Abstract
Oxidative injury of the root elongation zone is a primary event in aluminum (Al) toxicity in plants, but the injuring species remain unidentified. We verified the hypothesis that lipid peroxide-derived aldehydes, especially highly electrophilic α,β-unsaturated aldehydes (2-alkenals), participate in Al toxicity. Transgenic tobacco (Nicotiana tabacum) overexpressing Arabidopsis (Arabidopsis thaliana) 2-alkenal reductase (AER-OE plants), wild-type SR1, and an empty vector-transformed control line (SR-Vec) were exposed to AlCl3 on their roots. Compared with the two controls, AER-OE plants suffered less retardation of root elongation under AlCl3 treatment and showed more rapid regrowth of roots upon Al removal. Under AlCl3 treatment, the roots of AER-OE plants accumulated Al and H2O2 to the same levels as did the sensitive controls, while they accumulated lower levels of aldehydes and suffered less cell death than SR1 and SR-Vec roots. In SR1 roots, AlCl3 treatment markedly increased the contents of the highly reactive 2-alkenals acrolein, 4-hydroxy-(E)-2-hexenal, and 4-hydroxy-(E)-2-nonenal and other aldehydes such as malondialdehyde and formaldehyde. In AER-OE roots, accumulation of these aldehydes was significantly less. Growth of the roots exposed to 4-hydroxy-(E)-2-nonenal and (E)-2-hexenal were retarded more in SR1 than in AER-OE plants. Thus, the lipid peroxide-derived aldehydes, formed downstream of reactive oxygen species, injured root cells directly. Their suppression by AER provides a new defense mechanism against Al toxicity.
Aluminum (Al) is the most abundant metal in the earth's crust and is a major factor limiting plant growth and productivity in acid soils, which cover about 50% of the world's potentially arable land surface (Kochian, 1995; Kochian et al., 2004). The primary site of Al accumulation and toxicity is the root meristem, and inhibition of root elongation is the most notable symptom of Al toxicity (Delhaize and Ryan, 1995; Yamamoto et al., 2003). Al causes various adverse effects, such as disruption of signal transduction pathways, inhibition of cell division and ion fluxes, disruption of cytoskeletal dynamics, induced generation of reactive oxygen species (ROS), and disturbance of plasma membrane stability and function (Jones and Kochian, 1995; Blancaflor et al., 1998; Yamamoto et al., 2001, 2002; Kochian et al., 2004; Ma et al., 2007). Of all these toxic effects, the generation of ROS is observed rapidly and sustainably in roots after Al exposure. Al-induced generation of ROS has been shown in maize (Zea mays) and Allium cepa roots (Jones et al., 2006; Achary et al., 2008). Tahara et al. (2008) showed that ROS generated to a greater degree in Al-sensitive species than in Al-tolerant species. Yamamoto et al. (2002, 2003) have shown a correlation between ROS level and inhibition of growth capacity in cultured tobacco (Nicotiana tabacum) cells. Furthermore, ROS generation increases with increasing Al concentration (Achary et al., 2008; Xue et al., 2008). Generation of ROS appears to be a cause, rather than a result, of Al-induced cell injury, because high ROS scavenging ability resulted in enhanced Al tolerance (Devi et al., 2003; Ezaki et al., 2008). In addition, overexpression of genes encoding antioxidant enzymes (peroxidase and superoxide dismutase) conferred Al tolerance to the transgenic plants (Ezaki et al., 2000; Basu et al., 2001). Thus, ROS appears to be the primary factors that cause growth inhibition in Al-stressed roots.
Downstream of ROS generation, lipid peroxidation is a common symptom of Al toxicity (Yamamoto et al., 2001), and it increases with increasing Al concentration (Achary et al., 2008). From animal cell studies, it is now recognized that the toxicity of lipid peroxide (LOOH) is largely ascribable to LOOH-derived aldehydes. In particular, α,β-unsaturated aldehydes, such as 4-hydroxy-(E)-2-nonenal (HNE) and acrolein, are strong electrophiles and readily modify proteins and nucleic acids (Esterbauer et al., 1991; Taylor et al., 2002; O'Brien et al., 2005; Møller et al., 2007). HNE causes depletion of glutathione, a decrease in protein thiols, disturbance of calcium homeostasis, inhibition of DNA, RNA, and protein synthesis, lactate release, morphological changes of cells, and finally leading to cell death (Esterbauer et al., 1991; Burcham, 1998). Increase of HNE has been observed in a wide range of human diseases, including Alzheimer's disease, Parkinson's disease, and mitochondrial complex 1 deficiency (Poli and Schaur, 2000).
In plants, too, a close correlation between the level of LOOH-derived aldehydes (determined as thiobarbituric acid-reactive substances [TBARS]) and cellular damage has been shown under environmental stresses caused by heat, chilling, UV-B radiation, salinity, heavy metals, and Al (Ma et al., 2007; Ezaki et al., 2008). Their involvement in cellular damage has been demonstrated by the protective effects of the aldehyde-scavenging enzymes aldehyde dehydrogenase (Sunkar et al., 2003; Kotchoni et al., 2006) and aldehyde reductase (Oberschall et al., 2000; Hideg et al., 2003; Hegedüs et al., 2004) to confer tolerance against various environmental stresses when they were overexpressed in plants. In barley (Hordeum vulgare) roots, the formation of HNE in association with Al treatment was observed (Sakihama and Yamasaki, 2002). Occurrence of HNE in Arabidopsis (Arabidopsis thaliana) leaves under oxidative stress has been also deduced by detection of modified proteins in the mitochondria (Winger et al., 2007). HNE rapidly inhibited respiration in isolated potato (Solanum tuberosum) mitochondria by inactivating pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase, NAD-malic enzyme (Millar and Leaver, 2000), and alternative oxidase (Winger et al., 2005). HNE and other 2-alkenals also inactivated photosynthesis in isolated chloroplasts (Mano et al., 2009). Arabidopsis contains 2-alkenal reductase (AER; E.C. 1.3.1.74) that catalyzes the reduction of the α,β-unsaturated bond of 2-alkenals to produce n-alkanals (Mano et al., 2002). Overexpression of AER in tobacco (Mano et al., 2005) and in Arabidopsis (Papdi et al., 2008) improved the tolerance to photooxidative stress and NaCl stress, respectively. Thus, accumulated observation indicates that LOOH-derived aldehydes, especially 2-alkenals, are commonly involved in oxidative damage in plant cells. Considering the critical importance of ROS in Al toxicity to roots, it is expected that 2-alkenals are produced and mediate damage in the stressed root cells.
To evaluate the roles of LOOH-derived aldehydes in root injury under Al stress, we employed transgenic tobacco plants that overexpress the AER gene (AER-OE plants; Mano et al., 2005). With Al treatment, the roots of AER-OE accumulated Al and H2O2 to the same levels as those of the wild type, but they showed resistance to inhibition of elongation. Aldehyde analysis revealed that the Al treatment increased the contents of several toxic aldehydes, including HNE and acrolein in wild-type plants, but these aldehydes were significantly suppressed in the AER-OE plants. On the basis of these results, we propose that the inhibition of root growth by Al ions is induced by toxic aldehydes generated with ROS.
RESULTS
AER-OE Plants Show Al Tolerance
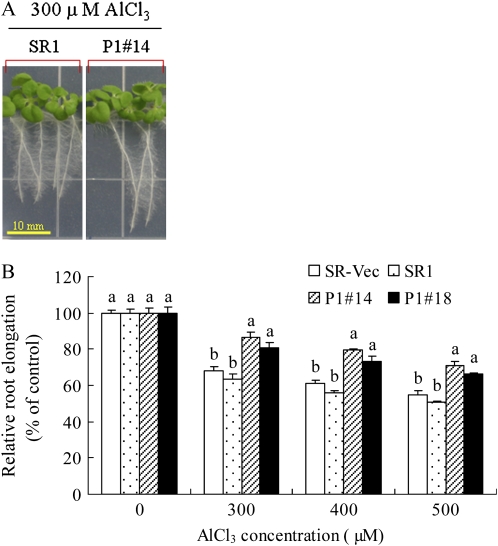
To examine whether AER overexpression improves Al tolerance in tobacco plants, root elongation was compared between the AER-OE lines (P1#14 and P1#18) and the two control lines (SR1 and SR-Vec). All lines showed similar root growth on Murashige and Skoog (MS) agar plates in the absence of AlCl3. When AlCl3 was added to the plates, root growth of the seedlings was inhibited; this inhibition increased with increasing Al concentration (Fig. 1). Notably, the inhibition was more severe in SR1 and SR-Vec plants than in AER-OE plants. When exposed to 300 μm AlCl3, the SR1 and SR-Vec plants showed a 35% decrease in root elongation, whereas AER-OE plants showed only a 16% reduction. Following 400 and 500 μm AlCl3 exposure, SR1 and SR-Vec plants showed 40% and 46% inhibition of root elongation, whereas in AER-OE plants, the inhibition was 20% and 28%, respectively. Thus, with respect to the root elongation, the AER-OE plants showed tolerance to Al.
Figure 1.
A, Root growth of SR1 and AER-OE line (P1#14). Seeds were grown for 14 d on one-sixth-strength MS agar plate (pH 4.2) containing 300 μm AlCl3. No difference in root elongation among SR and AER-OE lines was found without AlCl3 treatment. B, Effect of increasing AlCl3 concentration on root elongation. Seeds were grown on one-sixth-strength MS medium (pH 4.2) containing 0, 300, 400, or 500 μm AlCl3. Root length was measured after 14 d. Root elongation values at different levels of AlCl3 were represented as percentages of the values observed without AlCl3. Data are means ± se of three replications (each replication included 10 plants). Values followed by the same letter in the same AlCl3 concentration are not significantly different according to Tukey-Kramer test (P < 0.05).
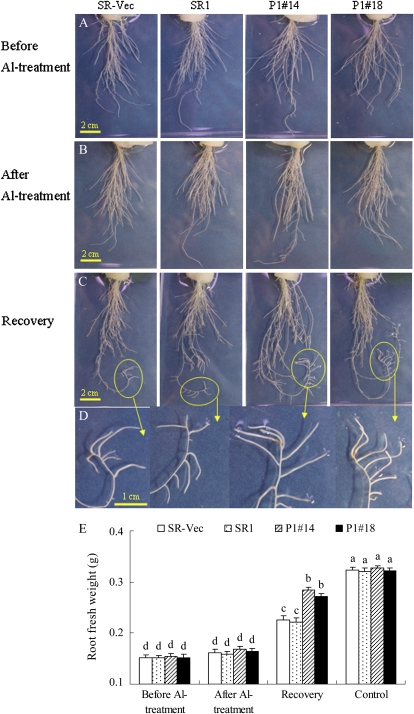
To evaluate the recovery of root growth after Al stress, hydroponically cultured plants were transiently treated with AlCl3 and then transferred to Al-free conditions. After treatment with 500 μm AlCl3 for 24 h, the root fresh weight per plant showed no difference among lines. After 3 d of recovery, the root fresh weight of AER-OE plants was 130% of that of SR1 and SR-Vec plants, indicating the less damage and quick recovery of AER-OE plants from Al stress (Fig. 2E). Furthermore, after recovery, several new, white adventitious roots emerged, and these new roots grew longer and thicker in AER-OE plants than in SR1 and SR-Vec plants (Fig. 2, C and D).
Figure 2.
Root morphology (A–D) and weight (E) of SR-Vec, SR1, and AER-OE lines (P1#14 and P1#18). Seeds were sown on MS agar plates, and the seedlings were cultured for 28 d and then transplanted into hydroponic medium and cultured for another 28 d. Seedlings were treated with 500 μm AlCl3 in one-sixth-strength HS for 24 h and then cultured in Al-free well aerated one-sixth-strength HS for 3 d to recover. Root morphology was recorded before (A) and after (B) Al treatment and 3 d after removal of AlCl3 (C and D). For fresh weight determination (E), roots were collected from the plants either before or after Al treatment, after the 3-d recovery, or without Al treatment. Data are means ± se (n = 8). Values followed by the same letter are not significantly different according to Tukey-Kramer (P < 0.05).
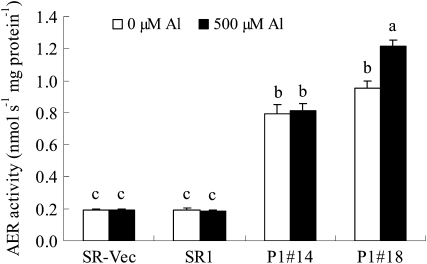
AER-OE plants showed higher AER activities in the roots compared with SR1 and SR-Vec plants (Fig. 3). AER activity in the roots of AER-OE plants was 400% to 600% of that in the SR1 and SR-Vec plants, irrespective of AlCl3 treatment. These results indicated that AER overexpression released from the Al-induced retardation of root growth.
Figure 3.
Activity of AER in roots. Seedlings were grown on MS agar plates for 28 d and then in hydroponic medium for 28 d. Seedlings were treated with or without 500 μm AlCl3 for 24 h. Proteins were extracted from the roots, and AER activity in the extract was determined as described in “Materials and Methods.” Data are means ± se (n = 3). Values followed by the same letter are not significantly different according to Tukey-Kramer test (P < 0.05).
AER-OE Plants Accumulate Al and H2O2
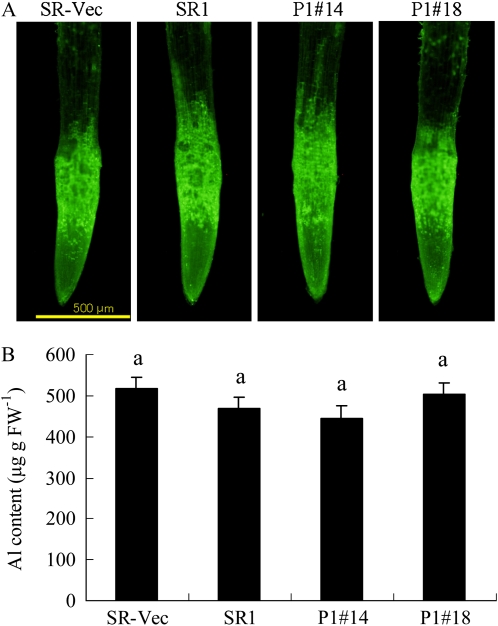
We then examined whether overexpression of AER affected Al accumulation and distribution in the roots. Localization of Al ions in the roots was determined with the fluorescent probe morin (Tice et al., 1992), which has a high specificity for Al3+ and is used widely to detect Al in plant tissues (Larsen et al., 1996; Jones et al., 2006; Ezaki et al., 2007). Roots without AlCl3 exposure showed no fluorescence (data not shown). Following exposure to 500 μm AlCl3 for 24 h, a marked increase in Al-induced morin fluorescence was observed, particularly in the region of 0 to 1 mm from the root tip (Fig. 4A). All the Al-treated plants showed an intense fluorescence signal in the root tips, and there was no difference among the SR1, SR-Vec, and AER-OE plants. The Al content in the root tips (0–10 mm), as determined with a plasma atomic emission spectrometer, also showed no difference among SR1, SR-Vec, and AER-OE plants (Fig. 4B). Thus, accumulation and distribution of Al in the roots were not affected by overexpression of AER.
Figure 4.
Al distribution and accumulation in roots. Seedlings were grown on MS agar plates for 28 d and then in hydroponic medium for 28 d. Seedlings were treated with or without 500 μm AlCl3 for 24 h, and then the roots were stained with morin (A). No fluorescence was observed in the roots prior to Al treatment. Al content in the root tips (0–10 mm) was measured by an inductively coupled plasma atomic emission spectrometer (B) as described in “Materials and Methods.” Data are means ± se (n = 3). Values followed by the same letter are not significantly different according to Tukey-Kramer test (P < 0.05).
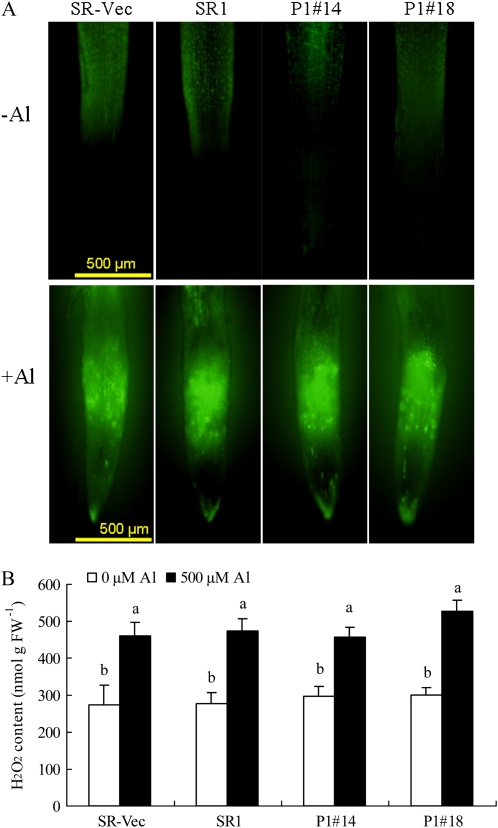
To evaluate ROS production in the roots, we used 2 ′ ,7′-dichlorofluorescein diacetate (DCF-DA) fluorescence, which indicates H2O2 accumulation. DCF-DA fluorescence was similarly low without AlCl3 treatment, and it was markedly increased by AlCl3 treatment in root apex, especially at the elongation zone (Fig. 5A). The increased levels and the position of H2O2 formation appeared to be similar among the four lines. Quantitative analysis of H2O2 in root tips (0–20 mm) by iodide oxidation assay confirmed that H2O2 content was increased by AlCl3 treatment (Fig. 5B); the levels did not differ among all the lines either before or after the treatment. These results showed that the Al-tolerant AER-OE lines accumulated Al and subsequently produced H2O2 at the root apex, to the same extent as the Al-sensitive control lines. In addition, overexpression of AER did not affect ROS-scavenging enzyme activities (SOD, APX, and catalase; Mano et al., 2005). Therefore, the tolerance of the AER-OE lines is attributable to a difference in some factor(s) downstream of ROS production.
Figure 5.
Distribution and accumulation of H2O2 in roots. Seedlings were grown on MS agar plates for 28 d and then in hydroponic medium for 28 d. Seedlings were treated with or without 500 μm AlCl3 for 24 h. Roots were treated with DCF-DA (A) or used for determination of H2O2 content in the tip regions (0–20 mm) by iodide oxidation (B) as described in “Materials and Methods.” Data are means ± se (n = 3). Values followed by the same letter are not significantly different according to Tukey-Kramer test (P < 0.05).
Differential Aldehyde Levels Are Correlated with Differences in Cell Death
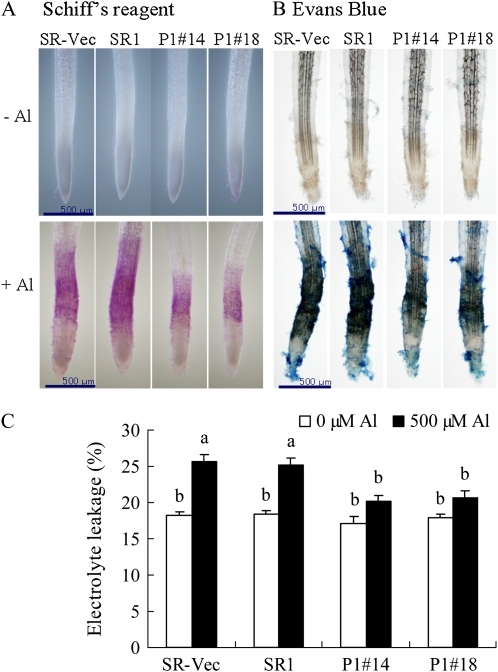
LOOH-derived aldehydes in plant tissues can be visualized with Schiff's reagent by the development of a pink dye (Yamamoto et al., 2001; Han et al., 2008). Without AlCl3 treatment, pink staining was barely observed in any of the lines (Fig. 6A). After exposure to AlCl3 for 24 h, the roots were clearly pink, mainly around the elongation zone, indicating aldehydes were produced at the site same to H2O2. The roots of AER-OE plants appeared a paler pink than those of SR1 and SR-Vec plants, indicating lower aldehyde contents in the former.
Figure 6.
Lipid peroxidation and membrane injury in roots. Seedlings were grown on MS agar plates for 28 d and then in hydroponic medium for 28 d. A and B, Seedlings were treated with or without 500 μm AlCl3 for 24 h and then stained with Schiff's reagent to visualize lipid peroxidation (A) or with Evans blue to detect cell death (B) as described in “Materials and Methods.” C, Electrolyte leakage. Data are means ± se (n = 3). Values followed by the same letter are not significantly different according to Tukey-Kramer test (P < 0.05).
Evans blue staining showed that extensive cell death was induced by AlCl3 treatment around the roots, and especially at the elongation zone (Fig. 6B), as reported previously (Yamamoto et al., 2001). As with the results of Schiff's reagent staining, stronger Evans blue staining was observed in the SR1 and SR-Vec lines than in the AER-OE lines. Electrolyte leakage assay confirmed that the membrane injury due to AlCl3 treatment was significantly suppressed in the AER-OE plants than in the SR1 and SR-Vec plants (Fig. 6C). These results revealed a close correspondence between Al-induced damage of the root and aldehyde accumulation therein.
Specific Aldehydes Are Suppressed in AER-OE Plants
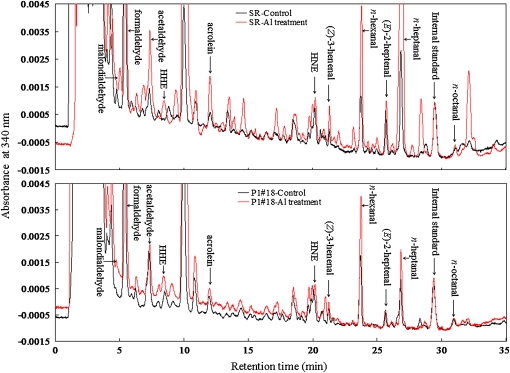
Individual aldehydes in the roots were identified and quantified in a reverse-phase HPLC after derivatization with 2,4-dinitrophenylhydrazine. Typical chromatograms for SR1 and P1#18 with and without AlCl3 treatment are shown in Figure 7. It was found that tobacco roots contained various aldehydes at considerable levels, even in the absence of Al stress, and some of them were increased by AlCl3 treatment. Aldehyde contents of SR1, P1#14, and P1#18 plants are summarized in Supplemental Tables S1 and S2. We distinguished 35 peaks of aldehydes, on their relative retention time as compared with that of the internal standard 2-ethyl-hexanal. In the absence of Al stress, the aldehyde contents of the roots did not differ significantly among SR1 and the two AER-OE lines. The most abundant aldehyde was formaldehyde (approximately 50 nmol g fresh weight [FW]−1) and the second were malondialdehyde and n-heptanal (2–4 nmol g FW−1). In addition, more reactive 2-alkenals, such as HNE, acrolein, and 4-hydroxy-(E)-2-hexenal (HHE), were present at approximately 1 nmol g FW−1. These values can be regarded as the basal physiological levels of these aldehydes (see “Discussion”).
Figure 7.
Typical chromatograms of DNP derivatives of aldehydes in the root tips (0–20 mm) of SR1 and P1#18 with (red lines) and without (black lines) AlCl3 (500 μm) treatment. Identified aldehydes are labeled at the top of each peak. HPLC conditions are described in “Materials and Methods,” and DNP derivatives of aldehydes were detected at 340 nm.
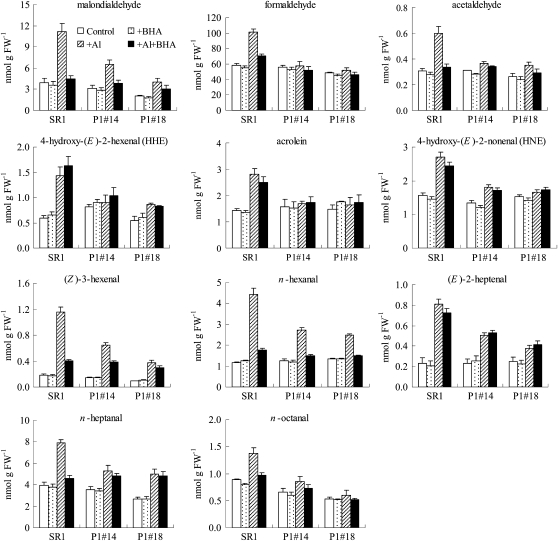
With AlCl3 treatment, the content of each aldehyde changed differently, and according to the mode of change, aldehydes were grouped into two: Group 1 (Supplemental Table S1) included aldehydes of which the contents after AlCl3 treatment were not lower in AER-OE lines than in the wild-type SR1. Identified in this group were crotonaldehyde, butyraldehyde, (E)-2-pentenal, n-pentanal, (E)-2-octenal, and n-nonanal, listed in order of elution. These aldehydes did not appear to be relevant to the protective effect of AER. Group 2 (Supplemental Table S2) included aldehydes of which the contents after AlCl3 treatment were significantly lower in AER-OE lines than in the wild-type SR1; identified in this group were malondialdehyde, formaldehyde, acetaldehyde, HHE, acrolein, butyraldehyde, phenylacetaldehyde, HNE, (Z)-3-hexenal, n-hexanal, (E)-2-hepenal, n-heptanal, and n-octanal.
The Group 2 aldehydes are candidates for the damage-causing molecules; indeed, they include highly reactive 2-alkenals, such as acrolein, HHE, and HNE. Their changes are represented in Figure 8. The increase in the contents of these aldehydes by AlCl3 treatment in SR1 ranged from 50% (n-octanal) to 540% [(Z)-3-hexenal]. The greatest absolute increase with AlCl3 treatment in SR1 plants was that of formaldehyde (40 nmol g FW−1; increased 75%), and the second highest was that of malondialdehyde (7.3 nmol g FW−1; increased 184%). For HNE, acrolein, and HHE, the Al-induced increases in SR1 were 1.2 nmol g FW−1 (increased 75%), 1.4 nmol g FW−1 (increased 100%), and 0.8 nmol g FW−1 (increased 140%), respectively. In contrast, in the AER-OE plants, the increases in the contents of these aldehydes were lower than in SR1 plants (see Supplemental Table S2 for statistical analysis). It should be noted that the observed increases here are diluted results because of the technical limitation; although the major injured part of Al toxicity was the root tip (0–2 mm), we had to include noninjured tissue also in the samples for the aldehydes analysis (0–20 mm from root tip) in order to collect the required amount (0.3 g for one analysis). Thus, the actual increase of the aldehyde contents in the Al-injured cells must be much greater than it appears in Figure 8.
Figure 8.
Contents of aldehydes in the root tips (0–20 mm) of SR1 and AER-OE lines P1#14 and P1#18. Seedlings were grown on MS agar plates for 28 d and in hydroponic medium for 28 d, and then they were treated with the following solutions: (1) one-sixth-strength HS (Control), (2) 10 μm BHA in one-sixth-strength HS (+BHA), (3) 500 μm AlCl3 in one-sixth-strength HS (+Al), and (4) 500 μm AlCl3 + 10 μm BHA in one-sixth-strength HS (+Al+BHA) for 24 h as described in “Materials and Methods.” Data are means ± se (n = 3).
Of these Group 2 aldehydes, only HNE, acrolein, and HHE are substrates for AER (Mano et al., 2002). Therefore, suppression of the other aldehydes in the AER-OE plants was an indirect effect of AER activity, probably through the scavenging of some precursor 2-alkenals (see “Discussion”). There were 12 unidentified aldehydes in Group 2, and they could be candidates for such precursors. It is also possible that some strongly toxic unknown aldehydes are included. All of these aldehydes are potentially toxic, and the increases in their contents could cause Al-induced damage of root tissues. Thus, overexpression of AER suppressed the increases in contents of these aldehydes via the direct enzymatic activity of AER or via indirect effects, thereby improving the tolerance of root tissues to Al toxicity.
2-Alkenals Inhibit Root Growth
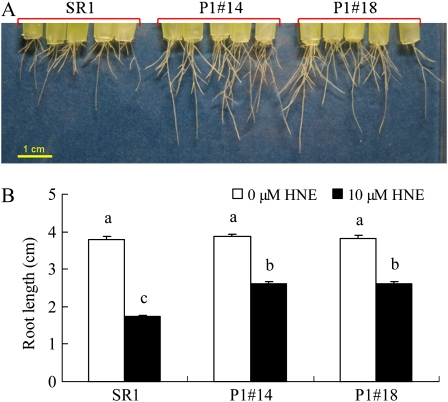
Toxicity of 2-alkenals to roots was verified by examining the effects of HNE and (E)-2-hexenal on root elongation. HNE at 10 μm inhibited root growth significantly, and the inhibition was severer in SR1 plants (55%) than in AER-OE plants (32%; Fig. 9). Similar results were obtained for (E)-2-hexenal (Supplemental Fig. S1); with increasing concentration from 10 to 300 μm, root growth inhibition was increased, and AER-OE plants suffered less. These results confirmed that 2-alkenals can be cause of root growth inhibition, and they were effectively detoxified in AER-OE plants.
Figure 9.
Effect of HNE on root growth. The 21-d-old seedlings were treated with or without 10 μm HNE in one-sixth-strength HS for 24 h and then cultured in HNE-free well aerated one-sixth-strength HS for 5 d to recover. A, Root growth of SR1 and AER-OE lines (P1#14 and P1#18) under HNE treatment. B, Length of the longest root of each plant was measured after recovery. Data are means ± se (n = 20). Values followed by the same letter are not significantly different according to Tukey-Kramer test (P < 0.05).
BHA Partly Protects Roots from Al Injury
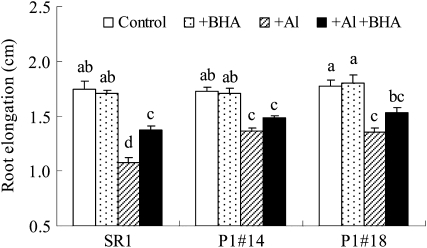
Yamamoto et al. (2001) previously suggested that lipid peroxidation was not the primary cause of elongation inhibition in pea (Pisum sativum) roots under Al stress, based on the result that butylated hydroxyanisole (BHA) suppressed the Al-induced increase in TBARS in roots but did not prevent the inhibition of root elongation in pea. In cultured tobacco cells, however, BHA could prevent Al-induced growth inhibition (Yamamoto et al., 2002). In order to investigate the effect of BHA in tobacco plants, BHA was applied to SR1 and AER-OE plants under Al stress. Our result showed that BHA could suppress the H2O2 production (Supplemental Fig. S2) and alleviated Al-induced root growth inhibition by 18% in SR1 plants (Fig. 10). Because BHA alleviated root elongation and growth capacity in both tobacco plants and cultured cells under Al stress, the effect of BHA in alleviating Al stress in tobacco might be different from that in pea (Yamamoto et al., 2001).
Figure 10.
Effect of AlCl3 and BHA on root growth of SR1 and AER-OE lines (P1#14 and P1#18). Seeds were sown on MS agar plates, the seedlings were cultured for 14 d, and then the seedlings were transferred into sterilized filter paper soaked by following solutions: (1) one-sixth-strength HS (Control), (2) 10 μm BHA in one-sixth-strength HS (+BHA), (3) 500 μm AlCl3 in one-sixth-strength HS (+Al), and (4) 500 μm AlCl3 + 10 μm BHA in one-sixth-strength HS (+Al+BHA) for 5 d as described in “Materials and Methods.” After the treatment, root elongation was measured. Data are means ± se of three replications (each replication included 10 plants). Values followed by the same letter are not significantly different according to Tukey-Kramer test (P < 0.05).
To investigate further the effect of BHA on root growth under Al stress in tobacco, individual aldehydes in AlCl3- and/or BHA-treated roots were identified and quantified as described above. With BHA treatment under Al stress, the content of each aldehyde changed differently (Fig. 8; Supplemental Tables S1 and S2). The contents of some aldehydes were decreased by BHA, including malondialdehyde, formaldehyde, acetaldehyde, crotonaldehyde, (Z)-3-hexenal, n-hexanal, n-heptanal, and n-octanal. The contents of other aldehydes were not affected by BHA, including HHE, acrolein, HNE, (E)-2-pentenal, (E)-2-heptenal, n-pentanal, (E)-2-octenal, and n-nonanal.
DISCUSSION
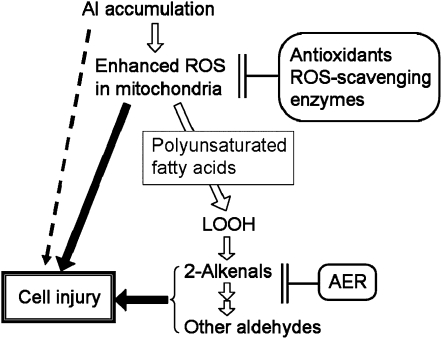
The overexpressed AER suppressed the LOOH-derived aldehyde levels without affecting the accumulation of Al and H2O2 (Figs. 4 and 5). Taking advantage of this, we could investigate the toxicity of aldehydes separately from that of the upstream ROS. Compared with the two types of control plants, AER-OE plants showed significantly higher relative rate of root elongation in the presence of AlCl3, as well as rapid root growth recovery after the removal of AlCl3 and effective maintenance of membrane integrity in the roots (Figs. 1, 2, and 6). Thus, overexpression of AER alleviated the Al-induced irreversible damage to root cells, especially to the elongation zone. This tolerance is attributed exclusively to suppression of the Al-induced increase in aldehyde contents. Increases in the contents of not only 2-alkenals, which are directly scavenged by AER, but also many other aldehydes that are incompatible with AER, were suppressed in the AER-OE plants (Fig. 8). Aldehydes accumulated around the root elongation zone, where cell death was most prominent, and the suppression of aldehyde accumulation at the elongation zone closely paralleled the alleviation of cell death (Fig. 6). When added exogenously, 2-alkenals inhibited the root elongation and its inhibition was alleviated by the overexpression of AER (Fig. 9; Supplemental Fig. S1). These results clearly indicate that, in Al stress, LOOH-derived aldehydes are involved in root cell injury.
Thus far, the alleviation of Al toxicity by the enhanced endogenous antioxidant levels (Ezaki et al., 2000; Basu et al., 2001) and by an exogenously added antioxidant (Yamamoto et al., 2002) has been explained as the detoxification of ROS. However, taking the formation and effects of aldehydes into consideration, the protection due to enhanced antioxidant levels can be partly explained as an indirect suppression of the downstream aldehyde production (Fig. 11). Of course, the above conclusion does not exclude the direct participation of ROS in the Al toxicity. Furthermore, our results also suggest the participation of some factors other than aldehydes in the root injury; in AER-OE roots, the increase of toxic 2-alkenals was totally suppressed, but the growth inhibition was only partially alleviated (Figs. 1 and 8). The protective effect of BHA in alleviating root growth inhibition in AER-OE plants also demonstrates that both ROS and aldehydes are involved in the Al toxicity (Fig. 10; Supplemental Fig. S2).
Figure 11.
Summary scheme of how LOOH-derived aldehydes act downstream of the formation of ROS and AER prevents Al-induced cell injury. In some Al-sensitive species like pea, ROS-independent Al toxicity can be critical.
It was previously stated that lipid peroxidation was not the primary cause of elongation inhibition in pea roots under Al stress (Yamamoto et al., 2001). This conclusion was derived solely from the observation that in pea plants, the antioxidant BHA failed to alleviate Al-induced inhibition of root elongation, although it effectively suppressed an increase in TBARS. While in tobacco cultured cells, BHA could protect from Al toxicity through inhibition of ROS generation (Yamamoto et al., 2002). Here, we observed in tobacco plants that BHA could suppress both ROS generation and TBARS increase and the inhibition of root elongation. Based on these apparent protecting effects of BHA, the Al toxicity in tobacco is at least partially ascribed to oxidative stress, in which LOOH-derived aldehydes are possibly involved.
Interestingly, BHA decreased some aldehyde contents but not others, including HHE, acrolein, and HNE, etc., which are the primary substrates for AER, and in AER-OE lines, the contents of these aldehydes were decreased (Fig. 8). Therefore, both BHA and AER can decrease the contents of aldehydes but different ones. Considering that both BHA and AER partially suppressed Al toxicity (Figs. 1, A and B, and 10), it is possible that BHA and AER contribute to Al tolerance through suppression of different aldehydes.
Among the detected aldehydes, malondialdehyde is a commonly studied marker of oxidative stress and has been shown to modify proteins by Schiff base addition (Fenaille et al., 2002; Taylor et al., 2004). Acrolein has DNA-damaging effects and inhibits enzymes with functional SH groups (Esterbauer et al., 1991). Recently, it was shown that acrolein inactivates the Calvin cycle enzymes phosphoribulokinase, glyceraldehyde-3-phosphate dehydrogenase, Fru-1,6-bisphosphatase, aldolase, and Rubisco and causes a rapid drop in the glutathione pool in chloroplasts in vitro (Mano et al., 2009). HNE is the most cytotoxic and abundant aldehyde generated through ROS-mediated lipid peroxidation; it is a highly reactive electrophile that forms Michael adducts via the C-3 atom and Schiff adducts via the C-1 aldehyde group; it modifies amino acids and forms cross-links in proteins, thus causing serious damage in cells (Winger et al., 2007). The toxicity of other aldehydes to plant components has been investigated less thoroughly.
The in vivo effect of an aldehyde depends on both its chemical reactivity and its intracellular concentration. Highly reactive 2-alkenals, such as acrolein, HNE, and HHE, affect cellular metabolism even at low levels, whereas less reactive aldehydes, such as malondialdehyde and formaldehyde, can be toxic only when their levels are much higher than those of the 2-alkenals (Esterbauer et al., 1991). We found that the level of HNE in SR1 increased by 1.2 nmol g FW−1 with Al treatment, whereas that of malondialdehyde increased by 7.3 nmol g FW−1 (Fig. 8). In light of the fact that the reactivity of malondialdehyde is one-tenth that of HNE (Esterbauer et al., 1975), the extent of the damage caused by malondialdehyde may be almost the same as that caused by HNE. In SR1, a large increase was also found in the content of formaldehyde, the content of which was 100 times that of acrolein under Al exposure. If we assume that formaldehyde is 400 times weaker than acrolein (from toxicity data observed in lettuce [Lactuca sativa] seed germination; Reynolds, 1977), the damaging effect of formaldehyde in Al-stressed roots should be one-fourth that of acrolein.
Several targets of aldehydes in plant cells have been identified. Mitochondrial lipoate enzymes, such as H-subunit of Gly decarboxylase and pyruvate dehydrogenase, are highly sensitive to HNE (Taylor et al., 2002) and most probably to other 2-alkenals. Winger et al. (2007) revealed that oxidative stress to Arabidopsis increased the HNE modification on various proteins, including ATP synthase β-subunit and malate dehydrogenase. They also showed that several enzymes were inactivated by the HNE modification. In Al-stressed roots, if these susceptible targets in the cells of elongation zone are attacked by HNE and other 2-alkenals, the energy metabolism will be stopped, resulting in the inhibition of growth. In addition, LOOH-derived aldehydes, such as malondialdehyde and HNE, can cause secondary membrane damage via avid binding to membrane proteins, eventually resulting in loss of membrane integrity (Esterbauer et al., 1991; Mueller, 2004; Halliwell, 2006). We found that the overexpressed AER alleviated membrane leakiness and cell death under AlCl3 stress in parallel with the suppression of aldehyde levels (Fig. 6, B and C); this suggests that the aldehydes affected membrane integrity under AlCl3 stress.
Overexpression of AER could lead to suppression of the production of a wide range of aldehydes, including malondialdehyde, formaldehyde, acetaldehyde, (Z)-3-hexenal, n-hexanal, (E)-2-heptenal, n-heptanal, and n-octanal, as well as the AER-substrate 2-alkenals, such as HHE, acrolein, and HNE (Fig. 8). This can be explained as a secondary effect of AER activity, as follows. There are multiple enzymatic pathways from polyunsaturated fatty acids to aldehydes that sometimes overlap each other (Blée, 1998), and many more reactions for nonenzymatic aldehyde formation are possible (Grosch, 1987). In these reactions, aldehydes are generally produced from the longer chain peroxides, which sometimes contain the α,β-unsaturated carbonyl structures (Esterbauer et al., 1991). AER could scavenge such long-chain precursors, thus suppressing generation of the descendant aldehydes. Although the substrate specificity of AER for long-chain compounds has not been tested extensively and the supposed precursors have yet to be identified, the enzyme prefers hydrophobic rather than hydrophilic aldehydes and can utilize C18 ketones as substrates (Mano et al., 2005). These results suggest that AER can act primarily at the upstream sites of aldehyde production pathways and regulate the global aldehyde composition of the cell.
In summary, tobacco plants overexpressing the Arabidopsis AER gene showed increased ability to tolerate Al stress. We ascribe this greater tolerance to a decrease in the production of aldehydes, which in turn resulted in reduced membrane damage and cell death in the roots, permitting improved root growth under Al stress. To the best of our knowledge, this is the first detailed report of the production of aldehydes under Al stress and the significance of aldehyde detoxification in enhancing Al tolerance in plants. Our findings should contribute to a better understanding of Al-induced aldehyde toxicity and provide a new strategy for improving Al stress tolerance in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Two transgenic tobacco (Nicotiana tabacum) lines, P1#14 and P1#18, that overexpress Arabidopsis (Arabidopsis thaliana) AER (AER-OE lines), the wild type Petit Havana SR1, and the empty vector-transformed line SR-Vec (Mano et al., 2005) were used. Plants were cultured in a growth chamber kept at 25°C with a 14-h photoperiod at 100 μmol m−2 s−1 photosynthetically active radiation.
Treatment with AlCl3
Seeds were sterilized in 1% (w/v) sodium hypochlorite for 20 min and sown on the surface of one-sixth-strength MS (Murashige and Skoog, 1962) agar (pH 4.2), containing 0, 300, 400, or 500 μm AlCl3, in square petri dishes (Ezaki et al., 2007). The petri dishes were placed in the growth chamber and positioned vertically for 4 d, by which time the seeds had germinated; there was no difference in germination between the SR1, SR-Vec, and AER-OE plants. The petri dishes were then tilted backward 45°, and the plants were allowed to grow for an additional 10 d. At the end of that period, the root length was measured for each treatment. Root elongation values under different levels of Al were presented as percentage of the value under control (no Al).
For further assay of Al tolerance, the plants were grown in a hydroponic system. Seeds of tobacco were first grown on MS agar plates (pH 5.7) for 28 d and then transferred to aerated one-sixth-strength Hoagland solution (HS; pH 5.7) and cultured for an additional 28 d. The uniformly grown plants (six to eight leaves) were selected and precultured for 24 h in one-sixth-strength HS (pH 4.2). They were then exposed to 0 μm (control) or 500 μm AlCl3 in one-sixth-strength HS (pH 4.2) for 24 h. Thereafter, one set of seedlings was retransplanted into well aerated one-sixth HS without AlCl3 and kept for 3 d, and the root morphology and fresh weight were observed. The other set of seedlings was used for determination of Al and H2O2 contents, electrolyte leakage, and AER activity. Those seedlings (six to eight leaves) were treated by Al and BHA for aldehyde analysis.
Distribution and Accumulation of Al
Root tips were excised and incubated in 5 mm ammonium acetate (NH4OAc) buffer (pH 5.0) for 10 min and then stained in 100 μm morin (Sigma-Aldrich) in NH4OAc buffer for 1 h, and finally washed with NH4OAc buffer for 10 min (Tice et al., 1992). Stained root tips were observed under an Olympus BX51 microscope (excitation wavelength 420 nm and emission 510 nm). A total of five to eight individual roots from five seedlings were examined for each time point, and the experiment was repeated three times. For determination of the Al content, 0.1 g root tip (0–10 mm) was washed three times with distilled water and dried and then digested with a concentrated acid mixture (HNO3:H2SO4, 1:1, v/v) at 160°C for 3 h. The Al content was quantified by using an inductively coupled plasma atomic emission spectrometer (Ciros CCD).
H2O2 Detection and Determination
H2O2 distribution in the root tips was detected by DCF-DA (Wako Pure Chemical; Jones et al., 2006). Root tips were excised and placed into a solution containing 200 μm CaCl2 (pH 4.4) and 10 μm DCF-DA for 15 min. The DCF-DA fluorescence was then detected under an Olympus BX51 microscope (excitation 488 nm and emission 530 nm). H2O2 content was determined according to the method of Ryan et al. (2009). Root tips (0.3 g, 0–20 mm) were frozen in liquid nitrogen, ground to powder in precooled mortars, and homogenized with 2 mL cold 0.1% (w/v) trichloroacetic acid. The homogenate was centrifuged at 12,000g for 30 min at 4°C, and 0.4 mL of the supernatant was added to 0.4 mL 10 mm potassium phosphate buffer (pH 7.0) and 0.8 mL 1 m KI. The absorbance of the mixture was read at 390 nm, which developed within 25 min and was stable for at least 2 h. The content of H2O2 was calculated against a calibration curve using H2O2 standards.
Visualization of Lipid Peroxidation and Cell Death
Aldehydes that originated from LOOH in the roots were visualized with Schiff's reagent as described by Yamamoto et al. (2001). Root tips were excised and stained with Schiff's reagent (Wako Pure Chemical) for 20 min, rinsed with a freshly prepared sulfite solution (0.5% [w/v] K2S2O5 in 0.05 m HCl), and then kept in the sulfite solution and observed instantly under a light stereomicroscope (Olympus SZX7). Cell death was detected by staining roots with Evans blue (Sigma) solution (0.025% [w/v] Evans blue in 100 μm CaCl2, pH 5.6) for 10 min (Yamamoto et al., 2001). Stained roots were washed three times with 100 μm CaCl2 (pH 5.6) and then observed under a light microscope (Olympus BX51). A total of five to eight individual roots from five seedlings were examined, and the experiment was repeated three times.
Electrolyte Leakage Assay
Loss of plasma membrane integrity was studied in terms of electrolyte leakage (EL) by measuring changes in electrical conductivity (Singh et al., 2007). Root tips (0.1 g, 0–20 mm) were incubated in distilled water at 25°C for 2 h in tubes, and the initial electrical conductivity (E1) of the medium was measured. The tubes containing the root material were then boiled for 30 min to release all the electrolytes, then cooled to 25°C, and the final electrical conductivity (E2) was measured. The EL was calculated as follows: EL = (E1/E2) × 100.
Assessment of Enzyme Activity
Roots were frozen and ground in liquid nitrogen with a precooled mortar and pestle, and then 50 mm potassium phosphate (pH 7.0) containing 1% protease inhibitor cocktail (P9599, for plant cell and tissue extraction; Sigma-Aldrich) was added. Homogenates were centrifuged at 8,000g for 10 min at 4°C, and the supernatant was concentrated on a Microcon filter (YM-10; Millipore) at 8,000g for 10 min. AER activity was assayed by the rate of oxidation of NADPH at 340 nm in a reaction mixture containing 50 mm MES-NaOH (pH 6.0), 0.1 mm NADPH, and 0.1 mm diamide as the electron acceptor (Mano et al., 2002, 2005). Protein was determined by the Bradford (1976) method, using bovine serum albumin as a standard.
Aldehyde Identification and Quantitation by HPLC
Seedlings (six to eight leaves) were treated with 0 or 500 μm AlCl3 under 0 or 10 μm BHA for 24 h. Then, roots of seedlings were used for aldehyde analysis. Aldehydes were extracted from the roots and derivatized with 2,4-dinitrophenylhydrazine and then identified and quantified by reverse-phase HPLC according to the method of Matsui et al. (2009), with a slight modification. Root tips (0.3 g, 0–20 mm) were frozen in liquid nitrogen, ground to fine powder by precooled motor and pestle, and then homogenized in 3 mL acetonitrile containing 1.5 nmol 2-ethylhexanal (as an internal standard) and 0.005% (w/v) butylhydroxytoluene. The slurry was incubated in a screw-capped glass tube at 60°C for 30 min. Then, an extract was collected through a glass filter in another glass tube. 2,4-Dinitrophenylhydrazine (final concentration of 0.5 mm) and formic acid (final concentration 0.5 m) were added, and the solution was mixed well and incubated at 25°C for 60 min. Then, 3 mL saturated NaCl solution and 0.3 g NaHCO3 were added to neutralize the formic acid. After centrifugation, the upper acetonitrile layer was collected and dried in vacuo. The residue was dissolved in 500 μL acetonitrile and passed through a BondEluteC18 cartridge (sorbent mass 200 mg; Varian), which had been prewashed with 2 mL acetonitrile. The material passed through the cartridge was collected, and 10-μL aliquots were subjected to HPLC in a Wakosil DNPH-II column (4.6 × 150 mm; Wako Pure Chemical). Wakosil DNPH-II Eluents A and B (Wako) were used to separate out the compounds, with 100% A (0–5 min), a linear gradient from 100% A to 100% B (5–20 min), and subsequently 100% B (20–35 min) at a flow rate of 1.0 mL min−1. Dinitrophenylhydrazone (DNP) derivatives of aldehydes were detected at 340 nm. Aldehydes were identified by their retention time, as compared with those of DNP derivatives of authentic aldehydes (Matsui et al., 2009). To determine the content of an aldehyde (nmol g FW−1) from its peak area, the ratio of the peak area to the peak area of the internal standard was first determined. The amount of an aldehyde was obtained by multiplying this ratio by the added amount of internal standard, i.e. 1.5 nmol 0.3 g FW−1. For identified aldehydes, the amount was further corrected for the DNP derivatization efficiency of the aldehyde and the extraction efficiency and absorption coefficient of the derivative relative to those of the internal standard (Matsui et al., 2009).
Effect of Exogenous Application of 2-Alkenal on Root Growth
Two aldehyde species were exogenously applied to evaluate the effect of 2-alkenals on root growth in tobacco. For HNE treatment, seeds were sown on a sponge with a holder in one-sixth-strength HS directly, and 21-d-old seedlings were treated with 0 or 10 μm HNE (Alexis Biochemicals) in the same medium for 24 h and then they were exposed to well aerated one-sixth-strength HS without HNE and kept for 5 d for recovery. After that, the maximum root length was measured. For (E)-2-hexenal treatment, seeds were first grown on MS agar plates (pH 5.7) for 28 d and then transferred to aerated one-sixth-strength HS (pH 5.7) and cultured for an additional 21 d. The uniformly grown plants were selected and exposed to 0, 10, 100, or 300 μm (E)-2-hexenal (Tokyo Chemical Industry) for 24 h, and then they were transplanted into well aerated one-sixth-strength HS without (E)-2-hexenal for 5 d for recovery. After that, root fresh weight and the maximum root length were measured.
Effect of BHA on Root Elongation under Al Stress
When the seedlings were treated with AlCl3 in the presence of BHA (Wako Pure Chemical), seeds were first sown on the surface of one-sixth-strength MS agar plate and grown for 14 d. Then seedlings with same root length (8–10 mm) were transferred into sterilized filter paper soaked with following four solutions: (1) one-sixth-strength HS, (2) 10 μm BHA in one-sixth-strength HS, (3) 500 μm AlCl3 in one-sixth-strength HS, and (4) 500 μm AlCl3 + 10 μm BHA in one-sixth-strength HS in petri dishes for 5 d. At the end of treatment, root elongation was measured.
Statistical Analyses
Experiments were performed three times. Data were analyzed by using the programs of Statistical Analysis System (SAS 8.0). Data were subject to ANOVA, and means were compared by Tukey-Kramer test (P < 0.05).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Root growth of SR1 and AER-OE lines (P1#14 and P1#18) under (E)-2-hexenal treatment.
Supplemental Figure S2. H2O2 content in root tips (0–20 mm) of SR1 and AER-OE lines (P1#14 and P1#18).
Supplemental Table S1. Aldehydes of which the contents after AlCl3 treatment were not lower in AER-OE lines (P1#14, P1#18) than in the wild-type SR1.
Supplemental Table S2. Aldehydes of which the contents after AlCl3 treatment were lower in AER-OE lines (P1#14, P1#18) than in the wild-type SR1.
References
- Achary VMM, Jena S, Panda KK, Panda BB. (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70: 300–310 [DOI] [PubMed] [Google Scholar]
- Basu U, Good AG, Taylor GJ. (2001) Transgenic Brassica napus plants overexoressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Environ 24: 1269–1278 [Google Scholar]
- Blancaflor EB, Jones DL, Gilroy S. (1998) Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maizes. Plant Physiol 118: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blée E. (1998) Phytooxylipins and plant defense reactions. Prog Lipid Res 37: 33–72 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Burcham PC. (1998) Genotoxic lipid peroxidation products: their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis 13: 287–305 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107: 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi SR, Yamamoto Y, Matsumoto H. (2003) An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. J Inorg Biochem 97: 59–68 [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schauer RJ, Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic Biol Med 11: 81–128 [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Zollner H, Scholz N. (1975) Reaction of glutathione with conjugated carbonyls. Z Naturforsch C 30: 466–473 [DOI] [PubMed] [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Matsumoto H. (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122: 657–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Kiyohara H, Matsumoto H, Nakashima S. (2007) Overexpression of an auxilin-like gene (F9E10.5) can suppress Al uptake in roots of Arabidopsis. J Exp Bot 58: 497–506 [DOI] [PubMed] [Google Scholar]
- Ezaki B, Nagao E, Yamamoto Y, Nakashima S, Enomoto T. (2008) Wild plants, Andropogon virginicus L. and Miscanthus sinensis Anders, are tolerant to multiple stresses including aluminum, heavy metals and oxidative stresses. Plant Cell Rep 27: 951–961 [DOI] [PubMed] [Google Scholar]
- Fenaille F, Tabet JC, Guy PA. (2002) Immunoaffinity purification and characterization of 4-hydroxy-2-nonenal- and malondialdehyde-modified peptides by electrospray ionization tandem mass spectrometry. Anal Chem 74: 6298–6304 [DOI] [PubMed] [Google Scholar]
- Grosch W. (1987) Reactions of hydroperoxides-products of low molecular weight. Chan HWS, , Autoxidation of Unsaturated Lipids. Academic Press, New York, pp 95–139 [Google Scholar]
- Halliwell B. (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141: 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang J, Chen X, Gao Z, Xuan W, Xu S, Ding X, Shen W. (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177: 155–166 [DOI] [PubMed] [Google Scholar]
- Hegedüs A, Erdei S, Janda T, Tóth E, Horváth G, Dudits D. (2004) Transgenic tobacco plants overproducing alfalfa aldose/aldehyde reductase show higher tolerance to low temperature and cadmium stress. Plant Sci 166: 1329–1333 [Google Scholar]
- Hideg É, Nagy T, Oberschall A, Dudits D, Vass I. (2003) Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B 280-320 nm stresses. Plant Cell Environ 26: 513–522 [Google Scholar]
- Jones DL, Blancaflor EB, Kochian LV, Gilroy S. (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29: 1309–1318 [DOI] [PubMed] [Google Scholar]
- Jones DL, Kochian LV. (1995) Aluminum inhibition of the inositol 1,4,5-triphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity?. Plant Cell 7: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV. (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D. (2006) Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ 29: 1033–1048 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Tai CY, Kochian LV, Howell SH. (1996) Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol 110: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma BH, Wan JM, Shen ZG. (2007) H2O2 production and antioxidant responses in seeds and early seedling of two different rice varieties exposed to aluminum. Plant Growth Regul 52: 91–100 [Google Scholar]
- Mano J, Belles-Boix E, Babiychuk E, Inzé D, Torii Y, Hiraoka H, Takimoto K, Slooten L, Asada K, Kushnir S. (2005) Protection against photooxidative injury of tobacco leaves by 2-alkenal reductase. Detoxication of lipid peroxide-derived reactive carbonyls. Plant Physiol 139: 1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J, Miyatake F, Hiraoka E, Tamoi M. (2009) Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 230: 639–648 [DOI] [PubMed] [Google Scholar]
- Mano J, Torii Y, Hayashi S, Takimoto K, Matsui K, Nakamura K, Inzé D, Babiychuk E, Kushnir S, Asada K. (2002) The NADPH:quinone oxidoreductase P1-ζ-crystallin in Arabidopsis catalyzes the α , β-hydrogenation of 2-alkenals: detoxication of the lipid peroxide-derived reactive aldehydes. Plant Cell Physiol 43: 1445–1455 [DOI] [PubMed] [Google Scholar]
- Matsui K, Sugimoto K, Kakumyan P, Khorobrykh SA, Mano J. (2009) Volatile oxylipins and related compounds formed under stress in plants. Armstrong D, , Methods in Molecular Biology ‘Lipidomics,’ Vol 580 Humana Press, Totowa, NJ, pp 17–28 [DOI] [PubMed] [Google Scholar]
- Millar AH, Leaver CJ. (2000) The cytotoxic lipid peroxidation product, 4-hydroxy-2-nonenal, specifically inhibits decarboxylating dehydrogenases in the matrix of plant mitochondria. FEBS Lett 481: 117–121 [DOI] [PubMed] [Google Scholar]
- Møller IM, Jensen PE, Hansson A. (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58: 459–481 [DOI] [PubMed] [Google Scholar]
- Mueller M. (2004) Archetype signals in plants: the phytoprostanes. Curr Opin Plant Biol 7: 441–448 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Oberschall A, Deák M, Török K, Sass L, Vass I, Kocács I, Fehér A, Dudits D, Horváth GV. (2000) A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chmical and drought stresses. Plant J 24: 437–446 [DOI] [PubMed] [Google Scholar]
- O'Brien P, Siraki AG, Shangari N. (2005) Aldehyde sources, metabolism, molecular toxicity mechanisms and possible effects on human health. Crit Rev Toxicol 35: 609–662 [DOI] [PubMed] [Google Scholar]
- Papdi C, Ábrahám E, Joseph MP, Popescu C, Koncz C, Szabados L. (2008) Functional identification of Arabidopsis stress regulatory genes using the controlled cDNA overexpression system. Plant Physiol 147: 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Schaur RJ. (2000) 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life 50: 315–321 [DOI] [PubMed] [Google Scholar]
- Reynolds T. (1977) Comparative effects of aliphatic compounds on inhibition of lettuce fruit germination. Ann Bot (Lond) 41: 637–648 [Google Scholar]
- Ryan A, Cojocariu C, Possell M, Davies WJ, Hewitt CN. (2009) Defining hybrid poplar (Populus deltoides × Populus trichocarpa) tolerance to ozone: identifying key parameters. Plant Cell Environ 32: 31–45 [DOI] [PubMed] [Google Scholar]
- Sakihama Y, Yamasaki H. (2002) Lipid peroxidation induced by phenolics in conjunction with aluminum ions. Biol Plant 45: 249–254 [Google Scholar]
- Singh HP, Batish DR, Kohli RK, Arora K. (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53: 65–73 [Google Scholar]
- Sunkar R, Bartels D, Kirch HH. (2003) Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35: 452–464 [DOI] [PubMed] [Google Scholar]
- Tahara K, Yamanoshita T, Norisada M, Hasegawa I, Kashima H, Sasaki S, Kojima K. (2008) Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant Soil 307: 167–178 [Google Scholar]
- Taylor NL, Day DA, Miller AH. (2002) Environmental stress causes oxidative damage to plants mitochondria leading to inhibition of glycine decarboxylase. J Biol Chem 277: 42663–42668 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Day DA, Miller AH. (2004) Targets of stress-induced oxidative damage in plant mitochondria and their impact on cell carbon/nitrogen metabolism. J Exp Bot 55: 1–10 [DOI] [PubMed] [Google Scholar]
- Tice KR, Parker DR, DeMason DA. (1992) Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol 100: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger AM, Millar AH, Day DA. (2005) Sensitivity of plant mitochondrial terminal oxidases to the lipid peroxidation product 4-hydroxy-2-nonenal (HNE). Biochem J 387: 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger AM, Taylor NL, Heazlewood JL, Day DA, Millar AH. (2007) The cytotoxic lipid peroxidation product 4-hydroxy-2-nonenal covalently modifies a selective range of proteins linked to respiratory function in plant mitochondria. J Biol Chem 282: 37436–37447 [DOI] [PubMed] [Google Scholar]
- Xue YJ, Tao L, Yang ZM. (2008) Aluminum induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. J Agric Food Chem 56: 9676–9684 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128: 63–72 [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. (2003) Oxidative stress triggered by aluminum in plant roots. Plant Soil 255: 239–243 [Google Scholar]
- Yamamoto Y, Yukiko Kobayashi Y, Matsumoto H. (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125: 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]