Abstract
Enantiomerically pure 2-C-methyl-D-erythritol 4-phosphate 1 (MEP) is synthesized from 1,2-O-isopropylidene-α-D-xylofuranose via facile benzylation in good yield. Subsequently, 1 is used for enzymatic synthesis of 4-diphosphocytidyl-2-C-methyl-D-erythritol 2 (CDP-ME) using 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase (IspD). The chemoenzymatically synthesized 2 can be used as substrate for assay of IspE and for high throughput screening to identify IspE inhibitors.
About 450,000 people a year are infected with multi-drug resistant tuberculosis (MDR-TB), which is resistant to the main first-line drugs isoniazid and rifampicin. In addition extensively drug resistant tuberculosis (XDR-TB), which is resistant to isoniazid and rifampin and resistant to any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin) has been reported in 37 countries in all regions of the world since 2006. Moreover human immunodeficiency virus – tuberculosis (HIV-TB) co-infection is also a big challenge besides the XDR-TB.1 Yet no new anti-TB drug had been introduced since the 1960’s. In this context, designing and developing a new anti-TB drug is very important.
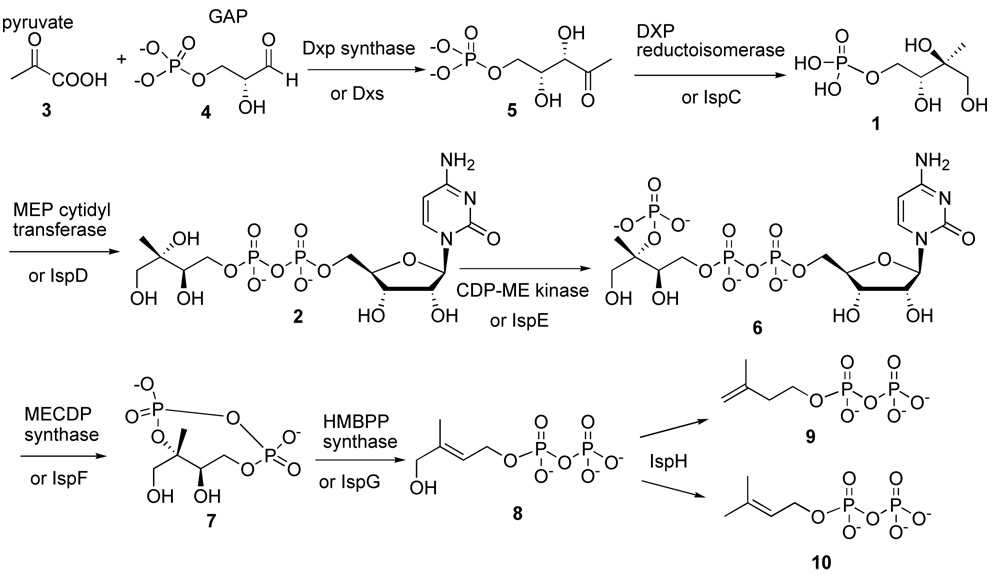
To date two different biosynthetic pathways have been reported leading to isopentenyl diphosphate, the universal precursor of isoprenoids. The mevalonate pathway2 is found in animals whereas the non-mevalonate or methylerythritol phosphate (MEP) pathway is found in many bacteria, some protozoa and plants (scheme 1).3 In the MEP pathway, 1-deoxy-D-xylulose 5-phosphate 5 (DXP) is made by condensing pyruvate 3 and glyceraldehyde 3-phosphate 4 catalysed by DXP synthase (DXS). Subsequently 1 is synthesized by intramolecular rearrangement and reduction of 5 catalysed by IspC. Then 1 is coupled with cytidine triphosphate (CTP) using IspD to produce 2. 2 is subsequently phosphorylated by 4-diphosphocytidyl-2-C-methyl-D-erythritol- 2-phosphate synthase (IspE) to form 4-diphosphocytidyl-2-C-methyl-D-erythritol-2-phosphate 6 (CDP-ME2P) and cyclized by IspF to form 2-C-methyl-D-erythritol 2, 4-cyclodiphosphate 7 (ME-CPP). The cyclic diphosphate is transformed into 1-hydroxy-2-methyl-2-E-butenyl 4-diphosphate 8 (HMBPP) by IspG. IspH (LytB) catalyzes the synthesis of isopentenyl diphosphate 9 (IPP) and its isomer dimethylallyl diphosphate 10 (DMAPP).
Scheme 1.
Isoprenoid biosynthesis via the MEP pathway
Since the MEP pathway is not found in mammalian cells, it is considered an attractive target for the development of antimicrobials, antimalarials and herbicidal agents3 a hypothesis that is being explored by an increasing number of researchers. A major difficulty hindering this research is the shortage of pure substrates. In this regard, access to MEP pathway intermediates and their analogues are essential to ongoing biochemical investigations and development of high throughput screens to attempt to identify leads for synthesis of new therapeutics. Recently we have described assays for mycobacterial DXS, IspC and IspD.4 In order to study mycobacterial IspE, we were in need of compound 2.
However, the reported chemical synthesis of 2 is only 50% enantiomerically pure5. Whereas, when synthesized enzymatically starting with the formation of 5 by condensation of 3 and 4 catalysed by DXS and the ultimate yield of 1 is very low.6 In addition this enzymatic method is of time consuming and expensive. Here in, we report a chemoenzymatic method to synthesize 2 in good yield.
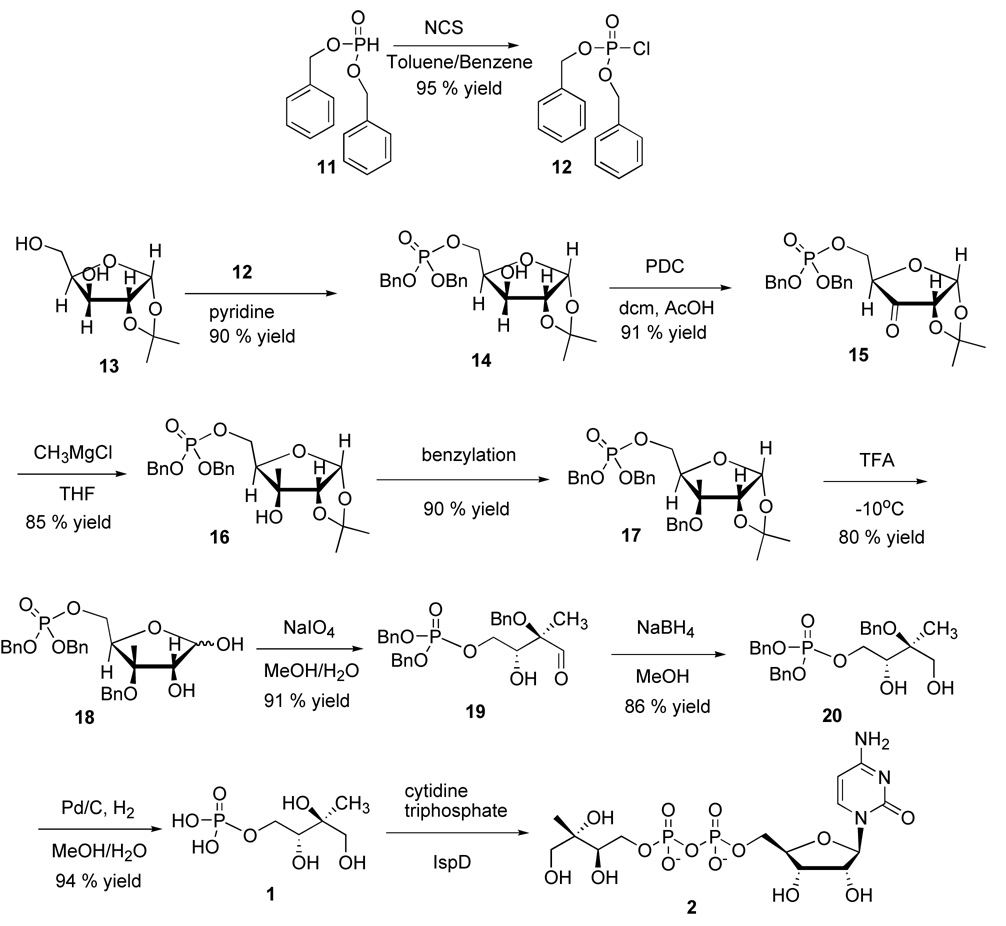
To initiate the synthesis of 2 (scheme 2), we synthesized enantiopure 1. Many procedures are available for the synthesis of 1. However, only one procedure is reported for synthesis of enantiomerically pure 1 from commercially available 1,2-O-isopropylidene-α-D-xylofuranose.7
Scheme 2.
Chemoenzymatic synthesis of CDPME
Dibenzyl phosphochloridate8 was synthesized by a modified procedure of chlorinating dibenzyl phosphite 11, in toluene and benzene using N-chlorosuccinamide (NCS). Dibenzyl phosphochloridate 12 in pyridine was used to selectively protect the primary alcohol of 1,2-O-isopropylidene-α-D-xylofuranose 13 yielding 5-dibenzylphosphate-1,2-O-isopropylidene-α-D-xylofuranose 14. Then the free secondary alcohol 14 was oxidized to ketone 15 quantitatively with pyridinium dichromate (PDC).7
Previously 3-C-alkyl ribofuranoses were obtained by a stereoselective addition reaction with the alkyl group on the β-face of the carbohydrate ring.9 Accordingly, in the ketone 15, addition of the methyl group occurs from the less hindered β-face, leading to the tertiary alcohol 16 with the desired stereochemistry. The alcohol 16 was protected by using benzyl bromide, after activating the hydroxyl group to yield 90% of benzylated 17.10
Acetonide deprotection was carried out using 90% aq. trifluoroacetic acid giving rise to two anomers 18 which underwent a sodium metaperiodate mediated glycol oxidative cleavage to the aldehyde 19. The aldehyde was reduced with sodium borohydride forming the MEP precursor,11 alcohol 20, followed by hydrogenolysis in water/methanol medium, without acid workup leading to enantiomerically pure 1 (Scheme 2). The chemically synthesized 1 was characterized by NMR, MS and optical rotation and the data were found to be identical with those previously reported in the literature.7,12,13 Subsequently, chemically synthesized 1 was used as a substrate for the enzymatic synthesis of 2.
Recombinant Rv3582c, M. tuberculosis IspD, was prepared as previously described.4a Briefly, Rv3582c was amplified using PCR primers, which was done using the expand high fidelity PCR system (Roche Molecular Biochemicals, Indianapolis, Indiana, USA) (Rv3582c–F: CAT ATG AGG GAA GCG GGC GAA GTA G and Rv3582c–R: CTC GAG TCA CCC GCG GAG TAT AGC TTG), containing NdeI and XhoI restriction enzyme sites (underlined), respectively. The PCR products were digested and ligated into the pET28a(+) vector (EMD Biosciences, Inc., San Diego, CA) and the ligation mixtures were used to transform E. coli DH5α cells (Life Technologies, Rockville, MD) creating DH5α[pET28a(+)∷Rv3582c] for amplification. The recombinant plasmids harboring Rv3582c were isolated using a Plasmid Miniprep Kit (Qiagen, Valencia, CA) and the sequences of the plasmids were confirmed by Macromolecular Resources (Colorado State University). Transformation of BL21 (DE3) (Novagen, Madison, WI) with pET28a(+)∷Rv3582c afforded the recombinant strain BL21(DE3)[pET28a(+)∷Rv3582c]. Protein expression was induced in the presence of 0.5 mM isopropyl–β–D–thiogalactopyranoside (IPTG) at 20°C for 10 hours. The recombinant protein carrying a hexa–histidine tag was purified by immobilized metal affinity chromatography on HIS-select™ Nickel affinity gel from Sigma-Aldrich using a linear gradient of 50 to 200 mM imidazole in washing buffer [50 mM 4–morpholine propane sulfonic acid (MOPS) (pH 7.9), 1 mM MgCl2, 10 % glycerol and 1 mMβ–mercaptoethanol].
2 was synthesized enzymatically14 with a maximum yield of 25% after incubation at 37 °C for 1 hr. Formation of 2 is followed by monitoring the formation of PPi released during catalysis by IspD (EnzChek® Phosphate Assay Kit, Invitrogen) although this is not required in production of 2. When examined by MS and 1H-NMR the product was found to generate spectra identical to reported data.5,13,15
Thus, we successfully synthesized enantiomerically pure 1, which could be utilized by mycobacterial IspD to synthesize 2 in satisfactory yields. Radiolabel could be introduced during the methylation or reduction steps if required. Pure 2 can be used to study the kinetic properties of IspE and for high throughput screening to identify IspE inhibitors. Experiments for M. tuberculosis IspE inhibitors are in progress.
Acknowledgments
This research was supported by NIH/NIAID grant AI65357-030010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Brennan PJ, Crick DC. Curr. Top. Med. Chem. 2007;7:475–488. doi: 10.2174/156802607780059763. [DOI] [PubMed] [Google Scholar]; (b) Editorial Lancet. 2006;368:964. [Google Scholar]; (c) Wright A. Morbidity and Mortality Weekly Report (MMWR) 2006;55:1176. [PubMed] [Google Scholar]; (d) World Health Organization. Geneva, Switzerland: WHO; WHO Report. 2007
- 2.Bochar DA, Friesen JA, Stauffacher CV, Rodwell VW. In: Comprehensive Natural Products Chemistry. Barton D, Nakanishi K, editors. Vol. 2. Oxford: Elsevier; 1999. pp. 15–44. [Google Scholar]
- 3.Rohmer M. Nat. Prod. Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 4.(a) Eoh H, Brown AC, Buetow L, Hunter WN, Parish T, Kaur D, Brennan PJ, Crick DC. J. Bacteriol. 2007;189:8922–8927. doi: 10.1128/JB.00925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dhiman RK, Schaeffer ML, Bailey AM, Testa CA, Scherman H, Crick DC. J. Bacteriol. 2005;187:8395–8402. doi: 10.1128/JB.187.24.8395-8402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bailey AM, Mahapatra S, Brennan PJ, Crick DC. Glycobiology. 2002;12:813–820. doi: 10.1093/glycob/cwf100. [DOI] [PubMed] [Google Scholar]
- 5.Koppisch AT, Poulter CD. J. Org. Chem. 2002;67:5416–5418. doi: 10.1021/jo025736o. [DOI] [PubMed] [Google Scholar]
- 6.Kuzuyama T, Takagi M, Kaneda K, Dairi T, Seto H. Tetrahedron Lett. 2000;41:703–706. [Google Scholar]
- 7.Hoeffler J-F, Grosdemange C-P, Rohmer M. Tetrahedron. 2000;56:1485–1489. [Google Scholar]
- 8.Gao F, Yan X, Shakya T, Baettig OM, Brunet SAM, Berghuis AM, Wright GD, Auclair K. J. Med. Chem. 2006;49:5273–5281. doi: 10.1021/jm060732n. [DOI] [PubMed] [Google Scholar]
- 9.(a) Nakatani K, Arai K, Terashima S. Tetrahedron. 1993;49:1901–1912. [Google Scholar]; (b) Nielsen P, Pfundheller HM, Olsen CE, Wengel J. J. Chem. Soc., Perkin Trans.1. 1997:3423–3433. [Google Scholar]
- 10.Crimmins MT, Brown BH. J. Am. Chem. Soc. 2004;126:10264–10266. doi: 10.1021/ja046574b. [DOI] [PubMed] [Google Scholar]
- 11.Data for 20: 1H-NMR (CDCl3, 300 MHz): δ7.27-7.19 (m, 15H), 5.00-4.96 (m, 4H), 4.43-4.41 (m, 2H), 4.26 (t, 1H, J = 9.9 Hz), 3.98-3.94 (m, 2H), 3.62-3.60 (m, 2H), 1.10 (s, 3H).; 13C-NMR (CDCl3, 75 MHz): 138.5, 135.7, 135.6, 128.6, 128.4, 128.1, 128.0, 127.6, 127.3, 78.2, 73.0, 69.6, 64.8, 64.2, 15.4.; IR (neat, cm−1): 3588, 2966, 2362, 2336, 1652, 1614. HRMS (ESI) C26H32O7P (M+H+) calculated 487.1880 and found 487.1870.; [α]D = 10.0 (c 0.5, CHCl3)
- 12.Koppisch AT, Blagg BSJ, Poulter CD. Org. Lett. 2000;2:215–217. doi: 10.1021/ol991299x. [DOI] [PubMed] [Google Scholar]
- 13.(a) Rohdich F, Schuhr CA, Hecht S, Herz S, Wungsintaweekul J, Eisenrich W, Zenk MH, Bacher A. J. Am. Chem. Soc. 2000;122:9571–9574. [Google Scholar]; (b) Richard SB, Bowman ME, Kwiatkowski W, Kang I, Chow C, Lillo AM, Cane DE, Noel JP. Nature structural biology. 2001;8:641–648. doi: 10.1038/89691. [DOI] [PubMed] [Google Scholar]
- 14.Reaction mixtures contained 50 mM Tris–HCl (pH 7.4), 200 µM 2–amino–6–mercapto–7–methylpurine ribonucleoside (MESG), 1 mM DTT, 100 µM 2–C–Methyl–D–erythritol 4–phosphate (MEP), 100 µM CTP, 0.03 U inorganic pyrophosphatase, 1U purine nucleoside phosphorylase and 11.6 pmols of purified M. tuberculosis IspD per 50 µl of reaction volume. Reactions were incubated at room temperature for 30 minutes after which their endpoint absorbance at 360 nm was determined. Chemically produced 1 was compared with authentic 1. Synthesized 2 was found to generate spectra identical to reported data.
- 15.Cassera MB, Gozzo FC, Alexandri FL, Merino EF, Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Weisner J, Jomaa H, Kimura EA, Katzin AM. J. Biol. Chem. 2004:51749–51759. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]