Abstract
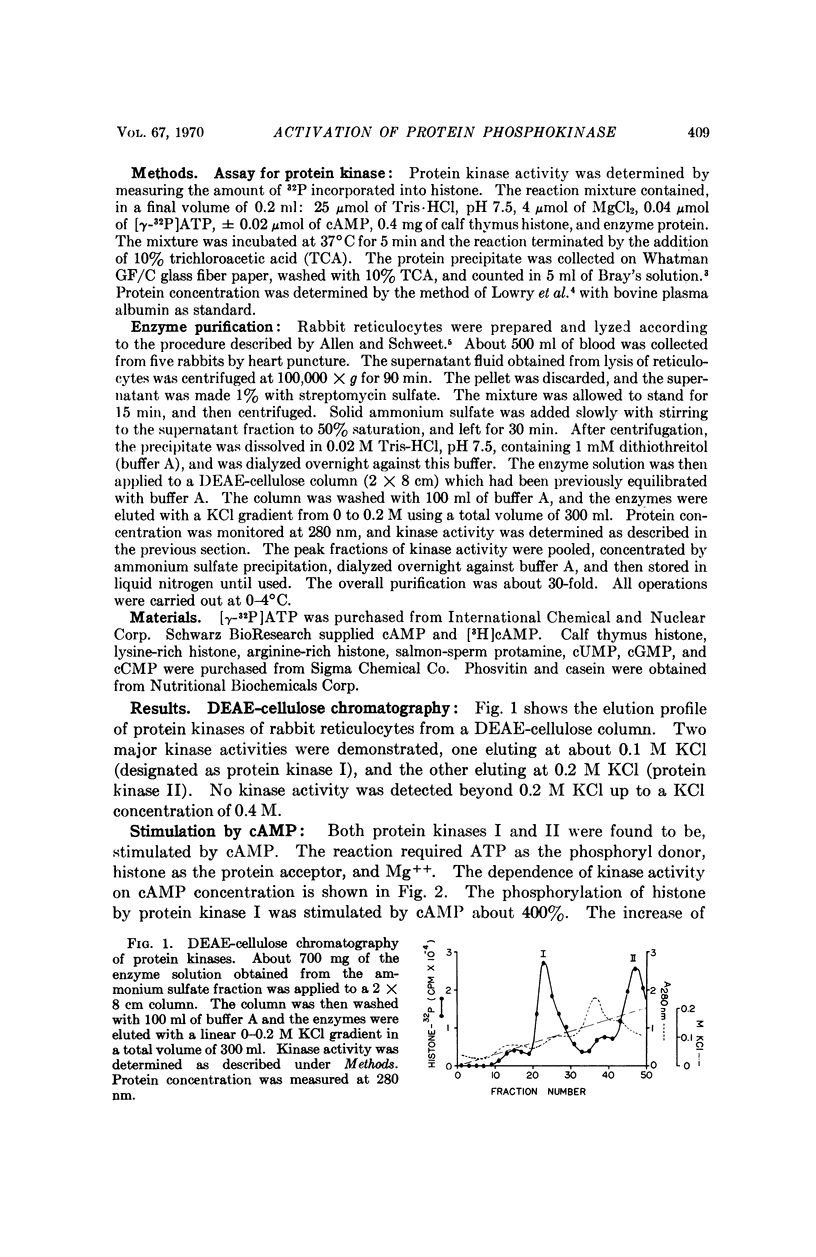
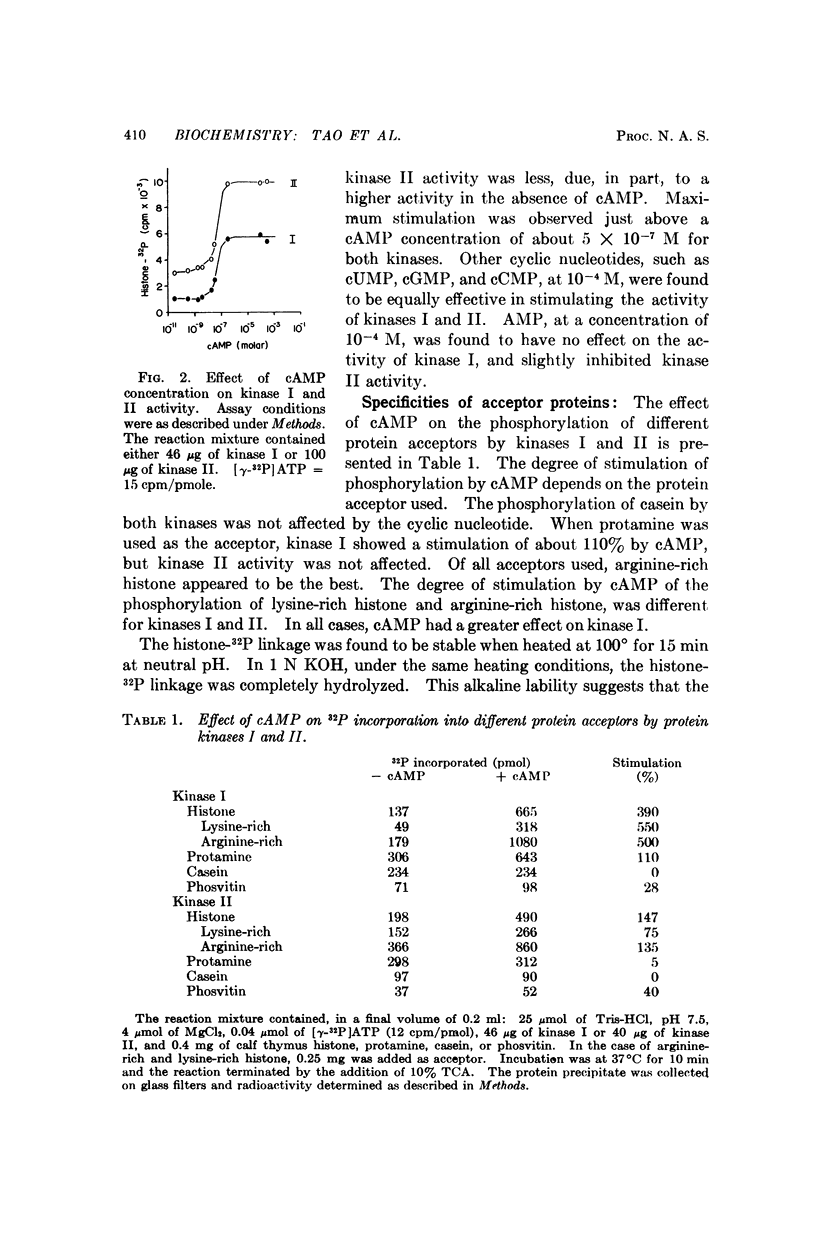
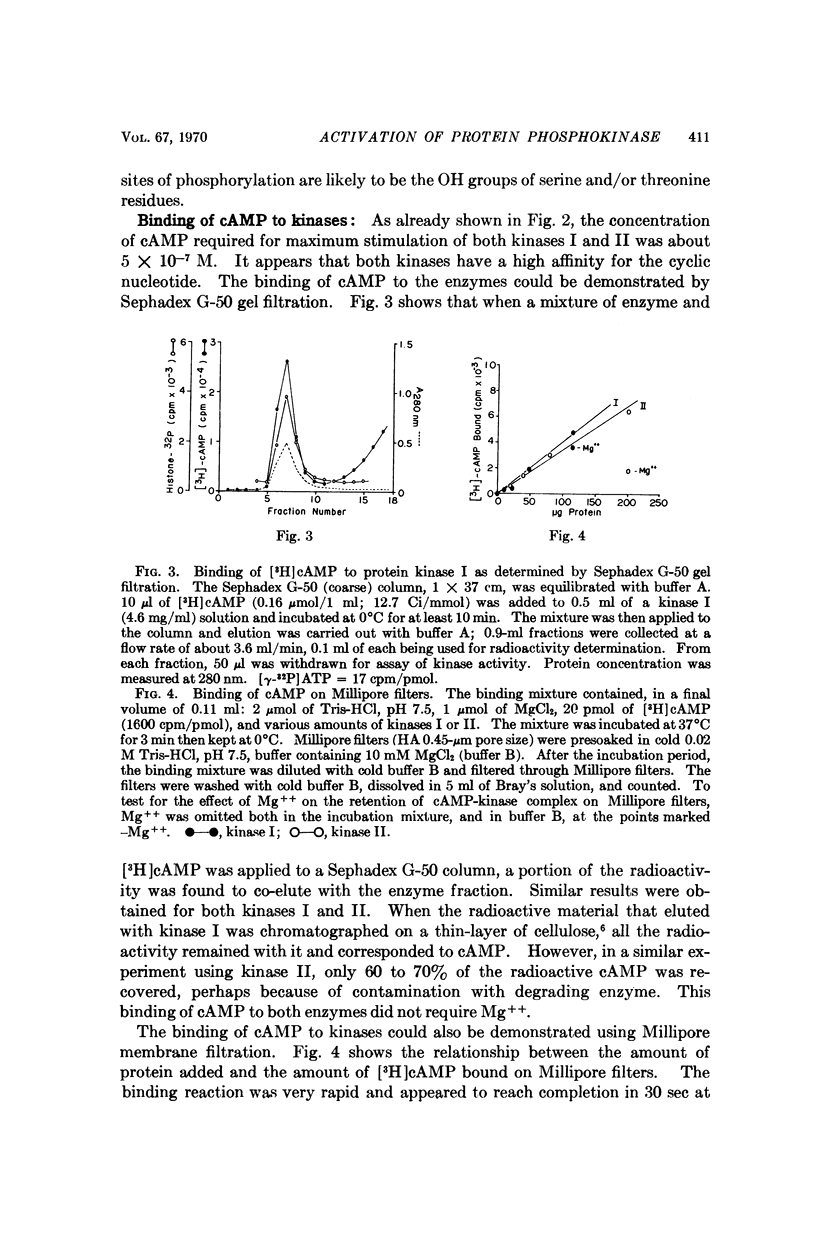
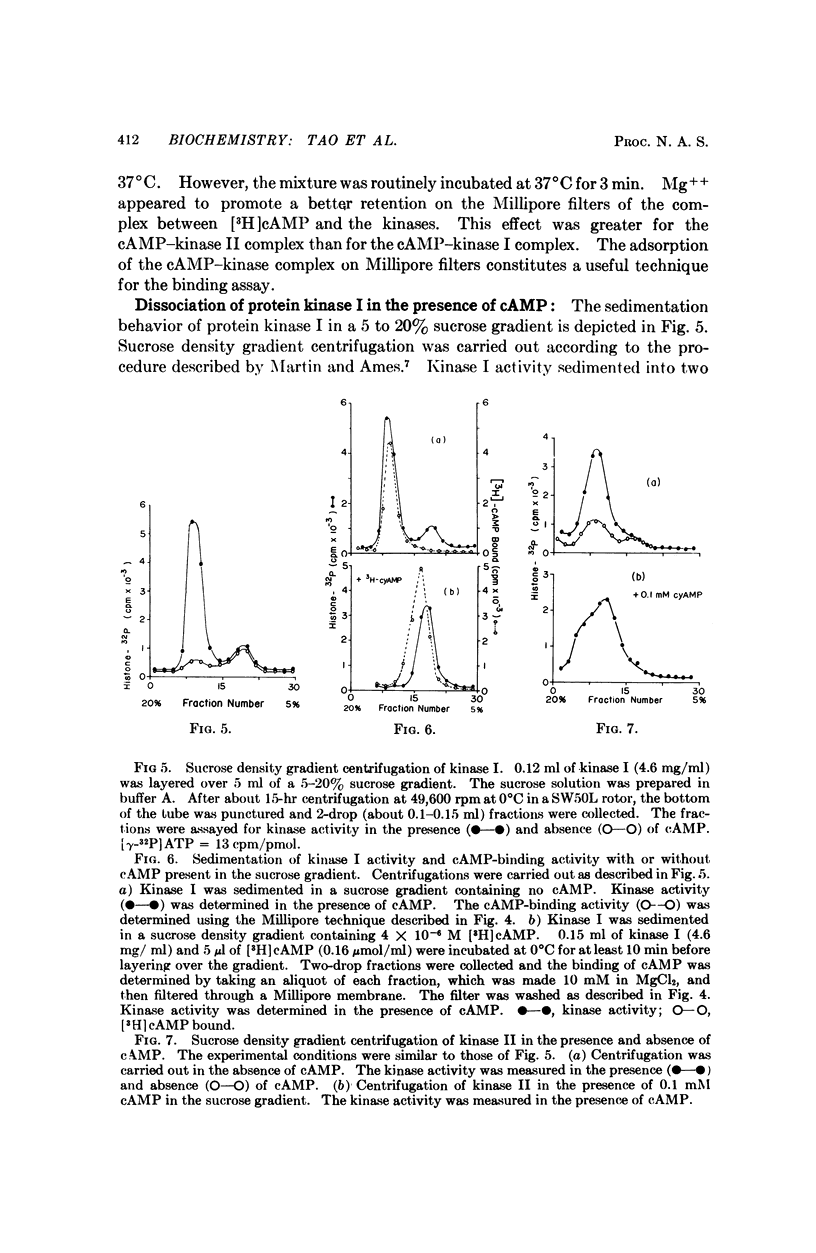
Two protein phosphokinases (EC 2.7.1.37) were found to be present in rabbit reticulocytes. The two enzymes were separated by DEAE-cellulose chromatography and called kinases I and II. Adenosien 3′:5′-cyclic monophosphate stimulated the activity of both enzymes. However, the degree of stimulation was different and depended on the protein acceptor used. In the presence of adenosine 3′:5′-cyclic monophosphate, protein kinase I dissociated into two subunits: a subunit binding adenosine 3′:5′-cyclic monophosphate, and a catalytic subunit. The component binding the cyclic nucleotide appeared to act as an inhibitory protein, regulating the activity of the catalytic subunit. The mechanism of action of the cyclic nucleotide on kinase II appeared to be different from that of kinase I.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. H., SCHWEET R. S. Synthesis of hemoglobin in a cell-free system. I. Properties of the complete system. J Biol Chem. 1962 Mar;237:760–767. [PubMed] [Google Scholar]
- Corbin J. D., Krebs E. G. A cyclic AMP--stimulated protein kinase in adipose tissue. Biochem Biophys Res Commun. 1969 Jul 23;36(2):328–336. doi: 10.1016/0006-291x(69)90334-9. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. A cyclic-3',5'-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970 May 11;39(3):335–343. doi: 10.1016/0006-291x(70)90581-4. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Malkin M., Lipmann F. A stimulation by cyclic 3',5'-adenosine monophosphate of amino acid activation and polymerization in reticulocyte hemolysates. Proc Natl Acad Sci U S A. 1969 Nov;64(3):973–980. doi: 10.1073/pnas.64.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]



