Abstract
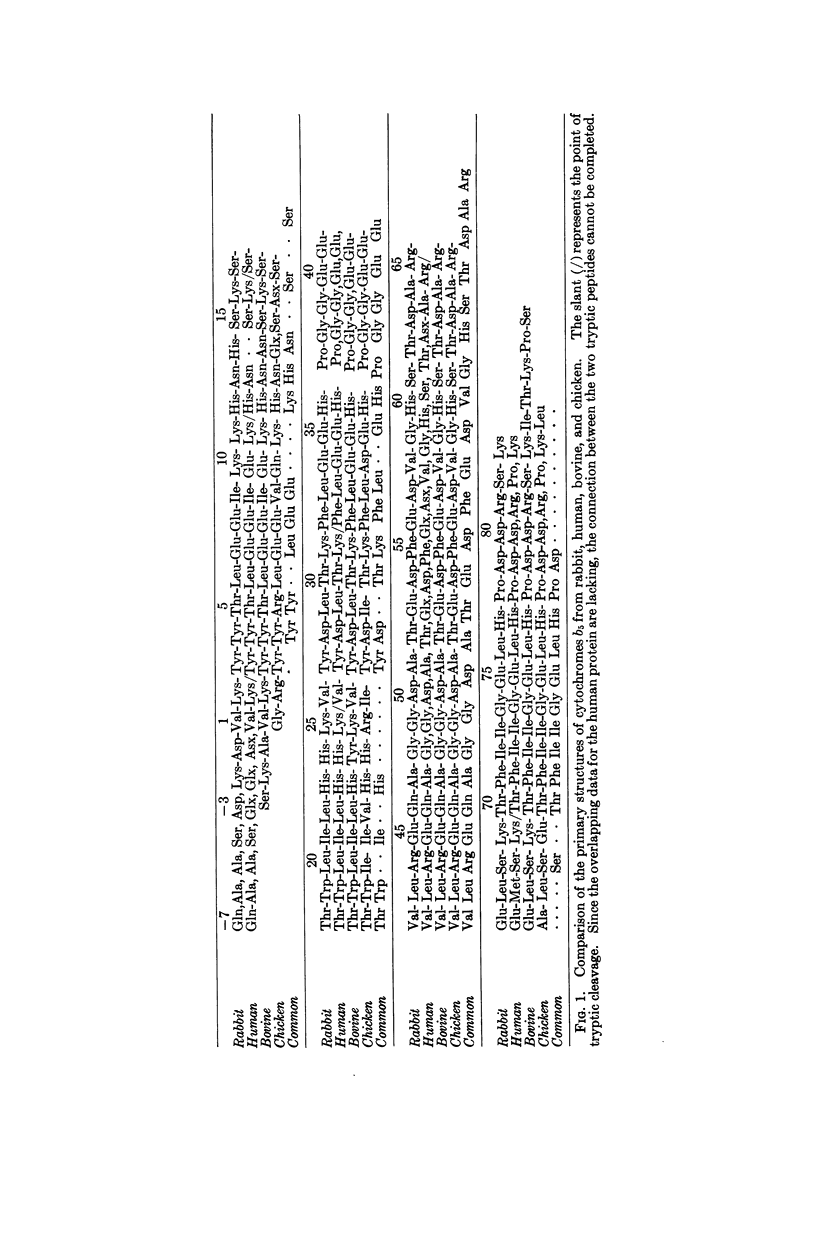
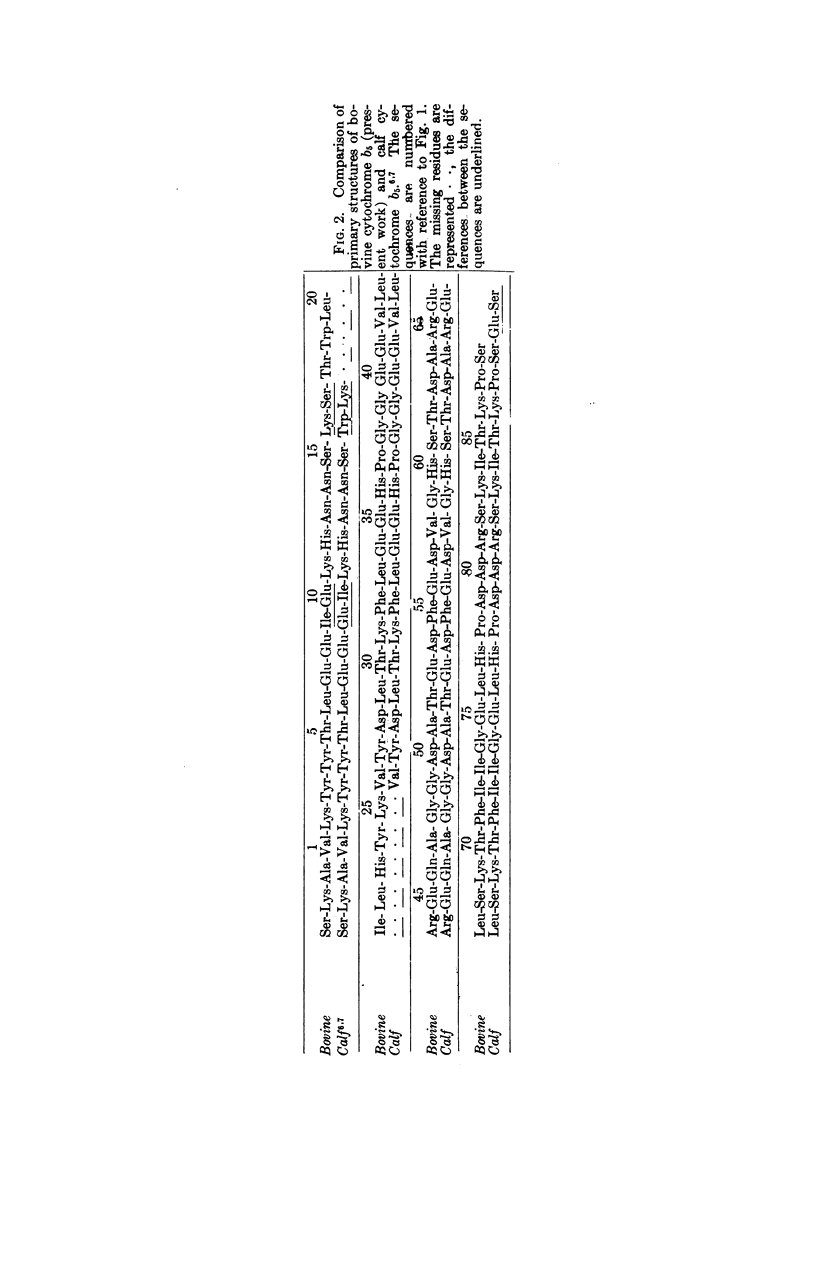
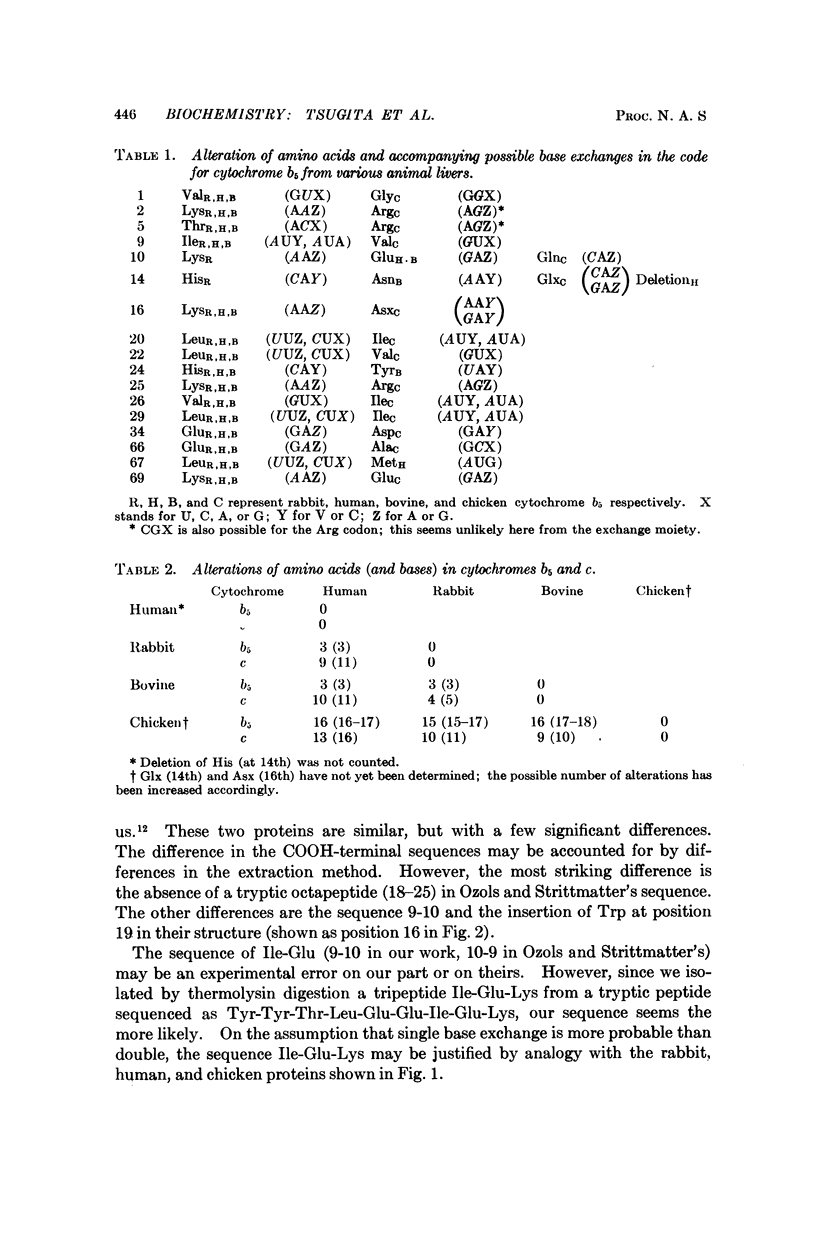
The primary structures of human, bovine, and chicken cytochrome b5 have been determined and compared with that of the previously studied rabbit protein. One peptide containing 31 amino acid residues and another containing 10 were found common to all four species. The substitutions of amino acids between species could be accounted for mainly by single base exchange, with a few exceptional double base exchanges for the chicken. Results for bovine cytochrome b5 differ significantly from those previously reported for calf cytochrome b5.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan S. K., Margoliash E. Amino acid sequence of chicken heart cytochrome c. J Biol Chem. 1966 Jan 25;241(2):507–515. [PubMed] [Google Scholar]
- Crick F. H. The genetic code--yesterday, today, and tomorrow. Cold Spring Harb Symp Quant Biol. 1966;31:1–9. [PubMed] [Google Scholar]
- GERALD P. S., EFRON M. L. Chemical studies of several varieties of Hb M. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1758–1767. doi: 10.1073/pnas.47.11.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDREW J. C. Myoglobin and the structure of proteins. Science. 1963 Mar 29;139(3561):1259–1266. doi: 10.1126/science.139.3561.1259. [DOI] [PubMed] [Google Scholar]
- Kajihara T., Hagihara B. Crystalline cytochrome b5. I. Preparation of crystalline cytochrome b5 from rabbit liver. J Biochem. 1968 Apr;63(4):453–461. doi: 10.1093/oxfordjournals.jbchem.a128797. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Hagihara B., Tsugita A. Crystallographic study of rabbit liver cytochrome b5. Biochim Biophys Acta. 1970 Feb 17;200(2):421–422. doi: 10.1016/0005-2795(70)90188-1. [DOI] [PubMed] [Google Scholar]
- MATSUBARA H., SMITH E. L. HUMAN HEART CYTOCHROME C. CHYMOTRYPTIC PEPTIDES, TRYPTIC PEPTIDES, AND THE COMPLETE AMINO ACID SEQUENCE. J Biol Chem. 1963 Aug;238:2732–2753. [PubMed] [Google Scholar]
- Mathews F. S., Strittmatter P. Crystallographic study of calf liver cytochrome b5. J Mol Biol. 1969 Apr;41(2):295–297. doi: 10.1016/0022-2836(69)90394-5. [DOI] [PubMed] [Google Scholar]
- Nakashima T., Higa H., Matsubara H., Benson A. M., Yasunobu K. T. The amino acid sequence of bovine heart cytochrome c. J Biol Chem. 1966 Mar 10;241(5):1166–1177. [PubMed] [Google Scholar]
- Needleman S. B., Margoliash E. Rabbit heart cytochrome c. J Biol Chem. 1966 Feb 25;241(4):853–863. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. Correction of the amino acid sequence of calf liver microsomal cytochrome b5. J Biol Chem. 1969 Dec 25;244(24):6617–6618. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The amino acid sequence of cytochrome b-5. J Biol Chem. 1968 Jun 25;243(12):3376–3381. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The amino acid sequence of the truptic peptides from cytochrome b5. J Biol Chem. 1968 Jun 25;243(12):3367–3375. [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The homology between cytochrome B-5, hemoglobin, and myoglobin. Proc Natl Acad Sci U S A. 1967 Jul;58(1):264–267. doi: 10.1073/pnas.58.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. The reactivity of the lysyl residues of cytochrome b5. J Biol Chem. 1966 Oct 25;241(20):4793–4797. [PubMed] [Google Scholar]
- PERUTZ M. F. X-ray analysis of hemoglobin. Science. 1963 May 24;140(3569):863–869. doi: 10.1126/science.140.3569.863. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Pure native globin from human hemoglobin: preparation and some physico-chemical properties. Biochim Biophys Acta. 1958 Apr;28(1):221–221. doi: 10.1016/0006-3002(58)90462-1. [DOI] [PubMed] [Google Scholar]
- STRITTMATTER P. The nature of the heme binding in microsomal cytochrome b5. J Biol Chem. 1960 Aug;235:2492–2497. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. The isolation and properties of microsomal cytochrome. J Biol Chem. 1956 Jul;221(1):253–264. [PubMed] [Google Scholar]
- Tsugita A., Kobayashi M., Kajihara T., Hagihara B. Primary structure of rabbit liver cytochrome b5. J Biochem. 1968 Nov;64(5):727–730. doi: 10.1093/oxfordjournals.jbchem.a128954. [DOI] [PubMed] [Google Scholar]



