Abstract
The interactions between dopamine and glutamate systems play an essential role in normal brain functions and neuropsychiatric disorders. The mechanism of NMDA receptor regulation through high concentrations of dopamine, however, remains unclear. Here, we show the signaling pathways involved in hyperdopaminergic regulation of NMDA receptor functions in the prefrontal cortex by incubating cortical slices with high concentration of dopamine or administering dopamine reuptake inhibitor 1-(2-[bis-(4-fluorophenyl)methoxy]ethyl)- 4-(3-phenylpropyl)piperazine (GBR12909) in vivo. We found that, under both conditions, the synaptic NMDA receptor-mediated currents were significantly attenuated by excessive dopamine stimulation through activation of D2 receptors. Furthermore, high dose of dopamine failed to affect NMDA receptor-mediated currents after blockade of NR2B subunits but triggered a dynamin-dependent endocytosis of NMDA receptors. The high-dose dopamine/D2 receptor-mediated suppression of NMDA receptors was involved in the increase of glycogen synthase kinase-3β (GSK-3β) activity, which in turn phosphorylates β-catenin and disrupts β-catenin–NR2B interaction, but was dependent on neither Gq11 nor PLC (phospholipase C). Moreover, the hyperdopamine induced by GBR12909 significantly decreased the expression of both surface and intracellular NR2B proteins, as well as NR2B mRNA levels, suggesting an inhibition of protein synthesis. These effects were, however, completely reversed by administration of either GSK-3β inhibitor or D2 receptor antagonist. These results therefore suggest that GSK-3β is required for the hyperdopamine/D2 receptor-mediated inhibition of NMDA receptors in the prefrontal neurons and these actions may underlie D2 receptor-mediated psychostimulant effects and hyperdopamine-dependent behaviors in the brain.
Introduction
The prefrontal cortex (PFC) is a brain region highly associated with cognition and psychiatric disorders (Harrison and Weinberger, 2005; Lewis and Gonzalez-Burgos, 2008). Dopamine (DA), as a major neurotransmitter in the PFC, has long been implicated in schizophrenia (Carlsson, 1978). Excess of DA or an elevated sensitivity to DA stimulation profoundly affects the synaptic transmission in the brain (Greengard, 2001; Seamans and Yang, 2004; Seeman, 2006). Recent studies suggested that many psychiatric disorders are strongly associated with abnormalities in both DA and glutamate systems. Indeed, in addition to DA, evidence supports the hypothesis that schizophrenia might be related to NMDA receptor hypofunction (Jentsch and Roth, 1999; Krystal et al., 2002; Moghaddam and Jackson, 2003). In the past decade, extensive studies have been focused on the interactions between DA and NMDA receptors (Goldman-Rakic et al., 2004; Yang and Chen, 2005; Cepeda and Levine, 2006). It is widely believed that optimal DA preferentially acts on D1 receptors to promote NMDA receptor function (Seamans et al., 2001; Flores-Hernández et al., 2002), possibly through receptor phosphorylation or trafficking (Dunah and Standaert, 2001; Dunah et al., 2004; Hallett et al., 2006; Gao and Wolf, 2008; Tong and Gibb, 2008) (Y. C. Li, G. Liu, J. Hu, Y. Q. Huang, and W. J. Gao, unpublished observation). In contrast, a high level of DA or activation of D2 receptors may attenuate NMDA receptor-mediated transmission (Zheng et al., 1999; Wang et al., 2003; Beazely et al., 2006; Liu et al., 2006; Jiao et al., 2007; Martina and Bergeron, 2008). The fundamental problem is that most of the studies focused on the D1–NMDA receptor interaction and interpreted it as a hypothetical inverted “U” curve for D1 action (Goldman-Rakic et al., 2000; Seamans and Yang, 2004). The mechanisms involved in the D2 receptor-dependent effects on NMDA receptor functions under conditions of hyperdopaminergic state are, however, not well investigated.
Recent studies have provided interesting insights into the involvement of glycogen synthase kinase-3β (GSK-3β) signaling in schizophrenia (Kozlovsky et al., 2000; Emamian et al., 2004; Koros and Dorner-Ciossek, 2007; Lovestone et al., 2007). The GSK-3β signaling is also particularly important in D2 receptor-mediated psychostimulant effects and DA-dependent behaviors (Beaulieu et al., 2005, 2007a,b; Bibb, 2005). In addition, antipsychotic agents can decrease the activity of GSK-3β by increasing GSK-3β Ser9 (Emamian et al., 2004; Li et al., 2007). GSK-3 is a serine/threonine protein kinase known for phosphorylating and thus inactivating glycogen synthase. GSK-3 contains two isoforms, GSK-3α and GSK-3β, but only GSK-3β is highly enriched in the brain (Woodgett, 2001). Because GSK-3β inhibitor is also involved in the regulation of NMDA receptor-dependent synaptic plasticity (Peineau et al., 2007, 2008; Zhu et al., 2007), we hypothesized that hyperdopamine/D2 receptor-induced inhibition of NMDA receptors may occur through GSK-3β-mediated NMDA receptor trafficking, which would result in NMDA receptor hypofunction. We tested this hypothesis using various approaches and demonstrated that elevated dopamine via D2 receptors triggered the internalization of NR2B subunits and this process required the activation of GSK-3β.
Materials and Methods
Electrophysiological recoding in prefrontal cortical slices.
Fifty Sprague Dawley rats at ages of postnatal day 12–30 were used for this study. The animals were treated under the National Institutes of Health (NIH) guidelines, and the experimental protocol was approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine. The detailed procedure can be found in our previous studies (Gao et al., 2001; Gao and Goldman-Rakic, 2003; Gao, 2007). The animals were deeply anesthetized with Euthasol (0.2 ml/kg; Virbac AH), and the brains were immediately removed and placed in ice-cold (<4°C) sucrose (in mm: 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 7.0 MgSO4, 213 sucrose, pH 7.4) solution buffered with 95% O2 and 5% CO2. The blocks of neocortex containing medial PFC (PrL) (Paxinos and Watson, 2005) were trimmed and were sectioned using Leica VT1000s Vibratome (Leica Microsystems). The horizontal brain slices at a thickness of 300 μm were incubated in oxygenated Ringer's solution at 35°C for 1 h. Ringer's solution contains the following ingredients (in mm): 128 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, 26 NaHCO3, and 10 dextrose, pH 7.4. The slices were then kept at room temperature until being transferred to a submerged chamber for recording. Whole-cell patch-clamp recordings were conducted in the medial PFC through an upright Zeiss Axioskop 2 microscope (Carl Zeiss) equipped with optics of infrared-differential interference contrast and a digital video camera system. The recordings were conducted at ∼35°C. Resistance of the recording pipette (1.2 mm borosilicate glass; Warner Instruments) was 7–9 MΩ. For voltage clamp, a Cs+ solution containing the following (in mm): 120 Cs-gluconate, 5 lidocaine (QX-314), 6 CsCl2, 1 ATP-Mg, 0.2 Na2GTP, and 10 HEPES at pH 7.3 (adjusted with CsOH), was used to block sodium and potassium channels. The NMDA receptor-mediated EPSCs were recorded at +60 mV by stimulating layer 2/3 with a bipolar electrode placed ∼200–500 μm from the recording neurons (single pulse; 0.1 ms; 10–100 μA; 0.1 Hz) in the presence of picrotoxin (50 μm) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (20 μm). To record the spontaneous NMDA receptor-mediated EPSCs (sEPSCs), the neurons were held at a membrane potential of +60 mV with bath perfusion of picrotoxin (50 μm) and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) (10 μm). All chemicals were purchased from Sigma-Aldrich unless specified (Sigma-Aldrich). The electric signals were amplified and filtered at 2 kHz in voltage-clamp mode with MultiClamp 700B (Molecular Devices) and acquired at sampling intervals of 20–50 μs through a DigiData 1322A (data acquisition system) and pCLAMP 9.2 software (Molecular Devices). The series resistances were compensated and were constantly monitored by injection of a hyperpolarization current pulse (−0.1 nA) at duration of 150 ms.
Only cells exhibiting stable membrane potentials without injection of holding current and producing stable EPSCs without rundown for at least 5 min were used for additional analysis of drug effects. The amplitudes of the evoked EPSCs were measured by averaging 30 traces from the onset to the peak of EPSC with Clampfit 9.2 software (Molecular Devices). The sEPSCs recorded in voltage-clamp mode were analyzed with Clampfit 9.2 (Molecular Devices). A typical sEPSC was selected to create a sample template for event detection within a data period. The frequency (event number) and amplitude of individual events were examined with Clampfit. The data were analyzed by Student's t test and were presented as mean ± SE.
Bis(sulfosuccinimidyl)suberate cross-linking assay.
Bis(sulfosuccinimidyl)suberate (BS3) cross-linking was performed as described previously (Grosshans et al., 2002a,b; Conrad et al., 2008). BS3 is a membrane-impermeable agent, which selectively cross-links cell surface proteins to form high-molecular-mass aggregates. Because intracellular proteins are not modified, they retain normal molecular mass. This enables surface and intracellular pools of a particular protein to be distinguished by SDS-PAGE and Western blotting. Brain tissues containing medial PFC were quickly sectioned as 400 μm slices with a Vibratome, and slices were incubated with BS3 (1 mg/ml; Pierce Biotechnology) in aerated artificial CSF (ACSF) (95% O2 and 5% CO2) at 4°C for 40 min with gentle agitation. The slices were then washed three times with ice-cold ACSF containing 20 mm Tris, pH 7.6, to quench the remaining BS3, and the surface expression was determined by Western blot analysis.
Immunoprecipitation.
Tissues containing PFC were microdissected and then homogenized in ice-cold lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% NP-40, protease inhibitor mixture) and centrifuged at 13,000 × g for 10 min at 4°C. Supernatant fractions (∼500 μg proteins) were incubated overnight with 2.5 μg of anti-β-catenin (Cell Signaling Technology). The immunocomplexes were isolated by addition of 20 μl of protein G-Sepharose beads (GE Healthcare Bio-Sciences), followed by incubation for 3–4 h at 4°C. The immunoprecipitates were then washed four times with lysis buffer, resuspended in Laemmli sample buffer, and boiled for 10 min. After they were centrifuged at 10,000 × g for 5 min, supernatant was collected. The immunoprecipitated proteins were analyzed by Western blot analysis with antibodies against NR2B (1:2000; Millipore Bioscience Research Reagents), β-catenin, and β-tubulin.
Western blot analysis.
PFC tissues were homogenized in a lysis buffer (20 mm Tris-HCl with pH 7.4, 200 mm NaCl, 1 mm Na3VO4, 10 mm NaF, and protease inhibitor mixture) and centrifuged at 13,000 × g for 2 min. Supernatant fraction was aliquoted and stored at −80°C. Equal amounts of proteins were subjected to 7.5% SDS-PAGE. Proteins were transferred to a nitrocellulose membrane (Bio-Rad Laboratories) and blocked with 5% nonfat milk in TBS-T (0.05% Tween 20 in 1× Tris-buffered saline). The blots were incubated with anti-NR2B, anti-NR2A (Millipore Bioscience Research Reagents), anti-GSK-3β, anti-phosphor-GSK-3β Ser9, anti-β-catenin, anti-phospho-β-catenin (Ser33/37/Thr41) (Cell Signaling Technology), or anti-phosphor-GSK-3β Tyr216 (Ambgood) at a dilution of 1:2000 in TBS-T with 5% milk. Anti-NR2B-pS1480 antibody (a gift from Dr. Richard L. Huganir, Johns Hopkins University, Baltimore, MD) was used at 1:200 (Chung et al., 2004). After several washes with TBS-T, the blots were then incubated in HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) at 1:2000 for 2 h. The immunopositive protein bands were detected with ECL Western Blotting System (GE Healthcare Bio-Sciences). After the exposure of membranes to Kodak Biomax film (Eastman Kodak), the band densities were measured with NIH ImageJ software. Final data were normalized to the levels of β-actin or total proteins (for phosphorylation). To minimize the interblot variability, each sample was analyzed four times. The mean value for each sample was calculated from all the replicates in different animals, and the results were presented as mean ± SE. Significance was determined with Student's t test or ANOVA.
RNA extraction and reverse transcription-PCR.
Total RNA was extracted from the homogenized lysis mixture directly using an RNAqueous Micro Kit and eventually was dissolved in 40 μl of elution buffer for the PFC tissue sample. RNA concentration was measured at wavelengths of 260 and 280 nm using the NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific). Only the RNA samples with 260/280 ratios of 1.8 to 2.0 were used for additional analyses. RNA extracted from the PFC tissue was directly diluted into RNase-free water at a concentration of 20 ng/μl. The QIAGEN one-step reverse-transcription PCR (RT-PCR) kit (QIAGEN) was used to perform RT-PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as housekeeping gene. The primers used are as follows: GAPDH (NM_017008) forward primer ccatcccagaccccataac, backward primer gcagcgaactttattgatgg, product 78 bp; GRIN2B (NR2B; NM_012574) forward primer tgagtgagggaagagagagagg, backward primer atggaaacaggaatggtgga, product 249 bp. The detailed RT-PCR set-up was as follows: 1.0 μl of RNA, 2.0 μl of NR2B forward primer (4 μm), 2.0 μl of NR2B backward primer (4 μm), 2.0 μl of GAPDH forward primer (4 μm), 2.0 μl of GAPDH backward primer (4 μm), 5.0 μl of Q buffer, 5.0 μl of 5× buffer, 1.0 μl of dNTP, 1.0 μl of enzyme, 4.0 μl of RNase-free water. The PCR protocol was as follows: (1) reverse transcription, 50°C for 30 min; (2) initial PCR activation, 95°C for 15 min; (3) three-step cycling (30 cycles), 94°C for 1 min, 55°C for 1 min, 72°C for 1 min; and (4) final extension, 72°C for 10 min. PCR products were evaluated by gel electrophoresis, and the mRNA expression levels for NR2B were normalized to the individual GAPDH level first, and then to the control level.
Results
High-dose DA consistently decreases NMDA receptor-mediated EPSCs in layer 5 pyramidal neurons
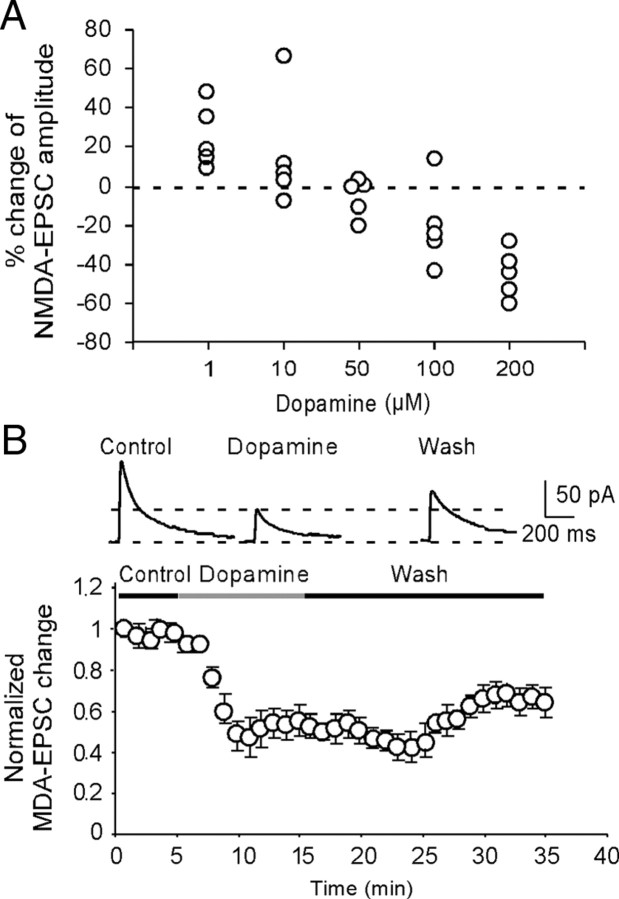
The synaptically evoked NMDA receptor-mediated EPSCs were recorded from layer 5 pyramidal neurons in the medial PFC by stimulating layer 2/3, with the membrane potential held at +60 mV and with the presence of AMPA receptor antagonist CNQX (20 μm) and GABAA receptor antagonist picrotoxin (50 μm). The NMDA receptor-mediated EPSCs were confirmed in some cases and were completely abolished by bath application of the NMDA receptor antagonist d-APV (50 μm) (data not shown). To examine the effects of high level of DA in NMDA receptors, we first bath-applied DA at different concentrations from 1 to 200 μm and compared the DA actions in NMDA receptor-mediated EPSCs. We found that DA exhibited bidirectional dose-dependent effects on NMDA receptor-mediated EPSCs in the layer 5 pyramidal neurons. As shown in Figure 1, low concentrations of DA (1 μm) significantly increased the NMDA receptor-mediated EPSCs by 25.1 ± 7.21% (n = 5; p < 0.01) (Fig. 1A), whereas medium doses of DA (10–100 μm) induced either increase or decrease of EPSC amplitudes. In contrast, at high concentration of 200 μm, DA consistently and significantly decreased the NMDA receptor-mediated EPSCs by an average of 42.2 ± 5.29% (n = 5; p < 0.01) (Fig. 1A,B). This suppressing effect on NMDA currents was long-lasting with little recovery even after 15–20 min washout, suggesting the possibility of NMDA receptor internalization under this condition. This dose-dependent bidirectional effect is in agreement with previous findings that puff NMDA-induced current is dose-dependently regulated by DA (Zheng et al., 1999).
Figure 1.
DA induces bidirectional dose-dependent effects in synaptically evoked NMDA-EPSCs in layer 5 pyramidal neurons. A, Dose–response relationship for the effects of DA. The data were average effects of DA on NMDA-EPSCs determined at 10 min after DA application (n = 5 for each dose). B, Top panel, Examples of the NMDA-EPSCs recorded in presence of NBQX (20 μm) and picrotoxin (50 μm) in control, dopamine, and washout period. The representative traces indicated that high-dose DA (200 μm) significantly reduced the amplitudes of NMDA-EPSCs. Bottom panel, Summary graph showing the time course of high-dose (200 μm) DA effects on the amplitude of NMDA-EPSCs. This suppressing effect on NMDA currents was long-lasting with little recovery even after 15–20 min washout, suggesting the possibility of NMDA receptor internalization under this condition. Note that the data were normalized to the baseline EPSCs recorded in the first minute. Error bars indicate SEM.
High-dose DA attenuates NMDA receptor-mediated EPSCs through activation of D2, but not D1, receptors
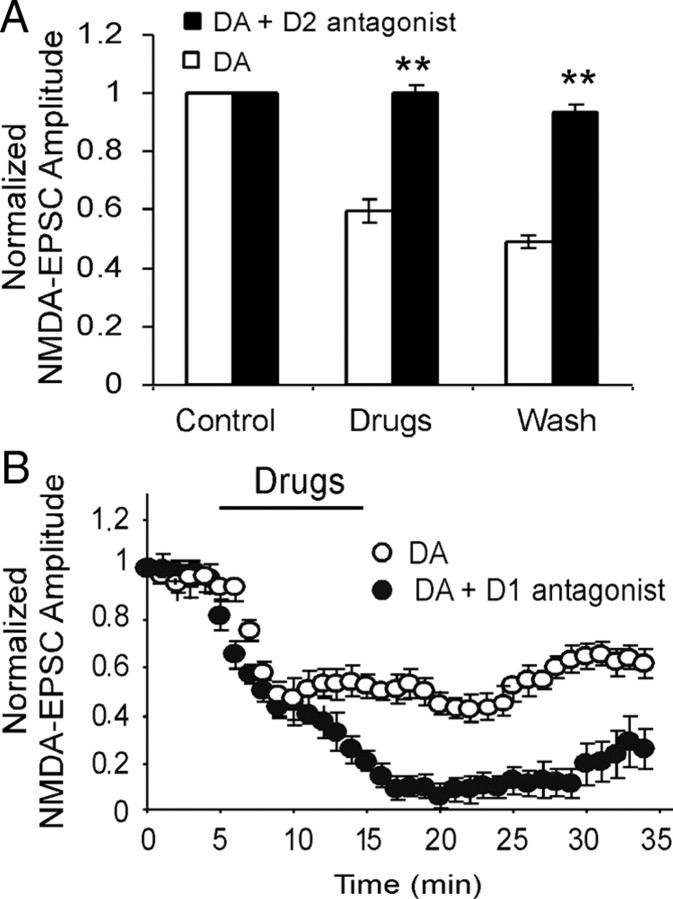
Numerous studies have been conducted involving low-dose DA or D1 agonists (Cepeda and Levine, 1998; Zheng et al., 1999; Seamans et al., 2001); therefore, we attempted to identify which DA receptor was mainly activated by high-dose DA to suppress the NMDA receptor-mediated EPSCs. Selective D2 antagonist (±)-3-[4-(4-chlorophenyl)-4-hydroxypiperidinyl]methylindole (L741,626) (10 μm) or D1 antagonist R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH 23390) (10 μm) was first applied for 5 min and then was coapplied with DA (200 μm). Under this condition, we found that neither L741,626 nor SCH 23390 exhibited significant effects on the NMDA receptor-mediated EPSCs (data not shown), supporting previous reports (Gao et al., 2001; Seamans et al., 2001; Tseng and O'Donnell, 2004). In contrast, L741,626 effectively abolished the suppressing effects of high-dose DA in NMDA receptor-mediated EPSCs in all of the neurons tested (n = 6; p < 0.01) (Fig. 2A), in agreement with a recent report in the amygdala (Martina and Bergeron, 2008). Surprisingly, additional attenuation of the NMDA receptor-mediated EPSCs was evident when the D1 antagonist SCH 23390 was coapplied with DA (n = 6; p > 0.05) (Fig. 2B) (Castro et al., 1999), suggesting that high dose of DA might activate both D1 and D2 receptors. The effect of D1 activation on NMDA receptors might be opposite to the effect produced by D2 activation. However, under our recording conditions, the D2 effect was much stronger and completely dominated the D1 effect when both receptors were coactivated.
Figure 2.
High-dose DA attenuates NMDA-EPSCs through activation of D2, but not D1, receptors. A, Coapplication of selective D2 receptor antagonist L741,626 (10 μm) with DA (200 μm) significantly abolished DA-induced attenuation of NMDA-EPSCs (n = 6; p < 0.01). B, On the contrary, coapplication of selective D1 receptor antagonist SCH 23390 (10 μm) with DA (200 μm) potentiated DA-induced attenuation of NMDA-EPSCs (n = 6; p < 0.05). *p < 0.05; **p < 0.01 for all figures. Error bars indicate SEM.
High-dose DA-induced reduction of NMDA receptor-mediated EPSCs is partially dependent on dynamin-mediated internalization of NR2B subunits
Recent studies conducted in the striatal (Dunah and Standaert, 2001; Dunah et al., 2004; Hallett et al., 2006; Tong and Gibb, 2008) and prefrontal neurons (Gao and Wolf, 2008) (Li, Liu, Hu, Huang, and Gao, unpublished observations) indicated that D1 receptor activation led to rapid insertion of NMDA receptors. We therefore attempted to examine whether high dose of DA-induced depression of NMDA currents also depends on the trafficking (e.g., internalization/endocytosis) of NMDA receptors. It has been reported that young prefrontal neurons, similar to other cortical neurons (Kumar and Huguenard, 2003; Liu et al., 2004; Harris and Pettit, 2007), contain a relatively high proportion of NR2B subunits (Wang et al., 2008). Because NR2B subunits exhibit significantly higher surface mobility and endocytosis than other NMDA receptor subunits (Roche et al., 2001; Lavezzari et al., 2004; Groc et al., 2006), we examined whether high dose of DA regulates NMDA receptor-mediated EPSCs mainly through NR2B-containing NMDA receptors. Selective NR2B subunit antagonist ifenprodil, which preferentially inhibits NR1/NR2B channels with high affinity (IC50 = 0.34 μm) (Williams, 1993; Tovar and Westbrook, 1999), was first applied to block NR2B component in NMDA receptor-mediated EPSCs. As shown in Figure 3A, ifenprodil (3 μm) significantly attenuated the EPSC amplitudes by an average of 41.7 ± 3.73% (n = 6; p < 0.01). The NR2B component in the evoked NMDA-EPSC appeared to be lower than that in monosynaptic connections between layer 5 pyramidal neurons (Wang et al., 2008) but was consistent with a previous study reported in primate PFC layer 2/3 pyramidal neurons (Gonzalez-Burgos et al., 2008) and our own findings (H. X. Wang and W. J. Gao, unpublished observation). In fact, there are likely laminar (Wang et al., 2008) or pathway-specific (Kumar and Huguenard, 2003) differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. Nevertheless, when ifenprodil and DA (200 μm) were continuously applied together, the EPSC amplitudes were reduced only ∼7% further (49.1 ± 4.42%), without significant difference (n = 6; p = 0.15). These data suggest that DA mainly interacts with NR2B subunits to achieve its depressive effects in the NMDA receptor-mediated EPSCs, although a lesser effect on NR2A or NR1 subunits cannot be excluded.
Figure 3.
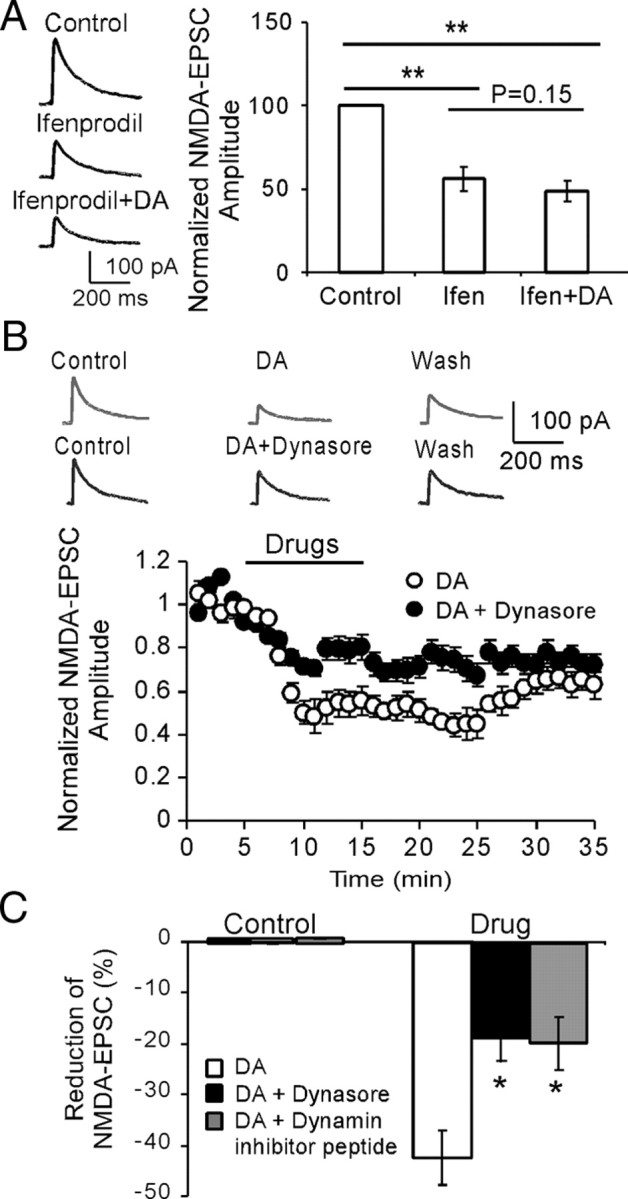
High-dose DA-induced reduction of NMDA-EPSCs is partially dependent on dynamin-mediated internalization of NR2B subunits. A, Left panel, Sample traces showing the effects of NR2B antagonist ifenprodil (3 μm) and ifenprodil plus dopamine (200 μm) on NMDA-EPSCs. Right panel, Initial application of NR2B antagonist ifenprodil significantly decreased the amplitudes of NMDA-EPSCs (p < 0.01), whereas followed coapplication of ifenprodil with DA did not cause significant attenuation on the NMDA-EPSCs (p = 0.15), suggesting the involvement of NR2B subunits. B, Sample traces (top panel) and time course data (bottom panel) showing the effects of dynasore, a potent membrane-permeable inhibitor of endocytosis, on DA-induced inhibition of NMDA receptors (n = 6). C, The inhibitory effects of DA on NMDA-EPSCs were significantly blocked by dynamin inhibitory peptide (50 μm in pipette) and dynasore (100 μm in bath), which prevent receptor endocytosis (n = 6; p < 0.05 for both). Error bars indicate SEM.
Previous studies have suggested a dynamin-dependent endocytosis of ionotropic glutamate receptors (Carroll et al., 1999; Newpher and Ehlers, 2008), particularly NMDA receptors (Nong et al., 2004; Montgomery et al., 2005). We therefore tested whether dynamin-dependent endocytosis of NMDA receptor trafficking underlies DA/D2 receptor-mediated downregulation of NMDA receptor currents in the PFC neurons. The dynamin inhibitory peptide (QVPSRPNRAP; Biomatik Corporation; 50 μm in pipette) was intracellularly applied to prevent the receptor endocytosis. We found that dynamin inhibitory peptide significantly attenuated the depressing effect of DA on NMDA receptor-mediated EPSCs by ∼50% (n = 6; p < 0.05) (Fig. 3B), consistent with a previous study (Montgomery et al., 2005). To investigate endocytosis further, we have tested the effects of another potent membrane-permeable inhibitor of endocytosis, dynasore (Tocris Bioscience), on DA-induced inhibition of NMDA receptors in the brain slices (Macia et al., 2006; Kirchhausen et al., 2008). Similarly, we found that dynasore (100 μm in bath solution) also blocked ∼50% of the inhibition induced by DA in NMDA receptor-mediated currents (n = 6; p < 0.05) (Fig. 3B,C). These data indicated that the inhibition of dynamin blocked the NMDA receptor internalization induced by DA and that the dynamin-dependent endocytosis of NMDA receptors plays an important role in DA-mediated suppression of NMDA responses in the prefrontal neurons (Tong and Gibb, 2008).
GSK-3β, but not Gq11, signaling pathway is involved in the high-dose DA/D2-induced depression of NMDA EPSCs
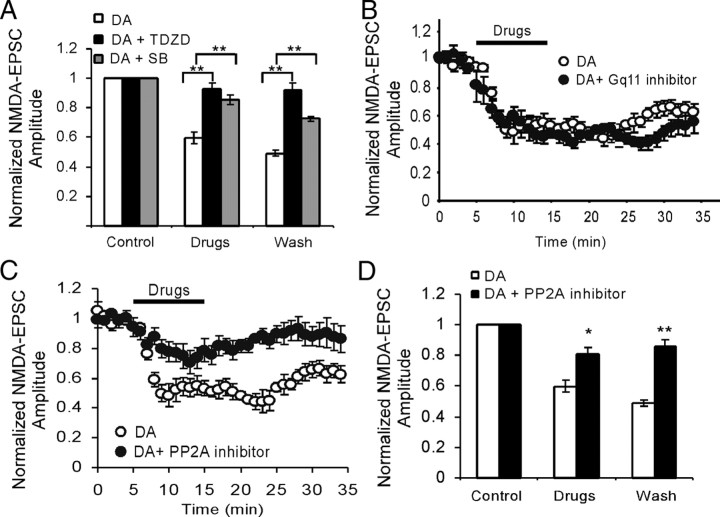
Several recent studies have indicated that D2 receptor-mediated GSK-3β signaling pathway is highly associated with hyperdopamine-dependent behaviors (Beaulieu et al., 2005, 2007b). In addition, other evidence suggested that elevated DA may couple to Gq11 signaling pathway by activating both D1 and D2 receptors in the striatal neurons (Lee et al., 2004; Rashid et al., 2007a,b). Because both D1 and D2 receptors are distributed in same neurons in the PFC, we tested the involvement of these two signaling pathways in the high-dose DA-mediated regulation of NMDA receptors. As shown in Figure 4, coapplication of DA (200 μm) with either of the two structurally different GSK-3β inhibitors, 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD) (10 μm) or 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216,763) (10 μm), completely abolished the DA-induced suppression in NMDA receptor mediated-EPSCs (n = 6; p < 0.01 for both). Both inhibitors themselves, however, did not show clear effects on the basal NMDA receptor-mediated transmissions (data not shown), as was reported in previous studies (Peineau et al., 2007, 2008; Zhu et al., 2007). In contrast, neither Gq11 inhibitor peptide [d-Trp7,9,10] Substance P (Arg-Pro-Lys-Pro-Gln-Gln-Trp-Phe-d-Trp-d-Trp-Met-NH2; Tocris Bioscience; 1 μm in pipette), the only inhibitor available for Gq11 blockade (Fig. 4B), nor phospholipase C (PLC) inhibitor U73122 (1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino] hexyl]-1H-pyrrole-2,5-dione) (10 μm bath application) (data not shown) affected the inhibitory effects of DA on the amplitude of the NMDA receptor-mediated EPSCs (n = 6; p > 0.05 for both). This negative effect was consistent with a previous study reported in synaptic NMDA-EPSC in lateral amygdala slices (Martina and Bergeron, 2008) but differs from that in puff-NMDA-induced current (Kotecha et al., 2002).
Figure 4.
PP2A-GSK-3β, but not Gq11, signaling pathway is involved in the high-dose DA/D2-induced depression of NMDA-EPSCs. A, Coapplication of GSK-3β inhibitors TDZD (10 μm) or SB216,763 (10 μm), the two structurally different GSK-3β inhibitors, completely abolished the DA-induced suppression in NMDA receptor mediated-EPSCs (n = 6; p < 0.01 for both). Both inhibitors themselves, however, did not show clear effects on the basal NMDA receptor-mediated transmissions (data not shown). B, Coapplication of Gq11 inhibitor (10 μm) with DA (100 or 200 μm), however, did not affect the effects of DA (n = 6; p > 0.05). C, D, PP2A inhibitor okadaic acid (100 nm) significantly blocked the DA effects on NMDA receptor-mediated EPSCs (n = 6; p < 0.05). Error bars indicate SEM.
Since the signaling complex containing protein phosphatase 2 (PP2A) directly mediates the action of D2-class dopamine receptors (Beaulieu et al., 2005) and PP2A is identified as an upstream regulator of GSK-3β (Beaulieu et al., 2009), we tested the effects of PP2A inhibitor okadaic acid (Tocris Bioscience) on DA-induced inhibition of NMDA receptor-mediated EPSCs. As shown in Figure 4, C and D, okadaic acid (100 nm) significantly blocked the DA effects (n = 6; p < 0.05). We also examined the role of phosphatidylinositol 3-kinase (PI3K), another upstream regulator of GSK-3β (Habas et al., 2006), in NMDA receptor-mediated EPSCs. Bath application of PI3K inhibitor LY294,002 [2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride] (20 μm; Tocris) only briefly reduced ∼50% of the DA-induced suppression in NMDA receptor mediated-EPSCs (19.9 ± 1.82% compared with that of 40.1 ± 3.95% in DA alone; n = 6; p < 0.05) (data not shown) for ∼5 min without continuous effects on the DA action (p > 0.05). These results suggest that the PP2A/GSK-3β, but not the Gq11/PLC, signaling pathway is likely involved in the high-dose DA/D2-induced depression of NMDA receptor-mediated EPSCs in the prefrontal neurons. The role of PI3K in DA action, however, is limited.
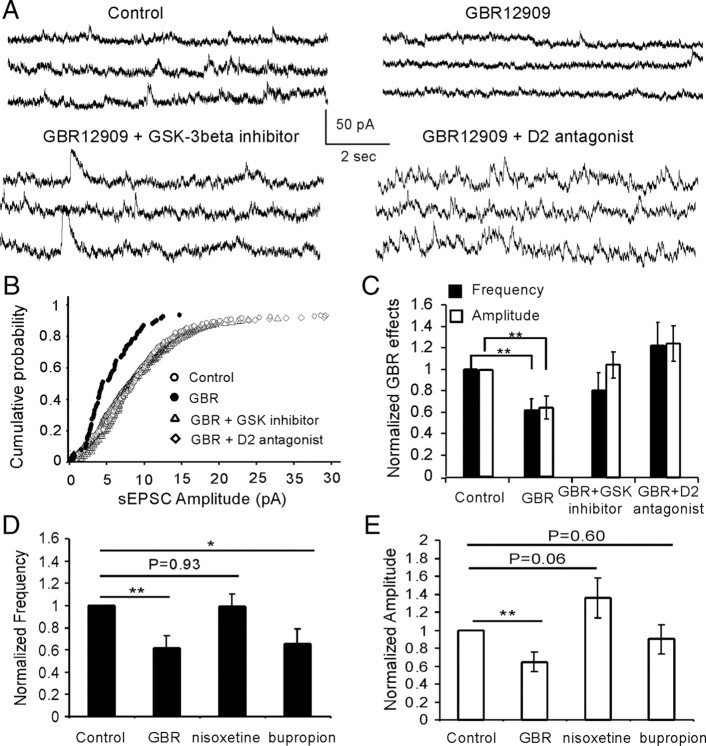
Hyperdopamine induced by systemic administration of GBR12909 attenuates spontaneous NMDA receptor-mediated EPSCs through activation of D2 receptors and GSK-3β in vivo
To investigate whether high concentration of DA also exhibits similar effects in NMDA receptor functions in vivo, 1-(2-[bis-(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine (GBR12909), a selective DA reuptake inhibitor, was systemically administered to animals to induce acute hyperdopamine state. A single dose of GBR12909 (10 mg/kg, i.p.) was injected with saline as vehicle control and the rats were killed for physiologic recording after 1 h. The 10 mg/kg GBR12909 is a widely accepted medium dose (range, 2.5–40 mg/kg) considered to be very effective in elevating DA levels in the brain (Zahniser et al., 1999; Camarero et al., 2002; Thakker et al., 2004). We found that GBR12909 significantly decreased both the frequency and amplitude of the spontaneous NMDA receptor-mediated EPSCs in the layer 5 pyramidal neurons compared with those in control (n = 6; p < 0.01) (Fig. 5A–C). These effects, however, were abolished by pretreatment with either D2 receptor antagonist L741,626 or GSK-3β inhibitor TDZD. L741,626 is a potent and selective D2 receptor antagonist and is biologically active after systemic administration in vivo. As shown in Figure 5, when L741,626 was injected (10 mg/kg, i.p.) 30 min before GBR12909 administration, no significant effects were detected, neither for frequency nor for amplitude, on the spontaneous NMDA receptor-mediated EPSCs (n = 6; p > 0.05) (Fig. 5). These data indicate that the suppressing effect of GBR12909 likely acts via the activation of D2 receptors (Caruana et al., 2006). Similarly, pretreatment with GSK-3β inhibitor TDZD (1 mg/kg, i.p.; 30 min before GBR12909 administration) also completely blocked the suppressing effects of GBR12909 on spontaneous NMDA EPSCs. Both frequency and amplitude of the spontaneous EPSCs were recovered to the control levels (n = 6; p > 0.05) (Fig. 5). It has been reported that, in the frontal cortex, DA might be at least partially, if not mainly, cleared by the norepinephrine transporter (NET) (Morón et al., 2002; Miner et al., 2003; Carboni and Silvagni, 2004; Williams and Steketee, 2004). We have therefore tested the effects of a selective NET inhibitior nisoxetine (10 mg/kg, i.p.; Tocris) and a nonselective DA and NE reuptake blocker bupropion (10 mg/kg, i.p.; Tocris) on NMDA currents. As shown in Figure 5, D and E, the selective NET inhibitor nisoxetine exhibited no effect on EPSC frequency but increased the amplitude of NMDA current, although not statistically significantly (n = 6; p = 0.93 for frequency and p = 0.06 for amplitude). In contrast, bupropion significantly decreased EPSC frequency (n = 6; p < 0.05), similar to GBR12909, but had no significant effects on the EPSC amplitude (n = 6; p = 0.60). The role of NET inhibitor on NMDA receptors in the neocortex remains unclear but seemed to be opposite from that of DA transporter inhibitor. In fact, bupropion acts as not only a norepinephrine and dopamine reuptake inhibitor but also a nicotinic antagonist (Slemmer et al., 2000). These results further confirmed the effects of GSK-3β on hyperdopamine-induced depression of NMDA receptors, consistent with the finding in PFC slice recording described above. However, the net effect of NET inhibitor and the interactive effect of NE and DA (dual reuptake blocker) on NMDA EPSCs remain to be explored.
Figure 5.
Hyperdopamine induced by systemic administration of GBR12909 significantly decreases the spontaneous NMDA-EPSCs, and the effects were completely blocked by systemic coadministration of GBR12909 and either D2 antagonist or GSK-3β inhibitor TDZD. A, Representative traces of the spontaneous NMDA-EPSCs. B, Cumulative probability showing the obvious shift of the spontaneous NMDA-EPSC in GBR12909 administration. C, The decreases of both spontaneous EPSC frequency (p < 0.01) and amplitude (p < 0.01) induced by GBR12909 were completely reversed by coadministration of GBR12909 with either D2 antagonist or GSK-3β inhibitor TDZD in vivo. D, E, Effects of selective NET inhibitior nisoxetine (10 mg/kg, i.p.) and a nonselective DA and NE reuptake blocker bupropion (10 mg/kg, i.p.) on NMDA currents. Nisoxetine exhibited no effect on EPSC frequency but increased the amplitude of NMDA current, although not statistically significantly (n = 6; p = 0.93 for frequency and p = 0.06 for amplitude). In contrast, bupropion significantly decreased EPSC frequency (n = 6; p < 0.05), similar to GBR12909, but had no significant effects on the EPSC amplitude (n = 6; p = 0.60). Error bars indicate SEM.
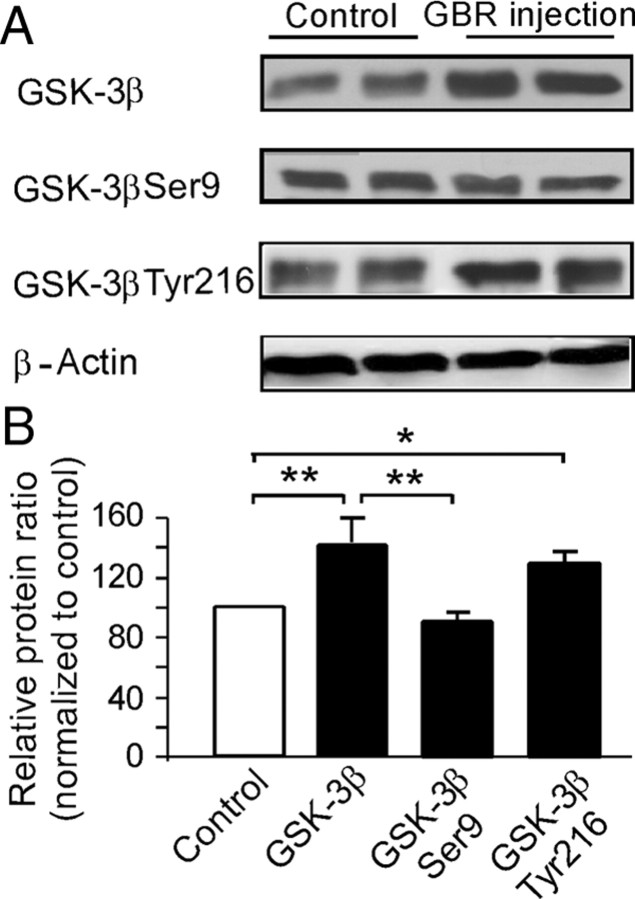
Hyperdopamine induced by systemic administration of GBR12909 significantly increases GSK-3β expression and phosphorylation of GSK-3β Tyr216 in the PFC in vivo
To further examine the role of GSK-3β in hyperdopamine-mediated depression of NMDA receptors, GSK-3β protein levels were measured using Western blotting. As shown in Figure 6, GBR12909 injection (10 mg/kg, i.p.; single dose; 1 h) significantly increased the total protein levels of GSK-3β by ∼40% compared with saline vehicle control (p < 0.01) (Fig. 6A,B). Because GSK-3β is activated via its phosphorylation at Tyr216 and is inactivated by phosphorylation at Ser9 through serine/tyrosine (Ser/Tyr) kinase, we further tested whether hyperdopamine induced by GBR12909 injection also affects the phosphorylation of GSK-3β. The phosphorylation state of GSK-3β was examined using Western blot with phosphor-specific anti-GSK-3β Ser9 and anti-GSK-3β Tyr216 antibodies. We found that the levels of GSK-3β Tyr216 in GBR12909 treatment were increased by ∼30% relative to saline vehicle control levels (p < 0.05). In contrast, the levels of phosphor-GSK-3β Ser9 in GBR12909 treatment remained unchanged compared with saline vehicle control levels (p = 0.67) and were significantly lower than total protein level of GSK-3β when the expression levels of phosphor-GSK-3β Ser9 in the GBR12909 treatment were normalized to total GSK-3β levels (p < 0.01). These results suggest an overall increase of GSK-3β activity in the GBR12909-induced hyperdopamine state, consistent with previous reports (Beaulieu et al., 2007b).
Figure 6.
Hyperdopamine induced by systemic administration of GBR12909 significantly increases GSK-3β expression in the PFC. A, Analysis by Western blots showed that a single dose of GBR12909 (10 mg/kg, i.p.) caused significant increase of total protein levels of GSK-3β as well as the levels of the phosphorylated form of GSK-3β Tyr216 but not GSK-3β Ser9 at 1 h after injection. B, Quantitative analysis showed that the total protein levels of GSK-3β were significantly increased by ∼40% after GBR12909 administrations relative to saline vehicle control (p < 0.01). A similar increase of ∼30% was observed in the levels of phosphor-GSK-3β Tyr216 relative to saline vehicle control (p < 0.05). In contrast, the levels of phosphor-GSK-3β Ser9 remained unchanged compared with control levels (p = 0.67) and but were significantly lower compared with total protein levels of GSK-3β (p < 0.01). Error bars indicate SEM.
Hyperdopamine induced by systemic administration of GBR12909 increases internalization and decreases protein synthesis of NR2B subunits in the PFC through GSK-3β activation
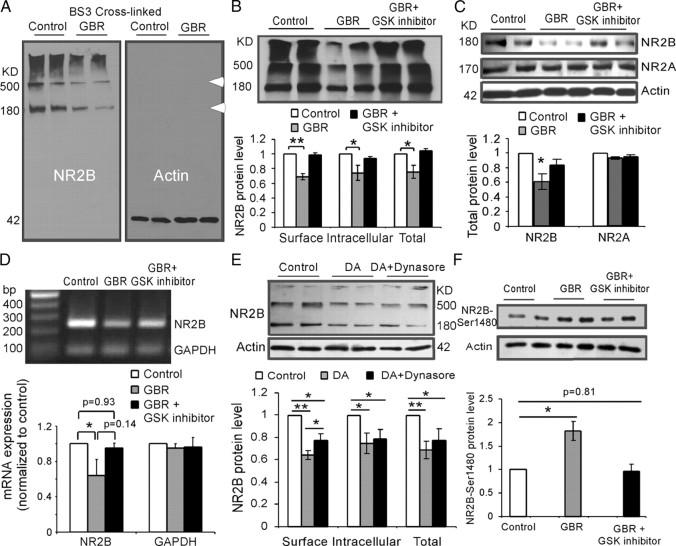
Because high dose of DA decreases the NMDA receptor-mediated EPSCs in the prefrontal cortical slices by inducing internalization of NMDA receptors, mostly NR2B subunits, we proposed that the surface expression of NMDA receptor proteins might be affected by hyperdopamine induced by systemic administration of GBR12909 (10 mg/kg, i.p.; single dose; 1 h). We tested this possibility by incubating the prefrontal cortical slices with membrane-impermeable cross-linking reagent BS3. Cross-linking assay has been used previously to measure the surface expression of glutamate receptors in brain slices (Grosshans et al., 2002a; Conrad et al., 2008). These studies have shown that incubation of brain slices with BS3 does not affect intracellular proteins such as β-actin. Thus, this technique enabled us to identify the relative changes of both surface and intracellular components of NMDA receptors. Here, we focused on the NR2B subunits because our in vitro slice physiology experiments (Fig. 3) suggested that NR2B subunits were mainly affected by DA. Our analysis revealed that GBR12909 injection (10 mg/kg, i.p.; 1 h) clearly decreased the protein expressions of both surface and intracellular NR2B subunits. Both the BS3 cross-linked surface NR2B (∼500 kDa) and the presumptive intracellular NR2B subunits (180 kDa) were significantly decreased by GBR12909 administration (Fig. 7A,B). In contrast, β-actin, which is not present on the cell surface, was not affected by BS3 cross-linking reagent in both control and GBR12909-treated rats, indicating the reliability of the BS3 cross-linking procedure (Fig. 7A). Overall, we found that both surface and intracellular expressions of NR2B subunits, as well as the total NR2B protein levels, were significantly decreased (p < 0.05) (Fig. 7B). These results indicate that GBR12909 significantly affected the trafficking of NMDA receptors, likely through a process involving inhibition of protein synthesis. This assumption was further confirmed by the evidenced significant decrease in both total protein level (p < 0.05) (Fig. 7C) and mRNA expression (p < 0.05) (Fig. 7D) of NR2B subunits in GBR12909-treated rat PFC. As shown in Figure 7C, GBR12909 (10 mg/kg, i.p.; 1 h) significantly decreased the total protein expression of NR2B (p < 0.05) but not NR2A (p > 0.05) detected with Western blot. Importantly, consistent with the electrophysiological changes in prefrontal cortical slices, pretreatment with GSK-3β inhibitor TDZD 30 min in advance (1 mg/kg, i.p.) completely reversed the decrease of protein expressions of both cell surface and intracellular NR2B subunits, the total protein level, as well as the mRNA expression of NR2B (p > 0.05) (Fig. 7B–D). These data further suggest that activation of GSK-3β is required for hyperdopaminergic regulation of NMDA receptor trafficking.
Figure 7.
Systemic administration of GBR12909 significantly decreases both surface and intracellular NR2B proteins, total NR2B, NR2B mRNA expression, as well as NR2B pS1480, in the PFC. A, Representative images of Western blot analysis from the PFC slices incubated with the BS3 cross-linking reagent. BS3-linked surface NR2B was presented as high-molecular-weight aggregates (>500 kDa), whereas the intracellular NR2B remained in a monomeric form represented by a single molecular weight band at 180 kDa. In contrast, β-actin was located exclusively within cytoplasm as a monomer band, confirming effectiveness of the cross-linking. B, Top panel, Representative blots showing the protein levels of NR2B subunits after systemic administration of GBR12909 and coapplication of GBR12909 and GSK-3β inhibitor TDZD. Bottom panel, Quantitative analysis showed that relative protein levels of the presumptive surface, intracellular, and total NR2B subunits relative to the control levels (*p < 0.05; **p < 0.01). C, GBR12909 (10 mg/kg, i.p.; 1 h) significantly decreased the total protein expression of NR2B (p < 0.05) but not NR2A (p > 0.05). D, The mRNA expression of NR2B subunits was significantly decreased by systemic injection of GBR12909 (p < 0.05) and this effect was reversed by coapplication of GBR12909 and GSK-3β inhibitor TDZD (p = 0.93). In contrast, the GAPDH expressions were stable, without clear change. E, The DA-induced endocytosis of NR2B subunit was further confirmed by incubating the prefrontal cortical slices with membrane-impermeable cross-linking reagent BS3. Both surface and intracellular protein levels of NR2B subunits were significantly decreased by DA treatment (200 μm; 10 min; n = 4; p < 0.01). When DA was coapplied with dynasore (100 μm), the effect of DA on surface, but not intracellular, expression of NR2B, was partially but significantly blocked (n = 4; p < 0.05). F, GBR12909 injection (10 mg/kg, i.p.; 1 h) significantly increased the expression of NR2B pS1480 (p < 0.05), and this increase was blocked by preinjection of GSK-3β inhibitor TDZD 30 min in advance (1 mg/kg, i.p.). Error bars indicate SEM.
The DA-induced endocytosis of NR2B subunit was further tested by incubating the prefrontal cortical slices with membrane-impermeable cross-linking reagent BS3 under conditions of DA incubation with and without dynasore. Both surface and intracellular protein levels of NR2B subunits were significantly decreased by DA treatment (200 μm; 10 min; n = 4; p < 0.05). When DA was coapplied with dynasore (100 μm), the effect of DA on surface, but not intracellular, expression of NR2B, was partially but significantly blocked (n = 4; p < 0.05) (Fig. 7E). In addition, we wondered whether NR2B phosphorylation plays a role in the D2-mediated suppression of NMDA receptors. Previous study indicated that phosphorylation of NR2B Ser1480 site disrupts the interaction of NMDARs with PSD-95 and SAP102 and decreases surface NR2B expression (Chung et al., 2004), whereas phosphorylation of NR2B at Tyr1472 was involved in synaptic insertion (Hallett et al., 2006; Gao and Wolf, 2008) (Li, Liu, Hu, Huang, and Gao, unpublished observation). We therefore tested whether the trafficking of NR2B induced by hyperdopamine is also through phosphorylation of Ser1480. As shown in Figure 7F, GBR12909 injection (10 mg/kg, i.p.; 1 h) significantly increased the expression of NR2B pS1480 (p < 0.05), and this increase was blocked by preinjection of GSK-3β inhibitor TDZD 30 min in advance (1 mg/kg, i.p.), indicating that phosphorylation of NR2B Ser1480 may also affect the surface NR2B expression. It is therefore possible that dynamin and NR2B pS1480 jointly regulate the endocytosis of NR2B subunits under condition of D2 receptor activation, although it is not clear whether dynamin is involved in surface expression of NR2B mediated by phosphorylation of NR2B Ser1480.
β-Catenin may mediate the action of GSK-3β in NMDA receptors
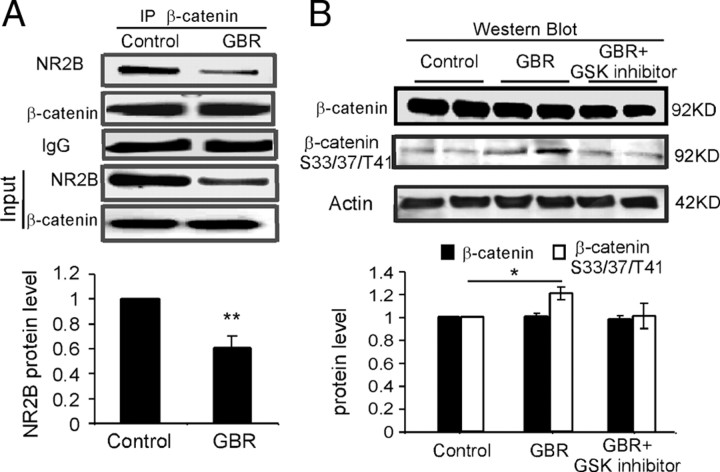
How does activation of GSK-3β affect the NMDA receptors? Previous studies indicated that the β-catenin-binding site lies close to the GSK-3β-binding site and that S33, S37, and T41 sites in β-catenin are strongly phosphorylated by GSK-3β (Daugherty and Gottardi, 2007; Xu and Kimelman, 2007). β-Catenin is known to be localized in postsynaptic density as a component of NMDA receptor multiprotein complex (Husi et al., 2000; Okabe et al., 2003; Al-Hallaq et al., 2007). GSK-3-mediated phosphorylation of β-catenin could allow β-catenin to go to the cell nucleus, interact with transcription factors, and thus regulate gene transcription (Aberle et al., 1997; Xu and Kimelman, 2007). We therefore speculated that hyperdopamine-induced decrease in expression of NR2B receptor protein and mRNA might be caused by regulating β-catenin through activation of GSK-3β. To test this possibility, we first examined the interaction between β-catenin and NR2B with immunoprecipitation. β-Catenin was immunoprecipitated with anti-β-catenin and was then probed for immunoprecipitated NR2B with specific anti-NR2B antibody. Under control conditions, we found that NR2B coprecipitated with β-catenin (Fig. 8A), whereas the negative control β-tubulin was not detected in β-catenin immunoprecipitates (data not shown), suggesting a potential protein interaction between β-catenin and NR2B (Husi et al., 2000; Okabe et al., 2003; Al-Hallaq et al., 2007). Consistently, the NR2B cobinding with β-catenin was significantly decreased by GBR12909 administration (10 mg/kg, i.p.) (p < 0.01) (Fig. 8A). The total protein level of β-catenin was, however, not changed by GBR12909 treatment, in both immunoprecipitation (p > 0.05) (Fig. 8A) and Western blot (p > 0.05) (Fig. 8B). The decreased binding between NR2B and β-catenin, however, might also be partially attributable to the decreased expression of NR2B subunits. How does the interaction between NR2B and β-catenin regulate NR2B expression? Although total protein of β-catenin was not significantly affected by GBR12909 administration, it is possible that β-catenin was phosphorylated and thus affected the NR2B expression by interfering with the mRNA expression of this subunit (Schmeisser et al., 2009). Indeed, the expression of phosphor-β-catenin detected with antibody against S33, S37, and T41 sites was significantly increased by GBR12909 treatment (p < 0.05) (Fig. 8B). These results thus suggest that phosphorylation of β-catenin mediates the action of GSK-3β in protein synthesis of NMDA receptors.
Figure 8.
Hyperdopamine induces phosphorylation of β-catenin and β-catenin–NR2B interaction. A, β-Catenin was immunoprecipitated with anti-β-catenin antibody and was then probed for the coimmunoprecipitated NR2B with specific anti-NR2B antibody. Under control conditions, NR2B coprecipitated with β-catenin, whereas β-tubulin was not detected in β-catenin immunoprecipitates (data not shown), suggesting a potential protein interaction between β-catenin and NR2B. Consistently, NR2B binding, as well as input NR2B expression, was significantly decreased by GBR12909 administration (10 mg/kg, i.p.) (p < 0.01). B, The total protein level of β-catenin was not changed by GBR12909 treatment in Western blot detection (p > 0.05). In contrast, the expression of phosphor-β-catenin detected with antibody against Ser33/37/Tyr41 sites was significantly increased by GBR12909 treatment (p < 0.05). These results indicate that β-catenin may mediate the action of GSK-3β in NMDA receptor trafficking and protein synthesis. Error bars indicate SEM.
Discussion
In recent years, there have been considerable efforts to understand the cellular mechanisms of DA effects on prefrontal neuronal communications. It is generally believed that DA induces a bidirectional regulation in synaptically evoked NMDA receptor-mediated EPSCs in the cortical neurons (Seamans and Yang, 2004; Cepeda and Levine, 2006; Castner and Williams, 2007). A large body of evidence indicates that activation of D1 receptors can antagonistically modulate NMDA responses via intracellular signaling cascades (Snyder et al., 1998; Flores-Hernández et al., 2002) or direct protein–protein interactions (Lee et al., 2002; Scott et al., 2002, 2006; Pei et al., 2004). The potentiation of NMDA receptor-mediated currents by low doses of DA or D1 agonists is primarily through the activation of D1/protein kinase A (PKA)/dopamine and cAMP regulated phosphoprotein 32 kDa (DARPP32) intracellular pathways (Cepeda and Levine, 1998; Zheng et al., 1999; Greengard, 2001; Seamans et al., 2001; Yang and Chen, 2005). The relationship between D1 stimulation and NMDA-EPSC changes evidenced at individual neurons in vitro is similar to the inverted “U” shape function for D1 receptor stimulation required for in vivo working memory performance (Zahrt et al., 1997; Floresco and Phillips, 2001; Seamans and Yang, 2004; Williams and Castner, 2006; Vijayraghavan et al., 2007). This biphasic/bidirectional modulation of NMDA receptors by D1 stimulation could therefore have important implications given the important role of NMDA receptors in the cellular aspects of working memory (Li et al., 1997; Lisman et al., 1998; Romanides et al., 1999; Durstewitz et al., 2000; Wang et al., 2008).
Despite the extensive study of D1–NMDA receptor interaction, the molecular mechanisms associated with the influences of hyperdopamine on glutamatergic systems, particularly NMDA receptors in the PFC, remain unclear. In this study, we show how elevated DA affects the NMDA receptor functions in the PFC by both in vitro and in vivo approaches. We confirmed that NMDA-EPSCs were potentiated by low doses of DA (≤10 μm) but significantly attenuated by higher doses of DA (≥100 μm), consistent with previous reports in the rat PFC (Law-Tho et al., 1994; Zheng et al., 1999; Seamans et al., 2001), striatum (Cepeda and Levine, 1998; Jiao et al., 2007), and amygdala (Martina and Bergeron, 2008). Importantly, we found that, under both in vitro and in vivo conditions, the synaptically evoked NMDA receptor-mediated currents in the rat prefrontal neurons were significantly attenuated by high concentration of DA stimulation through the activation of D2 receptors. The high-dose DA-induced inhibition of the NMDA-EPSCs recorded in the prefrontal slices was long-lasting with little recovery. How would D2 activation inhibit the NMDA-EPSCs in the prefrontal neurons? Although presynaptic D2 receptors have been implicated in suppressing glutamate synaptic transmission (Levine et al., 1996; Chen and Gurling, 1999), we, however, think that the D2-mediated inhibition of NMDA receptors was likely through postsynaptic mechanisms, such as receptor internalization/endocytosis. Indeed, high-dose DA failed to affect the NMDA-EPSCs after blockade of NR2B subunits. Intracellularly loaded inhibitory peptide of dynamin, a GTPase responsible for endocytosis, also significantly reduced the DA-induced inhibition of NMDA receptors. In addition, recent studies conducted on cultured prefrontal neurons reported a direct inhibition of puff-NMDA-induced current by activation of D2 or D4 receptors, via either platelet-derived growth factor (PDGF) receptor (Beazely et al., 2006) or PKA–PP1 (protein phosphatase 1) pathway (Wang et al., 2003). At very high concentration of DA (0.1–3 mm), a rapidly reversible and voltage-dependent blockade of inward NMDA-induced current had also been shown in cultured hippocampal neurons (Castro et al., 1999; Kotecha et al., 2002). However, whether the signaling pathways reported in cultured neurons are analogous to those in vivo remain to be determined.
Recent studies indicated that elevation of DA levels induced by administration of DA transporter inhibitors such as cocaine and amphetamine resulted in cognitive disorders via D2–GSK-3β pathway (Beaulieu et al., 2005, 2007b, 2009; Lute et al., 2008). The proposed role of GSK-3β in DA/D2 receptor-associated psychiatric disorders is intriguing, yet whether and how it will affect NMDA receptor-mediated synaptic transmission in vivo remains untested. Here, we provided the first evidence that hyperdopamine induced by DA reuptake inhibitor GBR12909 significantly decreases the expressions of both surface and intracellular NR2B proteins, as well as mRNA levels of NR2B, in the prefrontal cortical neurons, likely through the inhibition of protein synthesis. Furthermore, the high-dose DA/D2-mediated suppression of NMDA receptors required the activation of GSK-3β through increasing the phosphorylation of GSK-3β Tyr216 and decreasing the phosphorylation of GSK-3β Ser9, but neither dependent on Gq11 nor PLC signaling. Our data support several recent studies, which suggested that activation of GSK-3β is associated with NMDA receptor-dependent synaptic plasticity (Peineau et al., 2007, 2008; Zhu et al., 2007). Numerous studies have emphasized the importance of GSK-3β in NMDA receptor-mediated neuroprotection and neurodegeneration (Takadera et al., 2004; Szatmari et al., 2005; Habas et al., 2006; Hetman and Kharebava, 2006; Lei et al., 2008), as well as synaptic plasticity (Hooper et al., 2007; Peineau et al., 2007, 2008; Zhu et al., 2007). Previous studies indicated that phosphorylation of GSK-3β Tyr216 is required for basal activity, and increased phosphorylation of this residue results in increased activity of GSK-3β (Hughes et al., 1993), whereas phosphorylation of GSK-3β Ser9 leads to inactivation of GSK-3β, overriding the activation induced by phosphorylation of GSK-3β Tyr216 (Bhat et al., 2000). Based on our results, we therefore conclude that an increase in GSK-3β and GSK-3β Tyr216 levels together with the relatively lower expression of GSK-3β Ser9 are consistent with an overall increase of GSK-3β activity in the GBR12909-induced hyperdopaminergic state. The increased GSK-3β-mediated inhibition of NMDA receptors makes an ideal case for its role in the induction of long-term depression (Peineau et al., 2007, 2008) and inhibition of long-term potentiation (Zhu et al., 2007) because both synaptic processes are NMDA receptor dependent. Our result is also consistent with previous studies conducted in both in vivo and in vitro conditions, indicating that GSK-3β inhibitors had no clear effects on the basal NMDA receptor-mediated synaptic transmission (Peineau et al., 2007, 2008; Zhu et al., 2007). In another study using cultured neurons, however, GSK-3β inhibitor was found to slightly inhibit NMDA-induced current (Chen et al., 2007). It is likely that the discrepancy derived from the different neuronal preparations, and the dissociated neurons might be more sensitive to GSK-3β inhibitors.
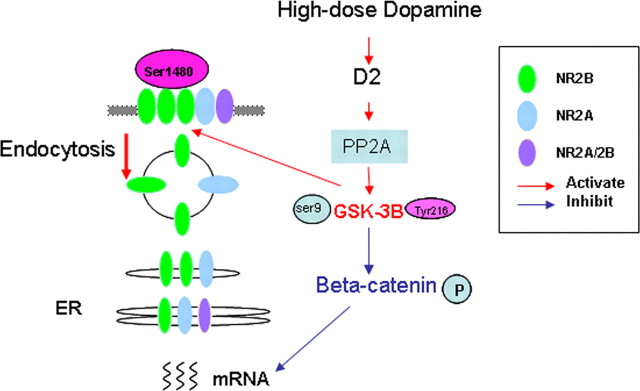
Previous studies indicated that activation of GSK-3β can induce phosphorylation and thus degradation of β-catenin (Liu et al., 2002; Daugherty and Gottardi, 2007). We, however, found that the total protein level of β-catenin was unaltered. This seemingly contradicts previous studies. Instead, we did observe increased phosphorylation of β-catenin S33/37/T41. It is possible that increase of GSK-3β activity induces phosphorylation of β-catenin that in turn results in the redistribution of β-catenin (Murase et al., 2002) instead of degradation (Liu et al., 2002; Daugherty and Gottardi, 2007). Another possibility is that degradation of β-catenin induced by phosphorylation occurred beyond the test time applied in our experiments, which was 1 h after GBA12909 administration. This proposition, however, needs additional exploration. Nevertheless, our data suggest that GSK-3β plays an essential role in regulating NMDA receptor functions in the prefrontal neurons. Based on our findings, we speculate that GSK-3β is likely activated by its upstream regulator PP2A (Beaulieu et al., 2009), and it in turn phosphorylates β-catenin, which interacts with NR2B, controls the NR2B gene transcription, and thus affects the protein synthesis of NR2B subunits (Daugherty and Gottardi, 2007; Xu and Kimelman, 2007). Consequently, the NR2B surface expression and NMDA receptor functions are regulated by D2 receptor activation (Fig. 9).
Figure 9.
Schematic graph showing the signaling pathway involved in hyperdopamine/D2-mediated inhibition of synaptic NMDA receptors. High concentration of DA/activation of D2 receptor activates GSK-3β via its upstream regulator PP2A. GSK-3β in turn phosphorylates β-catenin (Ser33/37/Tyr41), which interacts with NR2B, controls the NR2B gene transcription, and thus affects the protein synthesis of NR2B subunits. Phosphorylation of NR2B-Ser1480 and dynamin play an important role for NR2B endocytosis in the DA-induced inhibition of NMDA current.
Although concurrent changes of DA and NMDA receptors play an essential role in the pathogenesis of psychiatric disorders such as schizophrenia, little is known about the correlation between hyperfunction of the dopaminergic system and NMDA receptor functions in vivo. In this study, we present evidence that an animal model with persistently elevated dopamine levels is ideal for studying the action of hyperdopamine on NMDA receptor functions. We administered DA reuptake inhibitor GBR12909 to induce acute hyperdopamine state in rats (Carlsson et al., 1999). Numerous studies have shown that this potent and highly selective DA reuptake inhibitor can cause significant elevation of DA levels in the extracellular space (Karoum et al., 1994; Zahniser et al., 1999; Easton et al., 2007). In addition, GBR12909 selectively increases DA levels without affecting norepinephrine and serotonin because the affinity for the latter two transmitters is 100-fold lower (Andersen, 1989). The neurochemical and behavioral consequences of this blockade are similar to those obtained using DA transporter gene knockdown (Zhuang et al., 2001; Thakker et al., 2004; Cagniard et al., 2006), and thus it serves as a good model to study the hyperdopaminergic state. The increased DA neurotransmission arising from administration of GBR12909 results in the activation of D2 receptors, which in turn induces inhibition of NMDA receptors, particularly via NR2B subunit endocytosis and phosphorylation, through the constant activation of GSK-3β. These data support the involvement of GSK-3 as an important mediator of DA action in vivo and suggest that modulation of the PP2A–GSK-3β pathway might be relevant to DA-related disorders, such as schizophrenia and drug addiction (Beaulieu et al., 2007b, 2009; Koros and Dorner-Ciossek, 2007). In summary, our study suggests that GSK-3β signaling pathway is required for the hyperdopamine/D2 receptor-mediated inhibition of NMDA receptors in the prefrontal neurons and this action may underlie D2 receptor-mediated psychostimulant effects and hyperdopamine-dependent behaviors in the brain.
Footnotes
This study was supported by research fund of Drexel University College of Medicine, a grant from a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award, National Institutes of Health (NIH) Grants R21MH232307 and R01MH085666 (W.-J.G.); and research fund of Drexel University College of Medicine, NARSAD Young Investigator Award (2005) (Y.-Q.H.). We are grateful to Drs. Feng Yang and Victoria A. Zhukareva for their comments on this manuscript. Anti-NR2B-pS1480 antibody was gifted from Dr. Richard L. Huganir. The NIH had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication. W.-J.G. conceived the study, supervised the project, and wrote this manuscript. Y.-C.L performed the experiments and data analysis of electrophysiology, Western blots, immunoprecipitation, and wrote this manuscript. D.X. and J.R. performed the experiments of PCR and Western blots. Y.-Q.H. supervised the biochemical experiments and contributed to the manuscript preparation.
The authors declare no competing financial interests.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PH. The dopamine uptake inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007a;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J-M, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007b;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Beazely MA, Tong A, Wei W-L, Van Tol H, Sidhu B, MacDonald JF. D2-class dopamine receptor inhibition of NMDA currents in prefrontal cortical neurons is platelet-derived growth factor receptor-dependent. J Neurochem. 2006;98:1657–1663. doi: 10.1111/j.1471-4159.2006.04064.x. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA. Decoding dopamine signaling. Cell. 2005;122:153–155. doi: 10.1016/j.cell.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Camarero J, Sanchez V, O'Shea E, Green AR, Colado MI. Studies, using in vivo microdialysis, on the effect of the dopamine uptake inhibitor GBR 12909 on 3,4-methylenedioxymethamphetamine (“ecstasy”)-induced dopamine release and free radical formation in the mouse striatum. J Neurochem. 2002;81:961–972. doi: 10.1046/j.1471-4159.2002.00879.x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A. Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol. 2004;16:121–128. doi: 10.1615/critrevneurobiol.v16.i12.130. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Antipsychotic drugs, neurotransmitters, and schizophrenia. Am J Psychiatry. 1978;135:165–173. doi: 10.1176/ajp.135.2.165. [DOI] [PubMed] [Google Scholar]
- Carlsson ML, Martin P, Nilsson M, Sorensen SM, Carlsson A, Waters S, Waters N. The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice. J Neural Transm. 1999;106:123–129. doi: 10.1007/s007020050144. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Lüscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana DA, Sorge RE, Stewart J, Chapman CA. Dopamine has bidirectional effects on synaptic responses to cortical inputs in layer II of the lateral entorhinal cortex. J Neurophysiol. 2006;96:3006–3015. doi: 10.1152/jn.00572.2006. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV. Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn. 2007;63:94–122. doi: 10.1016/j.bandc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Castro NG, de Mello MC, de Mello FG, Aracava Y. Direct inhibition of the N-methyl-d-aspartate receptor channel by dopamine and (+)-SKF38393. Br J Pharmacol. 1999;126:1847–1855. doi: 10.1038/sj.bjp.0702479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;2006:pe20–pe24. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Chen AC, Gurling HM. D2 dopamine receptor but not AMPA and kainate glutamate receptor genes show altered expression in response to long term treatment with trans- and cis-flupenthixol in the rat brain. Brain Res Mol Brain Res. 1999;68:14–21. doi: 10.1016/s0169-328x(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates NMDA receptor channel trafficking and function in cortical neurons. Mol Pharmacol. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty RL, Gottardi CJ. Phospho-regulation of β-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-d-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Easton N, Steward C, Marshall F, Fone K, Marsden C. Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology. 2007;52:405–414. doi: 10.1016/j.neuropharm.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Flores-Hernández J, Cepeda C, Hernández-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem. 2008;106:2489–2501. doi: 10.1111/j.1471-4159.2008.05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ. Acute clozapine suppresses synchronized pyramidal synaptic network activity by increasing inhibition in the ferret prefrontal cortex. J Neurophysiol. 2007;97:1196–1208. doi: 10.1152/jn.00400.2006. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Goldman-Rakic PS. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci U S A. 2003;100:2836–2841. doi: 10.1073/pnas.262796399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci U S A. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002a;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 2002b;2002:pl8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Habas A, Kharebava G, Szatmari E, Hetman M. NMDA neuroprotection against a phosphatidylinositol-3 kinase inhibitor, LY294002 by NR2B-mediated suppression of glycogen synthase kinase-3beta-induced apoptosis. J Neurochem. 2006;96:335–348. doi: 10.1111/j.1471-4159.2005.03543.x. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6:787–799. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SGN. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D1 and D3 receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. J Neurochem. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Brinjak R, Hitri A, Wyatt RJ. Regional effects of amphetamine, cocaine, nomifensine and GBR 12909 on the dynamics of dopamine release and metabolism in the rat brain. Br J Pharmacol. 1994;113:1391–1399. doi: 10.1111/j.1476-5381.1994.tb17152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 2008;438:77–93. doi: 10.1016/S0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koros E, Dorner-Ciossek C. The role of glycogen synthase kinase-3beta in schizophrenia. Drug News Perspect. 2007;20:437–445. doi: 10.1358/dnp.2007.20.7.1149632. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry. 2000;157:831–833. doi: 10.1176/appi.ajp.157.5.831. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59:663–664. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. J Neurosci. 2003;23:10074–10083. doi: 10.1523/JNEUROSCI.23-31-10074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law-Tho D, Hirsch JC, Crepel F. Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res. 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chéry N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM. The role of Akt-GSK-3beta signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology. 2008;33:1343–1353. doi: 10.1038/sj.npp.1301511. [DOI] [PubMed] [Google Scholar]
- Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Sibley DR, Westphal H. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Li HB, Matsumoto K, Yamamoto M, Watanabe H. NMDA but not AMPA receptor antagonists impair the delay-interposed radial maze performance of rats. Pharmacol Biochem Behav. 1997;58:249–253. doi: 10.1016/s0091-3057(97)00015-4. [DOI] [PubMed] [Google Scholar]
- Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang X-J. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg G-H, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Lute BJ, Khoshbouei H, Saunders C, Sen N, Lin RZ, Javitch JA, Galli A. PI3K signaling supports amphetamine-induced dopamine efflux. Biochem Biophys Res Commun. 2008;372:656–661. doi: 10.1016/j.bbrc.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Martina M, Bergeron R. D1 and D4 dopaminergic receptor interplay mediates coincident G protein-independent and dependent regulation of glutamate NMDA receptors in the lateral amygdala. J Neurochem. 2008;106:2421–2435. doi: 10.1111/j.1471-4159.2008.05584.x. [DOI] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 2003;1003:131–137. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Selcher JC, Hanson JE, Madison DV. Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. BMC Neurosci. 2005;6:48. doi: 10.1186/1471-2202-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives β-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Salter MW. NMDA receptors are movin' in. Curr Opin Neurobiol. 2004;14:353–361. doi: 10.1016/j.conb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Okabe T, Nakamura T, Nishimura YN, Kohu K, Ohwada S, Morishita Y, Akiyama T. RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-d-aspartate receptor signaling. J Biol Chem. 2003;278:9920–9927. doi: 10.1074/jbc.M208872200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 5. Amsterdam: Elsevier Academic; 2005. [Google Scholar]
- Pei L, Lee FJ, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153:S428–S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007a;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, O'Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci. 2007b;28:551–555. doi: 10.1016/j.tips.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Romanides AJ, Duffy P, Kalivas PW. Glutamatergic and dopaminergic afferents to the prefrontal cortex regulate spatial working memory in rats. Neuroscience. 1999;92:97–106. doi: 10.1016/s0306-4522(98)00747-7. [DOI] [PubMed] [Google Scholar]
- Schmeisser MJ, Grabrucker AM, Bockmann J, Boeckers TM. Synaptic crosstalk between NMDA receptors and LAPSER1/beta-catenin at excitatory synapses. J Biol Chem. 2009;284:29146–29157. doi: 10.1074/jbc.M109.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Kruse MS, Forssberg H, Brismar H, Greengard P, Aperia A. Selective up-regulation of dopamine D1 receptors in dendritic spines by NMDA receptor activation. Proc Natl Acad Sci U S A. 2002;99:1661–1664. doi: 10.1073/pnas.032654599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjö S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci U S A. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari E, Habas A, Yang P, Zheng JJ, Hagg T, Hetman M. A positive feedback loop between glycogen synthase kinase 3beta and protein phosphatase 1 after stimulation of NR2B NMDA receptors in forebrain neurons. J Biol Chem. 2005;280:37526–37535. doi: 10.1074/jbc.M502699200. [DOI] [PubMed] [Google Scholar]
- Takadera T, Sakamoto Y, Ohyashiki T. NMDA receptor 2B-selective antagonist ifenprodil-induced apoptosis was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Brain Res. 2004;1020:196–203. doi: 10.1016/j.brainres.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Hüsken D, Maier R, Müller M, van der Putten H, Hoyer D, Cryan JF. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci U S A. 2004;101:17270–17275. doi: 10.1073/pnas.0406214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Gibb AJ. Dopamine D1 receptor inhibition of NMDA receptor currents mediated by tyrosine kinase-dependent receptor trafficking in neonatal rat striatum. J Physiol. 2008;586:4693–4707. doi: 10.1113/jphysiol.2008.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Characterization of dopamine transport in crude synaptosomes prepared from rat medial prefrontal cortex. J Neurosci Methods. 2004;137:161–165. doi: 10.1016/j.jneumeth.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:re12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Yang CR, Chen L. Targeting prefrontal cortical dopamine d1 and N-methyl-d-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11:452–470. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289:266–277. [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-d-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]
- Zhu L-Q, Wang S-H, Liu D, Yin Y-Y, Tian Q, Wang X-C, Wang Q, Chen J-G, Wang J-Z. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27:12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]