors. Handbook of Neurochemistry and Molecular Neurobiology: Practical Neurochemistry Methods. New York: Springer; 2007. pp. 1–18. [Google Scholar]
- 53.Budzinski H, Devier MH, Labadie P, Togola A. Analysis of hormonal steroids in fish plasma and bile by coupling solid-phase extraction to GC/MS. Anal Bioanal Chem. 2006;386:1429–39. doi: 10.1007/s00216-006-0686-9. [DOI] [PubMed] [Google Scholar]
- 54.Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31:1208–19. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Seippel L, Purdy RH, Backstrom T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–82. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 56.Chabbert Buffet N, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Front Neuroendocrinol. 1998;19:151–86. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- 57.Follesa P, Porcu P, Sogliano C, Cinus M, Biggio F, Mancuso L, Mostallino MC, Paoletti AM, Purdy RH, Biggio G, Concas A. Changes in GABAA receptor γ2 subunit gene expression induced by long-term administration of oral contraceptives in rats. Neuropharmacology. 2002;42:325–36. doi: 10.1016/s0028-3908(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 58.di Michele F, Longone P, Romeo E, Lucchetti S, Brusa L, Pierantozzi M, Bassi A, Bernardi G, Stanzione P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson's disease. Neurol Sci. 2003;24:172–3. doi: 10.1007/s10072-003-0115-1. [DOI] [PubMed] [Google Scholar]
- 59.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 60.George MS, Guidotti A, Rubinow D, Pan B, Mikalauskas K, Post RM. CSF neuroactive steroids in affective disorders: pregnenolone, progesterone and DBI. Biol Psychiatry. 1994;35:775–80. doi: 10.1016/0006-3223(94)91139-8. [DOI] [PubMed] [Google Scholar]
- 61.Naylor JC, Hulette CM, Steffens DC, Shampine LJ, Ervin JF, Payne VM, Massing MW, Kilts JD, Strauss JL, Calhoun PS, Calnaido RP, Blazer DG, Lieberman JA, Madison RD, Marx CE. Cerebrospinal fluid dehydroepiandrosterone levels are correlated with brain dehydroepiandrosterone levels, elevated in Alzheimer's disease, and related to neuropathological disease stage. J Clin Endocrinol Metab. 2008;93:3173–8. doi: 10.1210/jc.2007-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stenberg A. Developmental, diurnal and oestrous cycle-dependent changes in the activity of liver enzymes. J Endocrinol. 1976;68:265–72. doi: 10.1677/joe.0.0680265. [DOI] [PubMed] [Google Scholar]
- 63.Brambilla F, Biggio G, Pisu MG, Bellodi L, Perna G, Bogdanovich-Djukic V, Purdy RH, Serra M. Neurosteroid secretion in panic disorder. Psychiatry Res. 2003;118:107–16. doi: 10.1016/s0165-1781(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 64.Ströhle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–7. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- 65.Hill M, Parizek A, Bicikova M, Havlikova H, Klak J, Fait T, Cibula D, Hampl R, Cegan A, Sulcova J, Starka L. Neuroactive steroids, their precursors, and polar conjugates during parturition and postpartum in maternal and umbilical blood: 1. Identification and simultaneous determination of pregnanolone isomers. J Steroid Biochem Mol Biol. 2000;75:237–44. doi: 10.1016/s0960-0760(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 66.Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M, Casarosa E, Palumbo M, Petraglia F, Genazzani AR. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol. 2004;18:79–87. doi: 10.1080/09513590310001652955. [DOI] [PubMed] [Google Scholar]
- 67.Marx CE, Yuan P, Kilts JD, Madison RD, Shampine LJ, Manji HK. Neuroactive steroids, mood stabilizers, and neuroplasticity: alterations following lithium and changes in Bcl-2 knockout mice. Int J Neuropsychopharmacol. 2008:1–6. doi: 10.1017/S1461145708008444. [DOI] [PubMed] [Google Scholar]
- 68.Wang JM, Liu L, Irwin RW, Chen S, Brinton RD. Regenerative potential of allopregnanolone. Brain Res Rev. 2008;57:398–409. doi: 10.1016/j.brainresrev.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 69.He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–12. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Klak J, Hill M, Parizek A, Havlikova H, Bicikova M, Hampl R, Fait T, Sulcova J, Pouzar V, Kancheva R, Starka L. Pregnanolone isomers, pregnenolone and their polar conjugates around parturition. Physiol Res. 2003;52:211–21. [PubMed] [Google Scholar]
- 71.Schule C, Romeo E, Uzunov DP, Eser D, Di Michele F, Baghai TC, Pasini A, Schwarz M, Kempter H, Rupprecht R. Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3α-hydroxysteroid dehydrogenase activity. Mol Psychiatry. 2006;11:261–72. doi: 10.1038/sj.mp.4001782. [DOI] [PubMed] [Google Scholar]
- 72.Strohle A, Romeo E, Di Michele F, Pasini A, Hermann B, Gajewsky G, Holsboer F, Rupprecht R. Induced panic attacks shift gamma-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–8. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]