Abstract
Alkamides belong to a class of small lipid signals of wide distribution in plants, which are structurally related to the bacterial quorum-sensing signals N-acyl-l-homoserine lactones. Arabidopsis (Arabidopsis thaliana) seedlings display a number of root developmental responses to alkamides, including primary root growth inhibition and greater formation of lateral roots. To gain insight into the regulatory mechanisms by which these compounds alter plant development, we performed a mutant screen for identifying Arabidopsis mutants that fail to inhibit primary root growth when grown under a high concentration of N-isobutyl decanamide. A recessive N-isobutyl decanamide-resistant mutant (decanamide resistant root [drr1]) was isolated because of its continued primary root growth and reduced lateral root formation in response to this alkamide. Detailed characterization of lateral root primordia development in the wild type and drr1 mutants revealed that DRR1 is required at an early stage of pericycle cell activation to form lateral root primordia in response to both N-isobutyl decanamide and N-decanoyl-l-homoserine lactone, a highly active bacterial quorum-sensing signal. Exogenously supplied auxin similarly inhibited primary root growth and promoted lateral root formation in wild-type and drr1 seedlings, suggesting that alkamides and auxin act by different mechanisms to alter root system architecture. When grown both in vitro and in soil, drr1 mutants showed dramatically increased longevity and reduced hormone- and age-dependent senescence, which were related to reduced lateral root formation when exposed to stimulatory concentrations of jasmonic acid. Taken together, our results provide genetic evidence indicating that alkamides and N-acyl-l-homoserine lactones can be perceived by plants to modulate root architecture and senescence-related processes possibly by interacting with jasmonic acid signaling.
Plant growth and development require the integration of a variety of environmental and endogenous signals, which together with the intrinsic genetic program determine plant form and longevity. Lipids have long been recognized as signals that have the capacity to trigger profound physiological responses. In animals, ceramides and sphingosines are lipids that have proapoptotic and antiproliferative actions (Wymann and Schneiter, 2008). In plants, ceramides, sphingosines, and phosphatidic acid are involved in mediating plant growth, development, and responses to environmental stimuli (Worrall et al., 2003; Wang, 2004).
In the past few years, additional small lipids have been found to acts as plant signals, including alkamides and N-acyl-ethanolamines (NAEs). Alkamides comprise at least 200 amides with varied acyl chain lengths and saturation grades (for review, see López-Bucio et al., 2006; Morquecho-Contreras and López-Bucio, 2007). These compounds have been found to alter root and shoot system architecture in Arabidopsis (Arabidopsis thaliana; Ramírez-Chávez et al., 2004; Campos-Cuevas et al., 2008). NAEs represent compounds with aminoalcohol linked as an amide to the fatty acid. They are likely produced from the hydrolysis of N-acyl-phosphatidylethanolamines, a minor constituent of cell membranes, by phospholipase D (Chapman, 2004). NAEs have been found to accumulate in seeds of some higher plants, including cotton (Gossypium hirsutum), corn (Zea mays), Arabidopsis, soybean (Glycine max), tomato (Solanum lycopersicum), and pea (Pisum sativum), and their levels decline during germination (Wang et al., 2006).
Many gram-negative bacteria produce alkamide-related substances termed N-acyl-l-homoserine lactones (AHLs). These compounds participate in cell-to-cell signaling that is usually referred to as quorum sensing (Pearson et al., 1994). The AHL signals contain a conserved HL ring and an N-linked acyl side chain. The acyl-chain moiety of naturally occurring AHLs can differ in length and substitution at position C3, which is either unmodified or carries an oxo or hydroxyl group (Pearson et al., 1994; Parsek et al., 1999). These molecules are freely diffused through the bacterial membrane, which is to some extent dependent upon the length of the acyl side chain and the nature of any C3 substitutions and distribute within the rhizosphere (Pearson et al., 1999; Schuhegger et al., 2006; Scott et al., 2006). Evidence has accumulated indicating that plants are able to perceive AHLs. The application of AHLs to Medicago truncatula and Arabidopsis plants resulted in differential transcriptional changes in roots and shoots, affecting the expression of genes potentially involved in development (Mathesius et al., 2003; Von Rad et al., 2008). Ortíz-Castro et al. (2008) evaluated Arabidopsis growth responses to a variety of saturated AHLs ranging from four to 14 carbons in length, focusing on alterations in postembryonic root development. The compounds affected primary root growth, lateral root (LR) formation, and root hair development. While this information clearly indicates that plants are able to sense a variety of small lipid signals, including alkamides, NAEs, and AHLs, which modulate root architecture, the genetic mechanisms involved in signal perception to these compounds are unknown.
The Arabidopsis root system is an excellent model to dissect the genetic and developmental processes that determine plant architecture. It mainly consists of an embryonic primary root and postembryonic developed LRs (López-Bucio et al., 2005). LR formation is influenced by a wide range of environmental cues, such as nutrients and water availability in the soil (López-Bucio et al., 2003; Malamy, 2005; Nibau et al., 2008). The plasticity of LR formation is of critical importance, allowing plants to compete for resources and adapt to constantly changing growth conditions. LRs originate from pericycle founder cells located opposite to xylem poles, which undergo several rounds of anticlinal divisions to create a single-layered primordium composed of up to 10 small cells of equal length (termed stage I; Dolan et al., 1993; Malamy and Benfey, 1997; Dubrovsky et al., 2001). Further anticlinal and periclinal divisions create a dome-shaped primordium (spanning stages III–VII), which eventually emerges from the parental root (Malamy and Benfey, 1997; Casimiro et al., 2003; Péret et al., 2009).
The phytohormone auxin (indole-3-acetic acid [IAA]) plays an important role during each stage of LR formation (De Smet et al., 2006; Fukaki et al., 2007; Dubrovsky et al., 2008; Fukaki and Tasaka, 2009). Application of IAA or synthetic auxins such as 2,4-dichlorophenoxyacetic acid or naphthaleneacetic acid (NAA) stimulates LR formation (Celenza et al., 1995; Woodward and Bartel, 2005), whereas polar auxin transport inhibitors such as N-(1-naphthyl)-phthalamic acid and 2,3,5-triiodobenzoic acid prevent LR formation (Casimiro et al., 2001; Himanen et al., 2002). Consistently, Arabidopsis mutants with increased auxin levels, such as rooty and its alleles aberrant lateral root formation1 and superroot1, have increased numbers of LRs (Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995), while mutants with defective auxin transport, perception, or signaling, including aux1, axr1, and tir3/doc1/big, show reduced LR formation (Lincoln et al., 1990; Gil et al., 2001; Swarup et al., 2001). In contrast to auxin, less is known about the action of alkamides, AHLs, and other related small lipid signals on LR formation and whole plant development.
To identify the genetic components responsible for the root architectural responses to alkamides, we performed a visual screening for Arabidopsis mutants that under high N-isobutyl decanamide concentration do not manifest primary root growth reduction. We isolated an N-isobutyl decanamide-resistant mutant (decanamide resistant root [drr1]) defective in a single recessive trait. Detailed cellular and developmental studies of wild-type and drr1 plants indicate that drr1 mutants show resistance to primary root growth inhibition and LR growth promotion induced by both an alkamide (N-isobutyl decanamide) and a bacterial quorum-sensing signal (N-decanoyl-l-homoserine lactone [C10-AHL]). We further show that DRR1 is a crucial component of the regulation of plant senescence, which likely links alkamide and jasmonic acid (JA) in modulating plant longevity and LR development.
RESULTS
Isolation of drr1, an Arabidopsis Mutant with Altered Primary Root Growth Response to N-Isobutyl Decanamide
From a group of similar chain length alkamides and NAEs, López-Bucio and coworkers (2007) identified N-isobutyl decanamide, a C10 saturated alkamide that is naturally produced in Acmella radicans (Ríos-Chávez et al., 2003) and Cissampelos glaberrima (Laurerio-Rosario et al., 1996), as the most active compound in inhibiting primary root growth and stimulating LR formation in Arabidopsis.
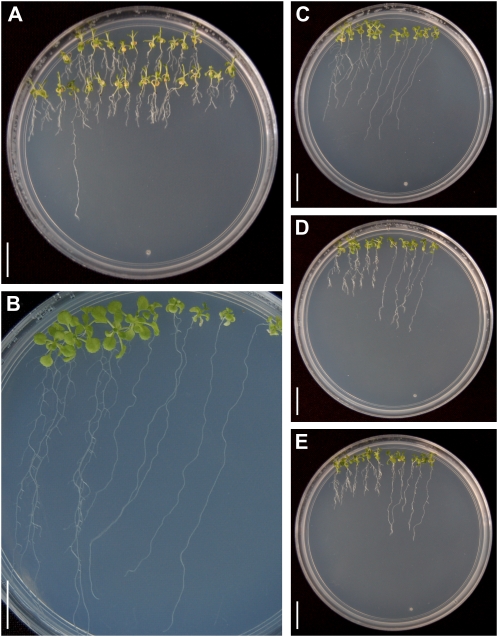
To investigate the genetic basis of plant responses to alkamides, we screened 25,000 lines from T-DNA insertion mutant collections (Krysan et al., 1999) by inspecting the root architecture of plants grown over the surface of 0.2 × Murashige and Skoog (MS) agar plates supplied with 30 μm N-isobutyl decanamide. A mutant line was isolated that, in contrast to the wild type, was able to sustain primary root growth under this inhibitory concentration of the alkamide (Fig. 1A). The mutant was backcrossed to wild-type plants (Wassilewskija [Ws] ecotype) three times prior to detailed phenotypical analysis. In F2 progeny from these crosses, in plants grown in medium supplied with 30 μm N-isobutyl decanamide, the line segregated the mutant phenotype in a 1:3 ratio (Table I). These results indicate that the primary root growth resistance to the alkamide resulted from a recessive single-gene mutation. We named this locus drr1. To further study the developmental alterations induced by N-isobutyl decanamide in wild-type and drr1 plants, we grew ecotype Ws and drr1 plants side by side on vertically oriented agar plates with varied alkamide contents. Wild-type plants grown in 0.2 × MS agar medium without N-isobutyl decanamide showed a typical root system, consisting of a long primary root with many LRs forming in a gradient from the root/shoot junction to the primary root tip (Fig. 1B). In the same medium, drr1 mutants developed a long primary root lacking visible LRs (Fig. 1B), thus indicating that DRR1 is important for normal LR development under normal growth conditions. In wild-type plants treated with 20, 25, or 30 μm N-isobutyl decanamide, there was a dose-dependent inhibitory effect of the alkamide on primary root growth, which correlates with an increase in LR formation. In these plants, multiple LRs developed, giving rise to a highly exploratory root system with different architecture from that observed in plants grown in medium without N-isobutyl decanamide (Fig. 1, C–E). In contrast, alkamide-treated drr1 mutants showed longer primary roots and reduced LR formation when compared with wild-type plants in most concentrations of N-isobutyl decanamide tested (Fig. 1, C–E).
Figure 1.
Genetic screen and phenotypic characterization of drr1 mutants. A, Photograph of an agar plate supplied with 30 μm N-isobutyl decanamide showing a putative drr1 mutant with long primary root. B, Five 14-d-old wild-type (Ws) and drr1 seedlings grown side by side on the surface of agar plates containing 0.2 × MS medium lacking N-isobutyl decanamide. C to E, Photographs of agar plates supplied with 20 μm (C), 25 μm (D), or 30 μm (E) N-isobutyl decanamide, showing five wild-type (left) and drr1 (right) plants grown side by side. Photographs in B and C are representative individuals of four plates per treatment. Bars = 1 cm. [See online article for color version of this figure.]
Table I. Segregation ratio of progeny resulting from crosses between drr1 mutant and wild-type seedlings.
| Generation | Phenotype of Progeny |
Ratio Obtained, Wild Type:drr1 | Ratio Tested, Wild Type:drr1 | χ2a | |
| Many LRs (Wild Type) | Few LRs (drr1) | ||||
| F1 | 128 | 0 | |||
| F2 | 730 | 260 | 2.81:1 | 3:1 | 0.84 |
With one degree of freedom and a critical value of 5%, the hypothesis is accepted if the χ2 is smaller than 3.841.
drr1 Mediates the Root Architecture Responses of Arabidopsis to N-Isobutyl Decanamide
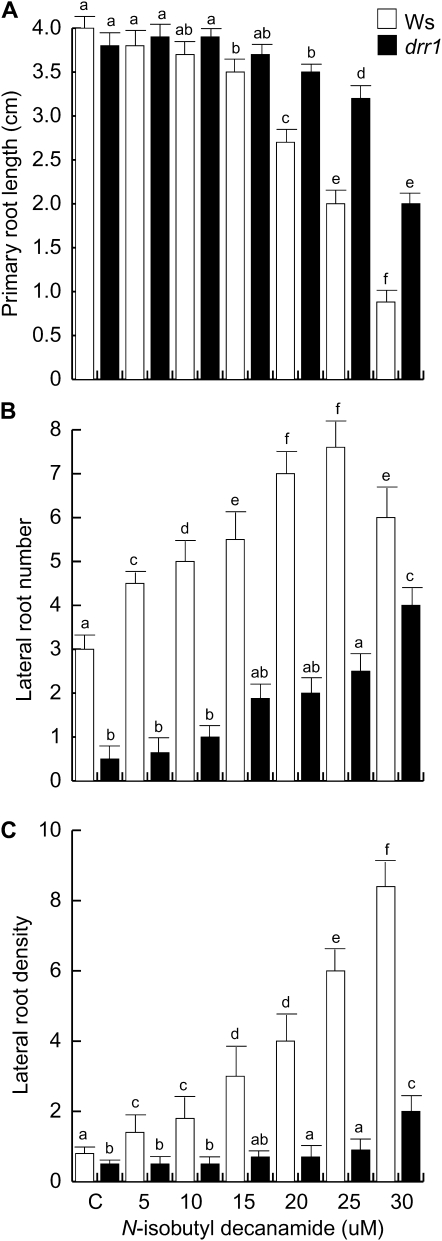
To more clearly define the alterations in the root architectural response to N-isobutyl decanamide caused by mutation in DRR1, we performed temporal and single-point measurements of primary root length, LR number per plant, and LR density in wild-type and drr1 mutants treated with varied concentrations of N-isobutyl decanamide. Primary root growth was similar in wild-type and drr1 plants in concentrations of up to 15 μm N-isobutyl decanamide, while in concentrations of 20, 25, and 30 μm of this compound, drr1 primary roots were significantly longer that wild-type plants (Fig. 2A). N-Isobutyl decanamide increased the number of emerged LRs in wild-type plants, while drr1 plants were resistant to this effect (Fig. 2B). The density of emerged LRs dramatically increased in response to alkamide treatment in wild-type plants, but the mutants again showed reduced responses. The most contrasting responses between wild-type and drr1 plants were observed in 25 μm N-isobutyl decanamide, in which wild-type plants showed a highly branched root system harboring second- and third-order LRs (Fig. 1, C–E), with a 6-fold increased density of LRs (Fig. 2C). In this alkamide concentration, drr1 mutant plants produced less than 15% of the LRs observed in wild-type plants. Interestingly, although drr1 mutants produced consistently fewer LRs compared with the wild type in most N-isobutyl decanamide treatments, exposure to 30 μm N-isobutyl decanamide caused an 8-fold increase in LR number and a 2-fold increase in LR density (Fig. 2, B and C), indicating that the mutants are not completely insensitive to the alkamide.
Figure 2.
Effects of N-isobutyl decanamide on the root system architecture of wild-type (Ws) and drr1 plants. A, Primary root length. B, Number of emerged LRs per plant. C, LR density expressed as the number of LRs per centimeter. Data were recorded at 12 d after germination. Values shown are means ± sd (n = 20). Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results.
drr1 Mutants Are Resistant to Inhibitory Effects of N-Isobutyl Decanamide on Cell Division in Primary Root Meristems
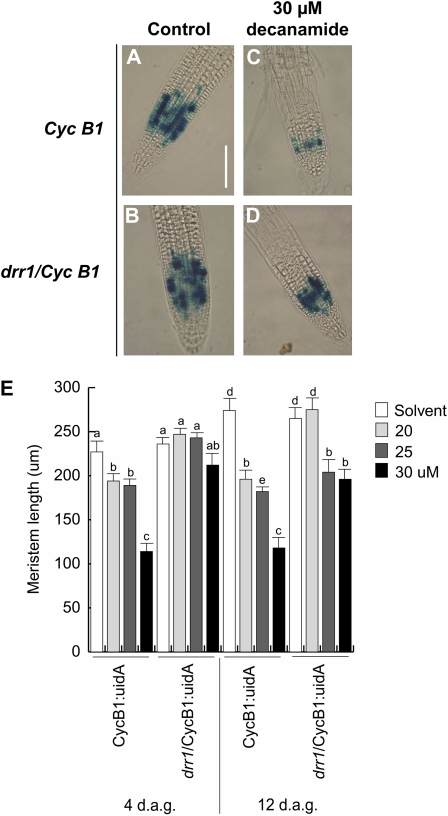
An important factor determining primary root growth reduction in wild-type seedlings grown in high N-isobutyl decanamide concentrations is the reduction in cell division in the root meristem (López-Bucio et al., 2007). To analyze the cell division responses of drr1 mutants to alkamide treatment, we crossed drr1 with a transgenic plant harboring the CycB1:uidA construct, which is expressed only in cells in the G2/M phase of the cell cycle and is a marker of mitotic activity (Colón-Carmona et al., 1999). CycB1:uidA seedlings and drr1 seedlings were grown in 0.2 × MS agar medium supplied with the solvent or with 20, 25, and 30 μm N-isobutyl decanamide. In both wild-type CycB1:uidA and drr1 mutant seedlings supplied with the solvent only, a patchy pattern of single cells expressing CycB1:uidA was observed in the primary root meristem (Fig. 3, A and B). In wild-type plants subjected to treatment with 30 μm N-isobutyl decanamide, GUS expression in the primary root tip decreased and root hairs were formed in close proximity to the root meristem (Fig. 3C). Interestingly, CycB1:uidA expression in the primary root apex of drr1 seedlings treated with the alkamide was not as much inhibited as in the wild type, and their root meristems were anatomically similar to those of solvent only-treated seedlings (Fig. 3D). Root hair formation close to the root meristem was not observed in drr1 seedlings treated with the alkamide (Fig. 3D). Next, we quantified the length of the primary root meristems in wild-type and drr1 plants at 4 and 12 d after germination. At these developmental stages, increased concentrations of N-isobutyl decanamide decreased the length of the meristem in wild-type plants, while drr1 mutants were resistant to this effect (Fig. 3E).
Figure 3.
CycB1:uidA expression in transgenic wild-type and drr1 seedlings. Twelve-hour GUS staining is shown for CycB1:uidA primary roots in wild-type and drr1 Arabidopsis seedlings grown on agar-solidified 0.2 × MS medium with or without N-isobutyl decanamide. A and B, Solvent-treated seedlings. C and D, Plants supplied with 30 μm N-isobutyl decanamide. E, Meristem length. Photographs are representative individuals of at least 20 stained seedlings. The experiment was repeated twice with similar results. Bar = 100 μ m. d.a.g., Days after germination. [See online article for color version of this figure.]
drr1 Is Defective in N-Isobutyl Decanamide-Induced LR Primordia Development
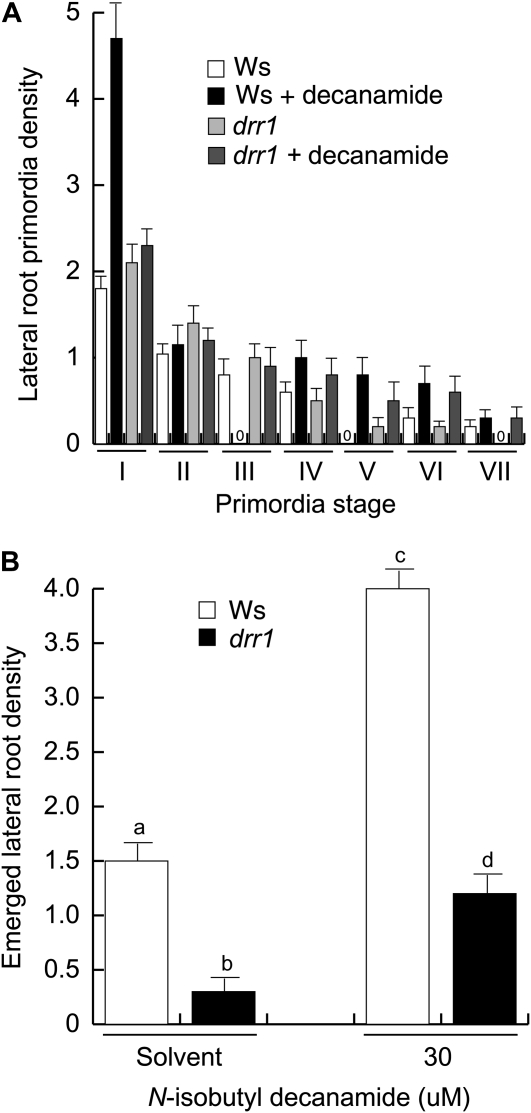
LR formation is a major determinant of root system architecture. Next, we investigated the effects of N-isobutyl decanamide on lateral root primordia (LRP) development and LR emergence in wild-type and drr1 plants. LRP originating in the primary root and emerged LRs were counted at 6 d after germination in plants grown in 0.2 × MS agar medium supplied with the solvent only or with 30 μm N-isobutyl decanamide. The developmental stage of each LRP was classified according to Malamy and Benfey (1997; see “Materials and Methods”). In solvent-treated wild-type plants, most LRP remained at an early developmental stage (stage I). Interestingly, N-isobutyl decanamide treatment increased both the number of LRP at stage I and the density of emerged LRs (Fig. 4). Solvent-treated drr1 mutants showed similar LRP density to wild-type plants (Fig. 4A) but dramatically decreased density of emerged LRs (Fig. 4B), indicating that the mutant is not inherently defective in LRP initiation but rather shows a retardation in the maturation of LRP. When treated with N-isobutyl decanamide, drr1 mutant seedlings did not show an increase in stage I LRP or in LRP emergence observed in wild-type plants (Fig. 4), indicating the DRR1 locus is involved in alkamide responses in the pericycle and during LRP development. Although alkamide treatment significantly increased the density of emerged LRs in drr1 mutants, drr1 always showed lower LR density than wild-type plants in the different growth conditions (Fig. 4B). These results indicate that N-isobutyl decanamide modifies root system architecture both by inducing more pericycle cells to form stage I LRP and by accelerating the emergence of LRP from the primary root to form mature LRs. Mutations in drr1 interfere with both of these processes.
Figure 4.
Effects of N-isobutyl decanamide on wild-type (Ws) and drr1 LR development. A, LRP stage distribution in 6-d-old primary roots grown on medium supplied with the solvent only or with 30 μm N-isobutyl decanamide (indicated as decanamide). B, Emerged LR density in the same experiment. Wild-type and drr1 seedlings were cleared, and the number and stage of LRP were recorded according to Malamy and Benfey (1997). Values shown are means ± sd (n = 15). Different letters represent means statistically different at the 0.05 level. This analysis was repeated twice with similar results.
drr1 Is Defective in Root Architectural Responses to C10-AHL, a Quorum-Sensing Signal from Bacteria
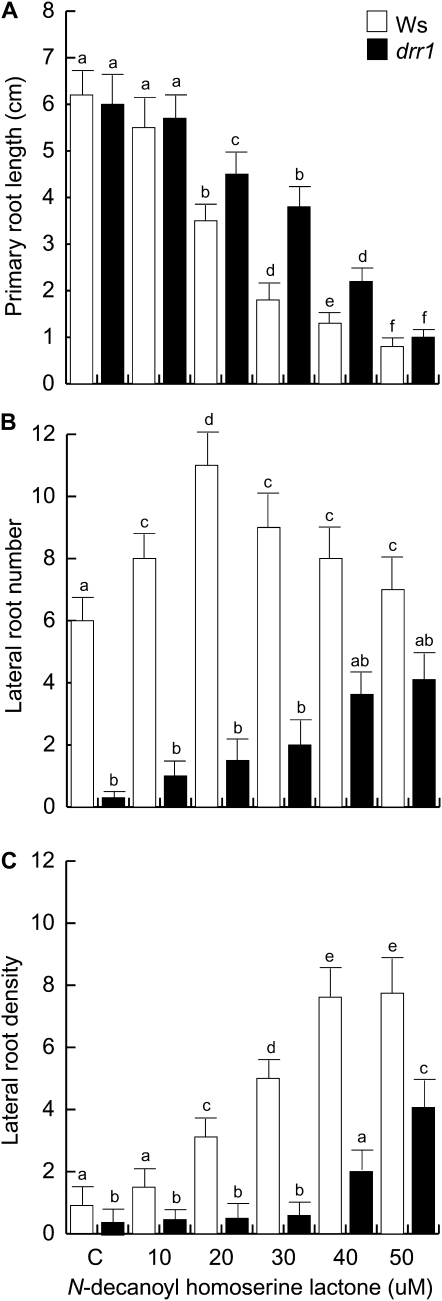
Previous studies documented that AHLs, a class of alkamide-related quorum-sensing signals from bacteria, modulate root system architecture in Arabidopsis (Ortíz-Castro et al., 2008; Von Rad et al., 2008). To determine if DRR1 is involved in AHL responses, we tested the primary root growth and LR responses of drr1 seedlings to C10-AHL over a range of concentrations of this compound as compared with wild-type plants. drr1 had a level of resistance to primary root growth inhibition by C10-AHL over most concentrations tested (Fig. 5A). At 30 μm C10-AHL in wild-type plants, about 60% inhibition of growth occurred, whereas in the mutant, it was about 30%. As previously reported (Ortíz-Castro et al., 2008), C10-AHL stimulated LR formation (Fig. 5B). A dose-dependent effect increasing LR density was observed (Fig. 5C), confirming the positive role of AHLs in LR induction. In contrast, drr1 mutants showed reduced LR formation when compared with wild-type seedlings over most concentrations of C10-AHL tested (Fig. 5, B and C).
Figure 5.
Effects of C10-AHL on the root system architecture of wild-type (Ws) and drr1 plants. A, Primary root length. B, Number of emerged LRs per plant. C, LR density expressed as the number of LRs per centimeter. Data were recorded at 14 d after germination. Values shown are means ± sd (n = 20). Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results.
drr1 Shows Normal Auxin Responses
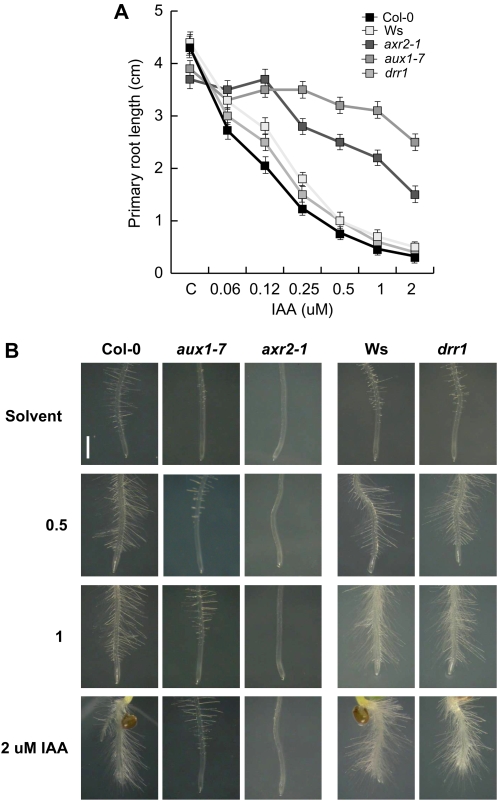
Several auxin-related mutants have been characterized in screens for primary root growth resistance to inhibitory amounts of IAA, which display alterations in LR formation (Rogg et al., 2001; Swarup et al., 2001; Fukaki et al., 2002). To determine if drr1 operates in a genetically defined auxin pathway, wild-type Arabidopsis seedlings (ecotype Columbia [Col-0] and Ws), drr1 seedlings, and the auxin-related mutants aux1-7 and axr2 were evaluated in primary root growth response assays to IAA. First, to confirm the auxin resistance of auxin-related mutant lines, homozygous aux1-7 and axr2-1 seedlings were screened for resistance to IAA based on primary root growth. In these experiments, aux1-7 and axr2 were resistant to the inhibition of primary root elongation by IAA when compared with wild-type Col-0 seedlings (Fig. 6A). These mutants also failed to form abundant root hairs at the root tip region in response to increasing IAA concentration in the medium, a phenotype associated with increased auxin resistance (Fig. 6B). In contrast, the auxin response in drr1 mutants was equally sensitive to IAA than the wild-type (Ws ecotype) both in primary root growth assays (Fig. 6A) and toward induction of root hair formation close to the root tip (Fig. 6B). Because drr1 mutants showed normal root responses to IAA, we conclude that auxin signaling is unaffected in the mutant.
Figure 6.
Auxin responses in wild-type and drr1 seedling roots. A, Primary root growth in 12-d-old primary roots of wild-type (Col-0 and Ws), axr2-1 and aux1-7 auxin-resistant mutants, and drr1 Arabidopsis mutants grown on medium supplied with the solvent only or with varied IAA concentrations. B, Morphology of root tips of wild-type and mutant lines exposed to IAA. Seedlings were photographed at 7 d after germination using a digital camera connected to a dissecting microscope. Values shown in A are means ± sd (n = 30). The experiment was repeated twice with similar results. [See online article for color version of this figure.]
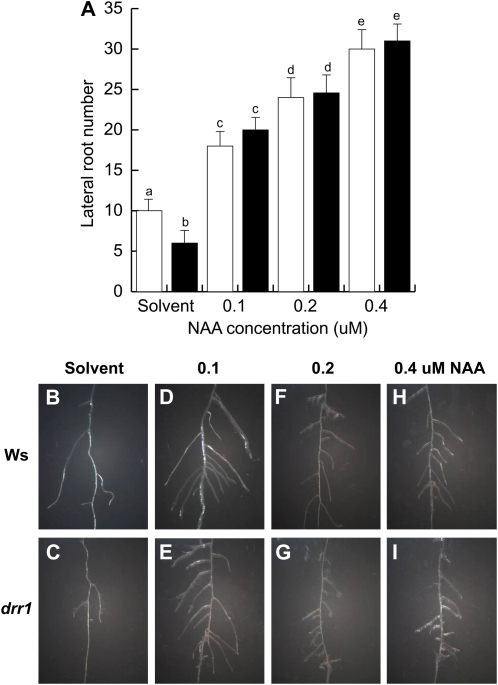
To better understand the role played by auxin in LR formation in wild-type and drr1 plants, we tested the effects of NAA to activate LR formation in a transfer assay. In these experiments, wild-type and drr1 plants were first germinated and grown for 7 d in 0.2 × MS agar medium. At day 7 after germination, plants were transferred to 0.2 × MS liquid medium supplied with the solvent or varied concentrations of NAA for an additional 4-d period. At this stage, the number and density of LRs were determined. As shown in Figure 7, NAA treatment caused a dose-response effect in LR formation (Fig. 7A), which was similar between wild-type and drr1 plants. Both wild-type and drr1 plants produced highly branched root systems with normal LR growth (Fig. 7, B–I). These results indicate that drr1 seedlings are not inherently defective in pericycle cell activation to form LRs and are able to correctly sense and respond to auxins.
Figure 7.
Auxin restoration of LR development in drr1 plants. A, Total LR number per plant in 11-d-old wild-type (Ws) and drr1 plants that were first grown for 7 d in 0.2 × MS agar medium and then transferred to 0.2 × MS liquid medium supplied with the solvent or with varied concentrations of NAA for an additional 4-d growth period. B to I, Representative photographs of wild-type and drr1 LRs in plants exposed to NAA. Values shown in A are means ± sd (n = 15). Different letters represent means statistically different at the 0.05 level. This analysis was repeated twice with similar results. [See online article for color version of this figure.]
drr1 Mutants Show Extended Longevity
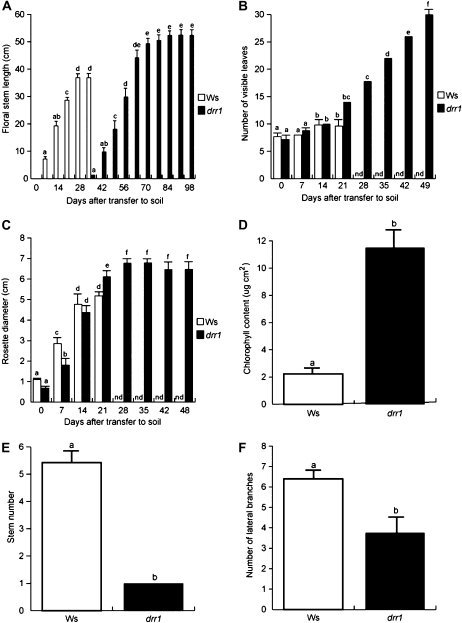
To study the role of DRR1 in plant growth and development, we compared the phenotype of wild-type and homozygous drr1 plants of the same age that were first germinated and grown for 10 d on 0.2 × MS agar medium and then transferred to soil. Wild-type and drr1 plants were grown side by side during their entire life cycle. The young and adult phenotypes of plants are shown in Figure 8. During the first 28 d after transfer, a general delay in the growth of drr1 mutants was observed, as illustrated by their delay in stem formation (Figs. 8, A and B, and 9A) and significantly decreased rosette size during early stages of vegetative growth (Fig. 9B). At 28 d after transfer, the rosette leaves of wild-type plants had already turned yellow and stem growth ceased, but drr1 leaves remained green, and 7 d later, the stems just started to be formed (Figs. 8, A–C, and 9, A and B). At 35 d after transfer, wild-type leaves had turned completely yellow and showed signs of death with drying (Fig. 8C). In contrast, the drr1 mutant leaves retained a significant amount of chlorophyll and maintained the integrity of the leaf shape (Fig. 8C). The extension of leaf longevity at a whole plant level dramatically increased in drr1 mutants with time. Delayed flowering was accompanied by the generation of new leaves, increased rosette size, and greater stem length in drr1 mutants when compared with wild-type plants (Figs. 8, D and E, and 9, A–C). drr1 sustained chlorophyll production for a longer time period (Fig. 9D). In addition, the shoot architecture of drr1 mutants was different from that observed in wild-type seedlings, producing only one primary stem with reduced branches, which suggests increased apical dominance in the mutants (Fig. 9, E and F). Aside from the delayed senescence and altered shoot architecture, drr1 mutants produced fertile flowers that yielded fruits with fully viable seeds (Fig. 8D). We determined that the longevity in drr1 mutants was extended by approximately 2-fold when compared with wild-type plants, which correlates with a 3- to 4-fold increase in the number of visible leaves and overall increased plant size (Fig. 9, A–C).
Figure 8.
Phenotypes of wild-type and drr1 plants grown in soil. A to D, Phenotypes of wild-type (Ws; left) and drr1 (right) plants grown side by side at 14, 28, 56, or 84 d after transfer to soil. E, Closeup of rosette leaves at 84 d after transfer. Plants were grown with a 16-h-light/8-h-dark cycle at 22°C in a growth chamber. The retarded leaf senescence in drr1 was related to the retarded emergence of floral stems and flowering time. [See online article for color version of this figure.]
Figure 9.
Age-dependent senescence symptoms and other developmental traits of wild-type (Ws) and drr1 plants grown in soil. A, Age-dependent stem size. B, Rosette diameter. C, Number of visible rosette leaves. D, Chlorophyll content in rosette leaves at 28 d after transfer to soil. E, Stem number. F, Number of stem branches. Plants were grown with a 16-h-light/8-h-dark cycle at 22°C in a growth chamber, and developmental traits were monitored during their entire life cycle. Values shown are means ± sd (n = 18). Different letters represent means statistically different at the 0.05 level. The experiment was repeated twice with similar results. nd, Not determined.
The drr1 Mutant Shows Delayed Senescence Symptoms in JA- and Alkamide-Induced Senescence
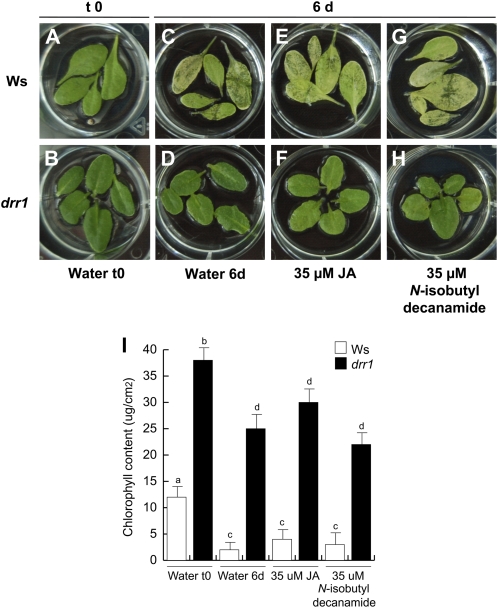
Leaf senescence is modulated by JA (Schommer et al., 2008). Therefore, the possibility was open that the drr1 mutant could be deficient in the JA-induced senescence program. We compared the effects of JA and N-isobutyl decanamide in wild-type and drr1 plants in a senescence-induced assay for detached leaves (Fig. 10). In this assay, after 6 d of incubation in water, wild-type detached leaves gradually lost chlorophyll content (Fig. 10, A and C). A deficient senescence program for detached drr1 plants incubated in water was evident (Fig. 10, B and D). In response to treatments with JA and N-isobutyl decanamide, wild-type leaves showed severe senescence symptoms that were reduced in drr1 mutants (Fig. 10, E–I). Taking together the increased drr1 longevity in soil and the delaying response to hormone-induced senescence, we conclude that DRR1 plays an important role in the senescence process modulated by JA and N-isobutyl decanamide as well as in age-dependent senescence.
Figure 10.
Hormone-dependent senescence symptoms in the drr1 mutant. Detached leaves of wild-type (Ws) and drr1 plants were incubated in 2-mL water solutions supplied with the indicated concentrations of compounds. The plates were included in a growth chamber (Percival ARR95L) under dark conditions, and representative photographs of leaves subjected to the different treatments were taken 6 d later (A–H) and chlorophyll determination was performed (I). The experiment included at least three independent samples of five leaves each and was replicated three times with similar results. [See online article for color version of this figure.]
drr1 Is Altered in Jasmonate-Mediated LR Induction
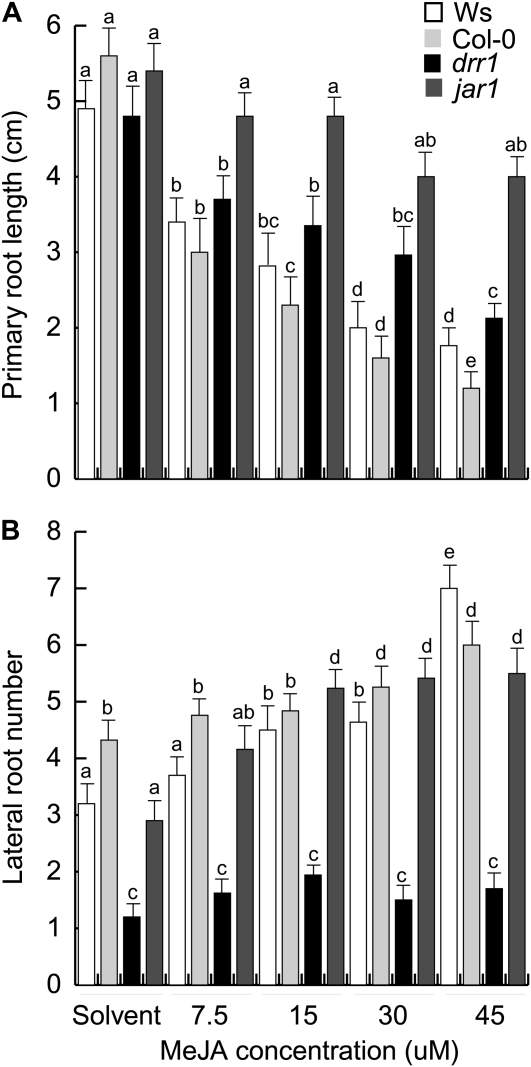
Jasmonates are signals involved in root system architecture modulation (Wasternack, 2007). The inhibitory effect of methyl jasmonate (MeJA) on primary root growth has been well recognized and widely employed as a useful trait to identify jasmonate-related mutants in Arabidopsis. Recently, it has been reported that MeJA also promotes LR formation (Sun et al., 2009). The MeJA dose response of drr1 in primary root growth and LR formation was compared with jar1, a MeJA-insensitive mutant, and wild-type seedlings of the Ws and Col-0 ecotypes; the Col-0 ecotype provided the genetic background for jar1 and therefore was included as an additional control. When compared with Ws and Col-0 plants, the jar1 mutant showed strong resistance to MeJA-induced primary root growth inhibition over most concentrations tested (Fig. 11A). The primary root growth inhibition in drr1 was essentially the same as in Ws seedlings (Fig. 11A). Interestingly, MeJA, at concentrations of 30 to 45 μm, increased emerged LRs in wild-type seedlings and in jar1 mutants by 70% to 150% (Fig. 11B; Supplemental Fig. S1). In the absence of MeJA, LR formation in drr1 was significantly reduced compared with wild-type and jar1 plants. However, drr1 mutants failed to produce increased numbers of LRs when grown on medium containing a range of concentrations of MeJA (Fig. 11B; Supplemental Fig. S1). Our data reveal that drr1 encodes a novel genetic locus modulating the effects of MeJA on LR formation.
Figure 11.
Effects of JA on primary root growth and LR development in wild-type (Ws and Col-0) and mutant (drr1 and jar1) lines. A, Primary root length. B, Number of emerged LRs per plant. Data were recorded at 12 d after germination. Values shown are means ± sd (n = 20). Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results.
DISCUSSION
drr1 Mutants Define a Locus Involved in Root Architectural Responses to Both Alkamides and AHLs
This report describes the identification and characterization of an Arabidopsis mutant that was defective in its root response to N-isobutyl decanamide but with additional characteristics, which suggest that alkamides play a role in plant longevity. Our characterization of root architectural responses in the wild type and drr1 to N-isobutyl decanamide provided insights into the genetic mechanisms mediating the responses to alkamides. While N-isobutyl decanamide inhibited primary root growth and promoted LR formation in wild-type seedlings, resistance to the repressive effect of this alkamide on primary root growth and the failure to increase LR formation typified the drr1 phenotype (Figs. 1 and 2).
Detailed cellular analysis of wild-type and drr1 plants showed that the mutants sustained almost normal root meristematic activity when grown under inhibitory concentrations of N-isobutyl decanamide, as revealed by cell counts and CycB1:uidA expression in the primary root meristem (Fig. 3). Interestingly, the typical increase in LR primordia initiation and LR emergence observed in wild-type plants treated with the alkamide was reduced in drr1 (Fig. 4). Several types of reported experimental evidence suggested that conditions that reduce primary root meristematic activity, including destruction of the primary root meristem by cell ablation and physical decapitation of the root tip, elicit an increase in LR number (Tsugeki and Fedoroff, 1999). Our findings that drr1 mutants grown in medium lacking alkamides sustain normal primary root growth but reduced LR formation (Figs. 1 and 2) indicate that LR proliferation in response to N-isobutyl decanamide is not a direct consequence of primary root growth inhibition but rather suggest a positive effect of the alkamide on pericycle cells to produce more LRP (Fig. 4).
Many bacterial species use small molecule signaling to communicate with each other and to coordinate their growth activities, a process commonly referred to as quorum sensing (Taga and Bassler, 2003; Reading and Sperandio, 2006). Diverse gram-negative bacteria produce AHLs, and these compounds contain a conserved HL ring and an N-linked acyl side chain. Our previous work indicated that saturated medium (C8–C14)-chained AHL compounds showed a dose-dependent effect on root architecture, inhibiting primary root growth and promoting LR formation (Ortíz-Castro et al., 2008). In this work, we used the drr1 mutant to determine whether alkamides and AHLs could be perceived by similar genetic mechanisms. We show that C10-AHL inhibited primary root growth and promoted LR formation in Arabidopsis wild-type seedlings (Fig. 5). drr1 mutants showed reduced sensitivity to both N-isobutyl decanamide and C10-AHL, indicating a potential genetic interaction in plant responses to alkamides and AHLs in roots. These results also suggest that plants have evolved the capacity to sense AHLs in order to activate developmental responses.
Several reports indicate that bacteria commonly associated with plants are capable to produce a variety of AHLs (Cha et al., 1998; Elasri et al., 2001; Khmel et al., 2002; D'Angelo-Picard et al., 2005). Several strains of Pseudomonas have been studied for their ability to colonize plant-related niches, such as the rhizosphere (i.e. P. aeruginosa, P. fluorescens, and P. putida), where they can act as plant growth-promoting rhizobacteria by antagonizing plant-deleterious microorganisms and through the production of traits that directly influence plant disease resistance and growth (Venturi, 2006). The rhizospheric P. putida plant beneficial strains WCS358 and IsoF produce 3-oxo-C12-AHL, whereas in the rhizosphere-colonizing biocontrol P. fluorescens strain F113, the production of three AHL molecules, including C10-AHL, has been reported (Laue et al., 2000; Venturi, 2006). Interestingly, C10-AHL and C12-AHL seem to be also produced in the nitrogen-fixing bacterial symbiont Sinorhizobium meliloti (Marketon et al., 2002; Teplitski et al., 2003). The marked resistance of drr1 to C10-AHL on root development opens new possibilities to identify novel genetic determinants involved in plant-bacteria interactions. Furthermore, the drr1 mutant can be used as a tool to identify novel plant growth-promoting bacterial strains, which could modulate root system architecture through AHL production.
Two recent reports suggested that auxin signaling might be involved in plant responses to AHLs (Mathesius et al., 2003; Von Rad et al., 2008). Auxins are signaling molecules that regulate the asymmetric pericycle cell divisions and thereby influence the patterning of newly initiated LRP. Thus, the possibility was open that altered auxin responses could be responsible for reduced LR formation in drr1 mutants. Our results, however, showed that drr1 mutants are not resistant to IAA or NAA treatments in terms of primary root growth inhibition or LR formation (Figs. 6 and 7), indicating that DRR1 might not be directly connected to the auxin response pathway to modulate plant growth and development. These results are in agreement with our previous research showing that both alkamides and AHLs modulate root system architecture, likely through auxin-independent signaling mechanisms (Ramírez-Chávez et al., 2004; Campos-Cuevas et al., 2008; Ortíz-Castro et al., 2008).
DRR1 Plays a Role in Senescence-Related Processes
Senescence is a developmental process that limits the longevity of an organism. Genetic studies of longevity mutants have also suggested that some common mechanisms, such as alterations in energy metabolism and oxidative damage, might play a role in determining life span in animals as divergent as nematodes, Drosophila, and mammals (Lin et al., 1998; Parkes et al., 1998). Plants also undergo a distinctive senescence process at the organ and/or organism level. A number of studies have provided evidence suggesting that leaf senescence is an active process controlled by a genetic program (Woo et al., 2001, 2002, Schommer et al., 2008). However, our understanding of how senescence and longevity are controlled at the whole plant level remains quite limited. Our results suggest that LR development and age-dependent plant senescence are directly connected through DRR1. Obvious alterations were seen in drr1 plants grown in soil under long days (16-h-light/8-h-dark conditions). In drr1 plants, leaf senescence was delayed by about 4 to 5 weeks when compared with wild-type plants (Figs. 8 and 9). The extended longevity of leaves was related to an extended growth period as well as to slower onset and/or progression of senescence after the maturation stage. The reduced growth observed at early stages of development in drr1 mutants opens the possibility that it may contribute to extended longevity. Consistent with this hypothesis, we observed that reduced LR formation was not caused by the failure of the pericycle to produce these structures but by the retarded development of LRP to emerge from the primary root (Fig. 4). In this way, the drr1 mutation shows a senescence character that differs from the delayed leaf senescence phenotype observed in the oresara (ore) and teosinte branched/cycloidea/PCF (tcp) mutants described previously (Woo et al., 2001, 2002; Schommer et al., 2008). In ore mutants, the retarded senescence phenotype seems to be specifically observed in leaves. For instance, the leaf longevity in ore9-1 was extended only by about 27%, without affecting other developmental traits such as flowering time and/or plant size (Woo et al., 2001). To our knowledge, no LR phenotypes have been reported for leaf senescence mutants such as the ore and tcp lines. Interestingly, the drr1 mutants also show that plants that bolt and senesce late produce more leaves and increase in size (Figs. 8 and 9), which could lead to potential agricultural applications. Together, our findings suggest that DRR1 may function normally as a positive regulator of senescence in Arabidopsis, limiting longevity at the whole plant level. Because the drr1 mutation affects a wide variety of age-dependent developmental and senescence responses (Fig. 10), DRR1 may function upstream in the regulatory cascade of senescence pathways.
drr1 Mutants Reveal Cross Talk between Alkamides and Jasmonate in LR Formation
Cross-resistance of mutants to multiple hormones is well documented (Wilson et al., 1990; Hobbie and Estelle, 1994; Tiryaki and Staswick, 2002) and suggests that the action of hormones is coordinated by common intermediates or modulators. Several phytohormones are involved in leaf senescence, including ethylene, cytokinin, and JA (Schommer et al., 2008). High concentrations of N-isobutyl decanamide have been found to induce callus formation in leaves and in roots (López-Bucio et al., 2007). Although not explicitly tested here, preliminary information shows that drr1 plants are also resistant to callus formation (data not shown). The proliferative growth activity elicited by N-isobutyl decanamide on callus formation in leaves and LR formation in roots was previously shown to be decreased or even absent in Arabidopsis mutants lacking one, two, or three of the putative cytokinin receptors CRE1, AHK2, and AHK3 (López-Bucio et al., 2007). The triple cytokinin receptor mutant cre1-12/ahk2-2/ahk3-3 was particularly insensitive to high alkamide concentrations in terms of developmental alterations, indicating that N-isobutyl decanamide requires, at least in part, a functional cytokinin-signaling pathway to control meristematic activity and differentiation processes. However, the primary root growth response of the drr1 mutants to kinetin, a highly active cytokinin in modulating root development, was similar to that observed in wild-type plants (Supplemental Fig. S2A), indicating that drr1 is not resistant to root inhibition by cytokinin. However, we cannot exclude the possibility that cross talk between alkamide and cytokinin responses may account for the increased longevity and/or reduced senescence of drr1 mutants. Abscisic acid and ethylene are two growth regulators also involved in senescence; DRR1 mutation rendered the drr1 seedlings more sensitive to the primary root growth inhibitory effect of low abscisic acid concentrations than wild-type seedlings (Supplemental Fig. S2B), whereas the ethylene precursor 1-aminocyclopropane-1-carboxylic acid similarly inhibited growth (Supplemental Fig. S2C).
The plant hormone JA plays a key role in the environmental stress responses and developmental processes of plants. A recent report has revealed an important role of JA in LR development. In such work (Sun et al., 2009), it was shown that exogenous MeJA promotes LR formation in Arabidopsis wild-type plants but not in anthranilate synthase1 mutants, leading to the proposal that localized auxin biosynthesis in response to jasmonate could be important for fine-tuned modulation of LR formation. Our detailed morphological comparison among wild-type, drr1, and jar1 plants indicated that, when grown on JA-free medium, LR development in drr1 was significantly lower than in wild-type or jar1 plants. Interestingly, while JA application led to increased LR numbers in wild-type and jar1 plants, it failed to activate LR formation in drr1 (Fig. 11; Supplemental Fig. S1). Comparison of the primary root response to JA also showed that drr1 behaves essentially different from jar1, which was very insensitive to primary root growth inhibition by JA but responded similarly to wild-type plants in LR induction by this compound. Therefore, drr1 shows alkamide resistance in terms of primary and LR growth, whereas it has resistance to jasmonate in LR formation only. Taking into account these results, it is tempting to speculate that further cross talk of alkamide signaling with phytohormones such as cytokinins or jasmonates might vary in different tissues or in a developmental context, possibly explaining why drr1 mutants exhibit no defects in primary root growth inhibition assays to these phytohormones.
In summary, we have provided evidence that alkamide and AHL signaling are under genetic control in Arabidopsis and that normal responses to these signals are important for plant development. Elucidation of the genetic identity of the DRR1 product is critical to understand the molecular mechanisms underlying the distinct effects of these and other small lipid signals on root architecture adjustment and their role in plant longevity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type plants (Col-0 and/or Ws ecotypes), the transgenic line CyCB1:uidA (Colón-Carmona et al., 1999), and the mutant lines jar1 (Tiryaki and Staswick, 2002), axr2-1 (Timpte et al., 1994), and aux1-7 (Picket et al., 1990) were used for all experiments. Seeds were surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach for 7 min. After five washes with sterile distilled water, seeds were germinated and grown on agar plates containing 0.2 × MS medium (Murashige and Skoog, 1962). MS medium (MS basal salts mixture; catalog no. M5524) was purchased from Sigma. The suggested formulation is 4.3 g L−1 salts for a 1 × concentration of medium; we used 0.9 g L−1, which we consider and refer to as 0.2 × MS. This medium lacks amino acids and vitamins. Phytagar (micropropagation grade) was purchased from Phytotechnology. Plants were placed in a plant grown chamber (Percival Scientific AR-95L) with a photoperiod of 16 h of light and 8 h of darkness, light intensity of 100 μ mol m−2 s−1, and temperature of 22°C.
Mutant Isolation Procedure
T-DNA lines (Ws; Krysan et al., 1999) were provided by the Ohio Arabidopsis Seed Stock Center. Seeds were surface sterilized and plated on 0.2 × MS medium supplied with 30 μm N-isobutyl decanamide. A number of approximately 25,000 T-DNA lines were screened for reduced LR formation by placing seeds on nutrient agar plates (20–25 seeds per plate). The seeds were distributed in two rows on the agar surface at a density of one seed per centimeter, stratified at 4°C for 48 h, and then incubated at 22°C. Fourteen days after germination, N-isobutyl decanamide-treated plants have a short primary root and a large number of LRs are formed. Putative mutants with long primary roots and a reduced number of LRs were selected, transferred to soil, and allowed to self-fertilize. Homozygous M3 seeds were rescreened for sustained primary root growth in medium supplied with 30 μm N-isobutyl decanamide, transferred to soil, and backcrossed three times to the wild type (Ws) to remove unlinked mutations.
Genetic Analysis of drr1 Mutants
To determine the segregation pattern of the drr1 phenotype, 990 F2 seedlings derived from the cross drr1 × Ws were analyzed in MS 0.2 × agar medium supplied with 30 μm N-isobutyl decanamide. A typical 3:1 recessive segregation was observed for the wild-type/drr1 phenotype. Cosegregation of primary root growth resistance and increased longevity was further confirmed in drr1 seedlings grown in soil.
Hormone Treatments
For all experiments, MS 0.2 × nutrient medium was supplemented with N-isobutyl decanamide, C10-AHL, or the indicated phytohormones. Ethanol-dissolved compounds were added to cooled (50°C) molten medium and poured onto plates. Control plates were supplied with the greatest concentration of solvent used in the treatments. For hormone-induced senescence, leaves at 22 d after leaf emergence were detached and floated on sterilized water in the presence or absence of 35 μm JA or 35 μm N-isobutyl decanamide for 6 d. All treatments were performed at 22°C under dark conditions. Chemicals were purchased from Sigma Chemical.
Analysis of Growth and Statistical Analysis
Growth of primary roots was registered using a rule. LR number and LR density were determined by counting the LRs present in the primary root from the tip to the root/stem transition. LR density was determined by dividing the LR number by the primary root length and expressed as LR density per centimeter. The length of the meristem was determined as the distance between the quiescent center and the cell file where cells started to elongate. For all experiments, the overall data were statistically analyzed in the SPSS 10 program (SPSS). Univariate and multivariate analyses with a Tukey's posthoc test were used for testing differences in growth and root developmental responses in wild-type and mutant lines. Different letters are used to indicate means that differ significantly (P < 0.05).
Determination of Developmental Stages of LRP
LRP were quantified at day 4 after germination. Seedling roots were first cleared to enable LRP at early stages of development to be visualized and counted. Each LR primordium was classified according to its stage of development as reported by Malamy and Benfey (1997). The developmental stages are as follows. Stage I, LRP initiation; in the longitudinal plane, approximately eight to 10 “short” pericycle cells are formed. Stage II, the formed LR primordium is divided into two layers by a periclinal division. Stage III, the outer layer of the primordium divides periclinally, generating a three-layer primordium. Stage IV, LR primordium with four cell layers. Stage V, the LR primordium is midway through the parent cortex. Stage VI, the LR primordium has passed through the parent cortex layer and has penetrated the epidermis. It begins to resemble the mature root tip. Stage VII, the LR primordium appears to be just about to emerge from the parent root.
Chlorophyll Determination
We used leaves from wild-type (Ws) and drr1 plants germinated and grown on 0.2 × MS medium and then transferred to soil for 35 d. Wild-type leaves were yellowed as a result of age-dependent senescence; drr1 leaves remained green at this stage. We used a hand-held chlorophyll meter (CCM-200; Opti-Sciences) to calculate a chlorophyll content index based on absorbance measurements at 660 and 940 nm on 15 independent leaves. Five separate measurements with the hand-held meter were made on each leaf. Chlorophyll content was finally determined as described previously (Richardson et al., 2002).
Histochemical Analysis of GUS Activity
Transgenic plants that express the uidA reporter gene (Jefferson et al., 1987) were stained in 0.1% 5-bromo-4-chlorium-3-indolyl- β -d-glucuronide in phosphate buffer (NaH2PO4 and Na2HPO4, 0.1 m, pH 7) with 2 mm potassium ferrocyanide and 2 mm potassium ferricyanide for 12 h at 37°C. Plants were cleared and fixed as described previously by Malamy and Benfey (1997). The processed roots were included in glass slips and sealed with commercial nail varnish. For each marker line and for each treatment, at least 10 transgenic plants were analyzed.
Microscopy
The Arabidopsis root system was analyzed with a stereoscopic microscope (MZ6; Leica Microsystems). Total LRs were counted at 30 × magnification. Primary root meristems were analyzed in semipermanent preparations of cleared roots using a composed microscope (Axiostar Zeiss Plus; Carl Zeiss) at 100 × or 400 × magnification. Images were captured with a Cyber-shot DSC-S75 digital camera (Sony Electronics) adapted to the microscope and processed with the Axio Vision 4AC software (Carl Zeiss).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. drr1 shows defective LR formation in response to JA treatment.
Supplemental Figure S2. Effects of kinetin, abscisic acid, and ethylene on primary root growth in wild-type and drr1 plants.
Acknowledgments
We thank Peter Doerner, Angel Arturo Guevara-García, and Plinio Guzmán for kindly providing us with seeds of transgenic and mutant lines. We gratefully acknowledge Jorge Molina-Torres and Enrique Ramírez-Chávez for kindly providing us N-isobutyl decanamide.
References
- Boerjan W, Cervera MT, Delarue M, Beeckman T, DeWitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagú M, Inzé D. (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Cuevas JC, Pelagio-Flores R, Raya-González J, Méndez-Bravo A, Ortiz-Castro R, López-Bucio J. (2008) Tissue culture of Arabidopsis thaliana explants reveals a stimulatory effect of alkamides on adventitious root formation and nitric oxide accumulation. Plant Sci 174: 165–173 [Google Scholar]
- Casimiro I, Beekman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett M. (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR. (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Cha C, Gao P, Chen YC, Shaw PD, Farrand SK. (1998) Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant Microbe Interact 11: 1119–1129 [DOI] [PubMed] [Google Scholar]
- Chapman KD. (2004) Occurrence, metabolism, and prospective functions of N-acylethanolamines in plants. Prog Lipid Res 43: 309–327 [DOI] [PubMed] [Google Scholar]
- Colón-Carmona A, You R, Haimovich-Gal T, Doerner P. (1999) Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20: 503–508 [DOI] [PubMed] [Google Scholar]
- D'Angelo-Picard C, Faure D, Penot I, Dessaux Y. (2005) Diversity of N-acyl homoserine lactone-producing and -degrading bacteria in soil and tobacco rhizosphere. Environ Microbiol 7: 1796–1808 [DOI] [PubMed] [Google Scholar]
- De Smet I, Vaneste S, Inzé D, Beeckman T. (2006) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60: 871–887 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. (1993) Cellular organization of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Rost TL, Colón-Carmona A, Doerner P. (2001) Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta 214: 30–36 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucially-Mendivil S, Ivanchenko MG, Friml J, Shiskova S, Celenza J, Benkova E. (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elasri M, Delorme S, Lemanceau P, Steward G, Laue B, Glickmann E, Oger PM, Dessaux Y. (2001) Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soil borne Pseudomonas spp. Appl Environ Microbiol 7: 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Okushima Y, Tasaka M. (2007) Auxin-mediated lateral root formation in higher plants. Int Rev Cytol 256: 111–137 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M. (2002) Lateral root formation is blocked by a gain-of-function mutation in the solitary root/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69: 383–396 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putrill J, Palme K, Estelle M, Chory J. (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vaneste S, de Almedida-Engler J, Inzé D, Beeckman T. (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. (1994) Genetic approaches to auxin action. Plant Cell Environ 17: 525–540 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusion: β -glucuronidase as a sensitive and versatile fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmel IA, Veselova MA, Metlitskaya AZ, Klein S, Lipasova VA, Mayatskaya AV, Chernin LS. (2002) Synthesis of signaling N-acyl-homoserine-lactone participating in quorum sensing regulation in rhizospheric and soil-borne bacteria Pseudomonas and Xanthomonas. Russ J Genet 38: 467–469 [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. (1995) A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7: 2023–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR. (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 12: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue BE, Jiang Y, Chlabra SR, Jacob S, Stewart GS, Hardman A, Downie JA, O'Gara F, Williams P. (2000) The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin N-(3-hydroxy-7-cis-tetradecenoyl-homoserine lactone, via HdtS, a putative novel N-acyl-homoserine lactone synthase. Microbiology 146: 2469–2480 [DOI] [PubMed] [Google Scholar]
- Laurerio-Rosario S, Silva A, Parente J. (1996) Alkamides from Cissampelos glaberrima. Planta Med 62: 376–377 [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. (1998) Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282: 943–946 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. (1990) Growth and development of the axr1 mutant of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Acevedo-Hernández G, Ramírez-Chávez E, Molina-Torres J, Herrera-Estrella L. (2006) Novel signals for plant development. Curr Opin Plant Biol 9: 523–529 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Pérez-Torres A, Ramírez-Pimentel JG, Sánchez-Calderón L, Herrera-Estrella L. (2005) Root architecture. Turnbull C, , Plant Architecture and Its Manipulation. Blackwell Annual Review Series; Blackwell, Oxford, pp 181–206 [Google Scholar]
- López-Bucio J, Millán-Godínez M, Méndez-Bravo A, Morquecho-Contreras A, Ramírez-Chávez E, Molina-Torres J, Pérez-Torres A, Higuchi M, Kakimoto T, Herrera-Estrella L. (2007) Cytokinin receptors are involved in alkamide regulation of root and shoot development in Arabidopsis. Plant Physiol 145: 1703–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JF. (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28: 67–77 [DOI] [PubMed] [Google Scholar]
- Malamy JF, Benfey PN. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Marketon MM, Gronquist MR, Eberhard A, González JE. (2002) Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J Bacteriol 184: 5686–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao MS, Teplitski M, Caetano-Anoles G, Rolfe BG, Bauer WD. (2003) Extensive and specific response of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA 100: 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquecho-Contreras A, López-Bucio J. (2007) Cannabinoid-like signaling and other new developmental pathways in plants. Int J Plant Dev Biol 1: 34–41 [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Nibau C, Gibbs DJ, Coates JC. (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179: 595–614 [DOI] [PubMed] [Google Scholar]
- Ortíz-Castro R, Martínez-Trujillo M, López-Bucio J. (2008) N-Acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31: 1497–1509 [DOI] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. (1998) Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet 19: 171–174 [DOI] [PubMed] [Google Scholar]
- Parsek MR, Val DL, Hanzelka BL, Cronan JE, Greenberg EP. (1999) Acyl-homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci USA 96: 4360–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91: 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JP, Van Delden C, Iglewski BH. (1999) Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol 181: 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, de Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett M. (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Picket FB, Wilson AK, Estelle M. (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Chávez E, López-Bucio J, Herrrera-Estrella L, Molina-Torres J. (2004) Alkamides isolated from plants promote growth and alter root development in Arabidopsis. Plant Physiol 134: 1058–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading NC, Sperandio V. (2006) Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett 254: 1–11 [DOI] [PubMed] [Google Scholar]
- Richardson AD, Duigan SP, Berlyn GP. (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153: 185–194 [Google Scholar]
- Ríos-Chávez P, Ramírez-Chávez E, Armenta-Salinas C, Molina-Torres J. (2003) Acmella radicans var. radicans: in vitro culture establishment and alkamide content. In Vitro Cell Dev Biol Plant 39: 37–41 [Google Scholar]
- Rogg LE, Lasswell J, Bartel B. (2001) A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 13: 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommer C, Palatnik J, Aggarwal P, Chetelat A, Cubas P, Farmer E, Nath U, Weigel D. (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6: 1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmid M, Van Breusegem F, Eber L, et al. (2006) Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29: 909–918 [DOI] [PubMed] [Google Scholar]
- Scott RA, Well J, Le PT, Williams P, Fray RG, Von Bodmann SB, Savka MA. (2006) Long-and-short chain plant-produced bacterial N-acyl-homoserine lactones become components of phyllosphere, rhizosphere and soil. Mol Plant Microbe Interact 19: 227–239 [DOI] [PubMed] [Google Scholar]
- Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, et al. (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21: 1495–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15: 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga ME, Bassler BL. (2003) Chemical communication among bacteria. Proc Natl Acad Sci USA 100: 14549–14554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitski M, Eberhard A, Gronquist MR, Gao M, Robinson JB, Bauer W. (2003) Chemical identification of N-acyl homoserine lactone quorum-sensing signals produced by Sinorhizobium meliloti strains in defined medium. Arch Microbiol 180: 494–497 [DOI] [PubMed] [Google Scholar]
- Timpte C, Wilson AK, Estelle M. (1994) The axr2-1 mutation of Arabidopsis thaliana is a gain-of-function mutation that disrupts an early step in auxin response. Genetics 138: 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiryaki I, Staswick P. (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeki R, Fedoroff NV. (1999) Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA 96: 12941–12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi V. (2006) Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30: 274–291 [DOI] [PubMed] [Google Scholar]
- Von Rad U, Klein I, Dobrev PI, Kottova J, Zazimalova E, Fekete A, Hartmann A, Schmitt-Kopplin P, Durner J. (2008) Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229: 73–85 [DOI] [PubMed] [Google Scholar]
- Wang X. (2004) Lipid signaling. Curr Opin Plant Biol 7: 329–336 [DOI] [PubMed] [Google Scholar]
- Wang YS, Shresta R, Kilaru A, Wiant W, Enables BJ, Chapman KD, Blancaflor E. (2006) Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines. Proc Natl Acad Sci USA 103: 12197–12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. (1990) A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet 222: 377–383 [DOI] [PubMed] [Google Scholar]
- Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG. (2001) ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13: 1779–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HR, Goh CH, Park JH, Teyssendier B, Kim JH, Park YI, Nam HG. (2002) Extended leaf longevity in the ore4-1 mutant of Arabidopsis with a reduced expression of a plastid ribosomal protein gene. Plant J 31: 331–340 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action and interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall D, Ng CKY, Hetherington AM. (2003) Sphingolipids, new players in plant signaling. Trends Plant Sci 8: 317–320 [DOI] [PubMed] [Google Scholar]
- Wymann MP, Schneiter R. (2008) Lipid signaling in disease. Nat Rev Mol Cell Biol 9: 163–176 [DOI] [PubMed] [Google Scholar]