Abstract
This paper presents a novel unified theory of the structure activity relationship of opioids and opioid peptides. It is hypothesized that a virtual or known heterocyclic ring exists in all opioids which have activity in humans, and this ring occupies relative to the aromatic ring of the drug, approximately the same plane in space as the piperidine ring of morphine. Since the rings of morphine are rigid, and the aromatic and piperidine rings are critical structural components for morphine’s analgesic properties, the rigid morphine molecule allows for approximations of the aromatic and heterocyclic relationships in subsequent drug models where bond rotations are common. This hypothesis and five propositions are supported by stereochemistry and experimental observations.
Proposition #1 The structure of morphine provides a template. Proposition #2 Steric hindrance of some centric portion of the piperidine ring explains antagonist properties of naloxone, naltrexone and alvimopam. Proposition #3 Methadone has an active conformation which contains a virtual heterocyclic ring which explains its analgesic activity and racemic properties. Proposition #4 The piperidine ring of fentanyl can assume the morphine position under conditions of nitrogen inversion. Proposition #5 The first 3 amino acid sequences of beta endorphin (l-try-gly-gly) and the active opioid dipeptide, l-tyr-pro, (as a result of a peptide turn and zwitterion bonding) form a virtual piperazine-like ring which is similar in size, shape and location to the heterocyclic rings of morphine, meperidine, and methadone. Potential flaws in this theory are discussed.
This theory could be important for future analgesic drug design.
Keywords: opioid, stereochemistry, analgesic, heterocyclic
Introduction
During the last half century, medicinal chemists have searched for improved opioid analgesics. Hundreds of compounds have been synthesized and tested for improvements of alkaloids derived from the opium poppy. The simplest synthetic compounds which have extensive clinical use are meperidine and methadone. Researchers continue to search for improved analgesics with fewer side effects, increased potency, and less risk of tolerance.1
Endogenous opioids such as beta endorphin bind to the same receptor as the opium alkaloids. The conformational similarities between morphine, meperidine, fentanyl, methadone and the endorphins are still speculative. Although the endorphins are potent analgesics they have limited clinical use because they are inactivated during ingestion and cannot cross the blood brain barrier.2 Effect is only achieved with intrathecal administration.
It is hypothesized that a virtual or known heterocyclic ring exists in all opioids which have activity in humans and this ring occupies relative to the aromatic ring of the drug, approximately the same plane in space as the piperidine ring of morphine.
General Premises of the Argument
In humans, a single mu opioid receptor exists as defined by that structure of the central nervous system which binds morphine and endorphin and facilitates analgesia.
- The clinical, animal, experimental, and computational information pertaining to opioid and opioid peptides is vast and spans two centuries. Some of the data may be inaccurate because laboratory and computing technologies have been refined during this time period. In order to develop a theory applicable to human pharmacology, the author chose to prioritize data in the literature. For example, conflicting activity data from homogenate receptor studies will not supercede data from in vivo human studies and conflicting structural determinations from computational chemistry will not supercede results from stereochemistry, crystallography or NMR studies. Thus, observations from the literature can be weighted from most significant to least in the following manner:
- Agonist/antagonist activity-human, in vivo potency > animal, in vivo potency (tail flick, hot plate) > guinea pig ileum or mouse vas deferens preparations > receptor homogenates
- Crystallography, NMR, stereochemistry > molecular modeling (molecular mechanics and/or quantum mechanics)
Conclusions are based on the inductive argument. Exceptions to the propositions may exist because numerous opioid and opioid peptides have been synthesized prior to recognition of multiple opioid receptors. Also refinements in laboratory techniques may have changed data interpretation. However, this argument applies to active opioids and opioid peptides which produce effects in humans. These opioids are referred to as “first class” opioids whereas as all other opioid like analgesics are referred to as “second class” opioids.
This theory relies heavily on the stereochemistry of the opioids to explain pharmacologic activity of opioids and opioid peptides. Although computational measurements from other authors are considered, the focus has been to describe the pharmacologic activities through comparisons of enantiomers which become evident in the presence of virtual or known heterocyclic rings. Steric effects hindering the heterocyclic ring by various isomers explain agonist and antagonist characteristics of the molecule. Further work in the form of computational chemistry and experimental pharmacology may support or refute this theory.
Previous Structure Activity Theories
Beckett and Casy proposed that an aromatic and a basic amine, which is protonated at physiologic pH, exists to form a 3 point model consisting of an anionic site (N), hydrophobic region of a piperidine ring, and a phenolic site (tyrosine).3,4 Their theory predates the discovery of opioid peptides.
Kane et al described an opioid agonist model suggesting that multiple epitopes exist for ligand binding. 5 Their work led to a theory of a more complex structure activity mechanism of “message and address” sites in fentanyl and related second class opioids. They did not extend their theory to opioid peptides.
Cometta-Morini et al proposed a structure activity relationship for the fentanyl classes of compounds which relied upon four key moieties. 1) a protonated amine nitrogen, 2) a polar group capable of hydrogen bonding, 3) an aromatic ring, 4) a second aromatic ring.6
Martin and Andrews, using a computational analysis, favored a protonated nitrogen, an aromatic ring, and a lipophilic group as the essential components of opioid agonists.15 Most of their computations were conducted with second class opioids and they did not extrapolate their theory to opioid peptides.
Rationale for Selection of Opioids to Investigate
The opioids considered for this paper represent a subset of those compounds known to produce analgesia through the mu opioid receptor in man excluding many of the analgesics where bioavailability, lipid solubility, or metabolism may predict differences in action. Just as clinical data is prioritized in reviews or meta-analysis, the selected compounds of highest priority (clinical response in man) were investigate which by- passed the in vitro–in vivo correlation discrepancies. Many second class opioids were excluded which were investigated in earlier years before subpopulations of opioid receptors were discovered. The conclusions from this argument may or may not explain some of the prior experimental observations, particularly of second class opioids.
Proposition #1 The structure of morphine provides a template
In humans, within the central nervous system, morphine is a mu receptor agonist.
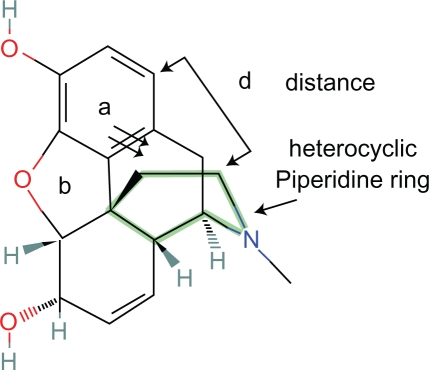
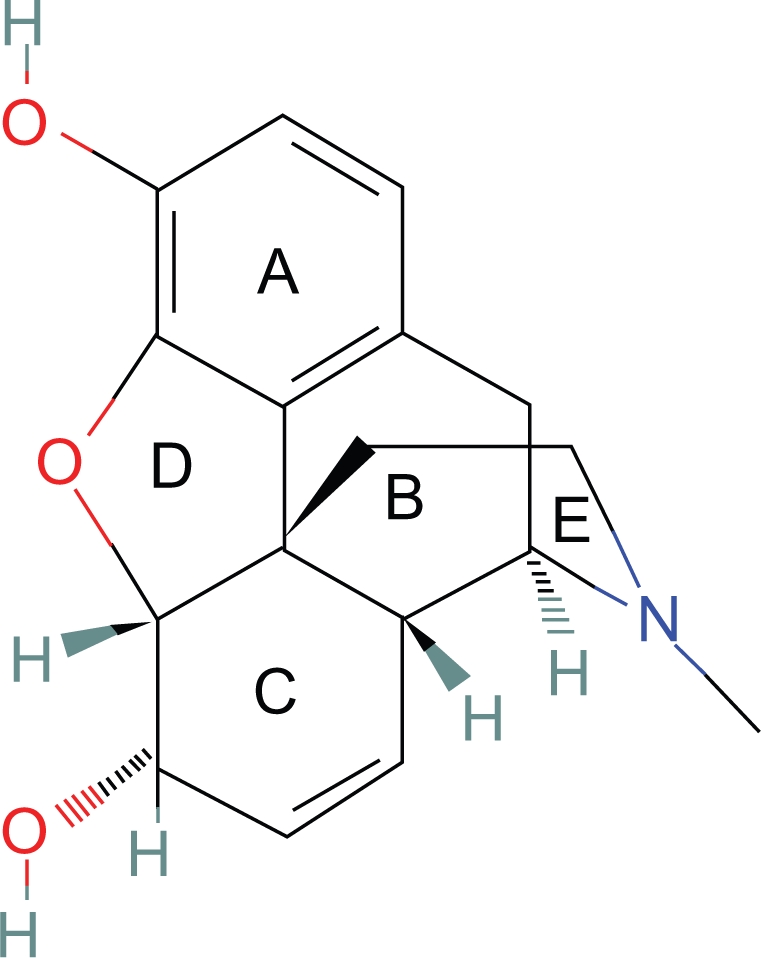
It is fortuitous that the morphine rings are nearly rigid with little rotational movement and therefore can be considered a template.
The aromatic and heterocyclic ring incorporating nitrogen (piperidine ring) are essential for analgesic activity. The B and C rings can be eliminated with minimal loss of activity.7 If the D ring is also eliminated, the molecule has limited activity as the position of the heterocyclic ring is significantly less rigid with more degrees of freedom of movement (Fig. 1).
One cannot say whether the relationships of the aromatic and heterocyclic rings in morphine are ideal, but if they are not, they must be close to ideal because of morphine’s analgesic potency. Furthermore, natural selection may have favored organisms with opioid receptors which are responsive to naturally occurring opioids.
The plane of the heterocyclic ring is defined by two vectors originating from the plane of the aromatic ring and the distance between two points on each plane (Fig. 2).
The primary state of the heterocyclic ring is the lower energy chair conformation.
Figure 1.
Morphine.
Figure 2.
Morphine.
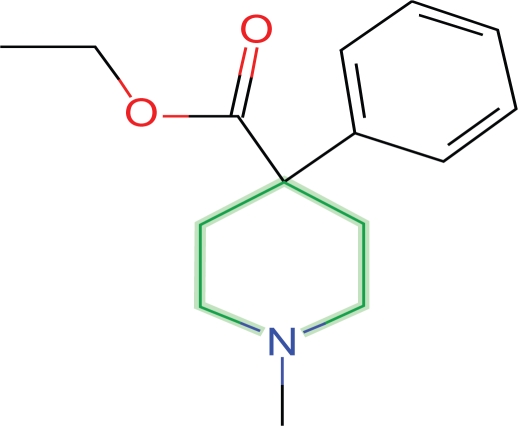
Meperidine is the simplest active opioid and conforms to the morphine model
Meperidine is the simplest active opioid and is comprised of an aromatic ring and piperidine ring. With the aromatic ring of meperidine congruent with the aromatic ring of morphine the piperidine ring can assume a position very closely approximating the position of the morphine piperidine ring (Fig. 3).
Figure 3.
Meperidine.
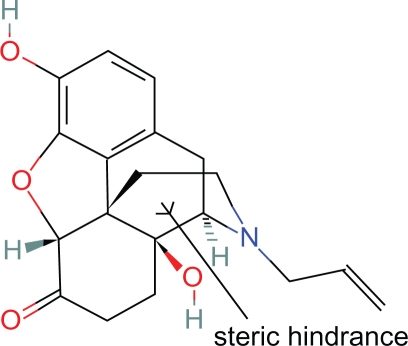
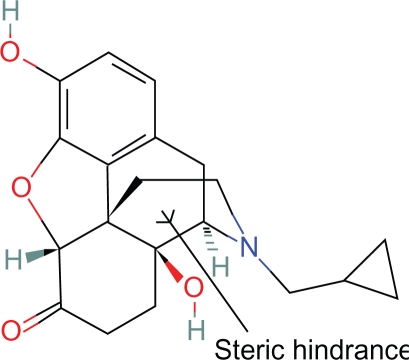
Proposition #2 Steric hinderance of some centric portion of the piperidine ring explains antagonist properties of naloxone, naltrexone and alvimopam
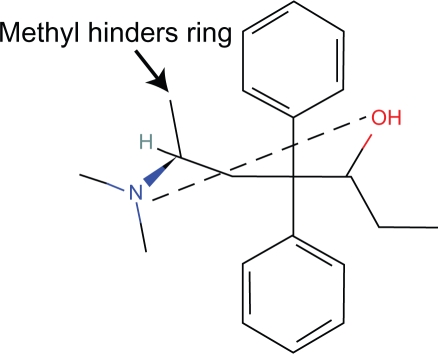
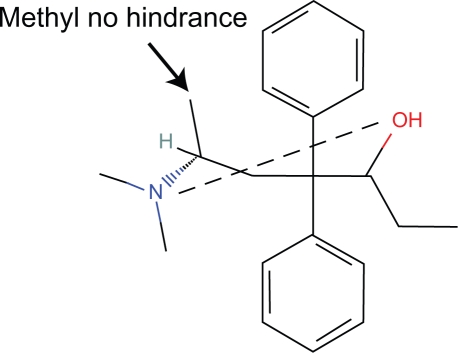
These steric effects are caused by the OH and allyl side chains which block more than a peripheral portion of the piperidine ring (Fig. 4).
In the guinea pig ileum preparation, naltrexone with a bulkier side chain is more antagonistic than naloxone8 (Fig. 5).
In humans, alvimopam is an antagonist and there is significant hindrance of the piperidine ring by the aromatic ring (Fig. 6).
Figure 4.
Naloxone.
Figure 5.
Naltrexone.
Figure 6.
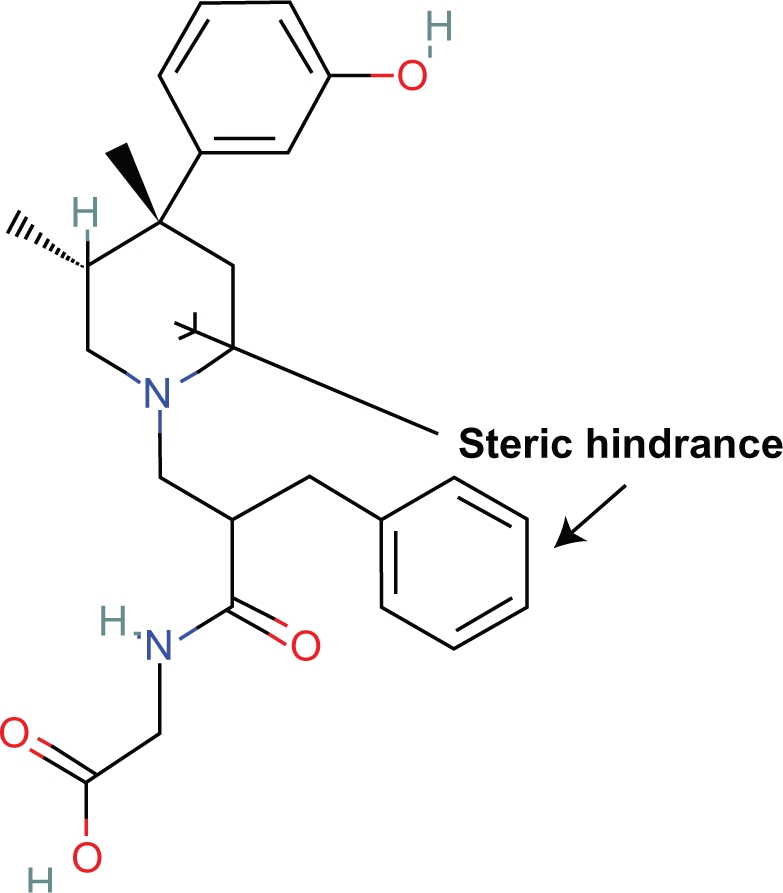
Alvimopam.
Proposition #3 Methadone has an active conformation which contains a virtual heterocyclic ring which explains its analgesic activity and racemic properties
Methadone has a unique structure compared to other opioids since it does not possess a heterocyclic ring. (Fig. 7) Attempts to explain the structure activity relationship of methadone have invoked unusual steric changes and rearrangements which have never been very convincing.9 An argument that a pharmacologically active methadone conformation includes a “virtual heterocyclic ring” is based on the following assumptions:
1. Methadone contains a ketone group which also exists in equilibrium as an enol tautomer (Fig. 8).
2. The OH in the enol tautomer can form an intramolecular hydrogen bond with the tertiary nitrogen and produce a seven member heterocyclic ring. According to Pauling, the N-H-O bond is near linear. 10 Therefore, the virtual ring has characteristics of a 6 member nitrogen containing ring which can be shown to be positioned in a plane similar to the piperidine ring of morphine. The position of this ring can be easily modeled (Figs. 9, 10).
-
3. The formation of the heterocyclic ring positions a methyl group connected to the chiral carbon which has steric influences on activity. In the chair conformation of the d isomer, the methyl group hinders the heterocyclic ring and the medication has minimal analgesic activity, but in the l isomer there is no ring hindrance and substantial analgesic effects. Similar to the steric blocking effects observed with naloxone and naltrexone, these steric effects explain why l-methadone is active and d-methadone is relatively inactive (Fig. 11).
This observation is consistent with prior work showing that the stereospecific potency of methadone is intrinsic.11
4. The presence of a virtual ring also explains prior observations that racemic threo-5-methyl methadone is inactive where as (–) erythro-5 methyl-methadone is highly active. This occurs because of the steric blocking effects from methyl groups on the virtual ring.12
5. NMR and circular dichroic studies are “not inconsistent” with the existence of intramolecular hydrogen bonding within methadone and a heterocyclic ring conformation.13,14
6. Loew et al using quantum chemical studies showed evidence that methadone conforms to a low energy heterocyclic ring as described by Portoghese.13
7. Even though the methadone molecule contains two aromatic groups and the quaternary carbon connecting these groups is not chiral, there is one preferred conformation incorporating the virtual ring. This becomes more apparent when the molecule is viewed from the plane of the aromatic ring and this supports the specificity of the stereochemistry.
Figure 7.
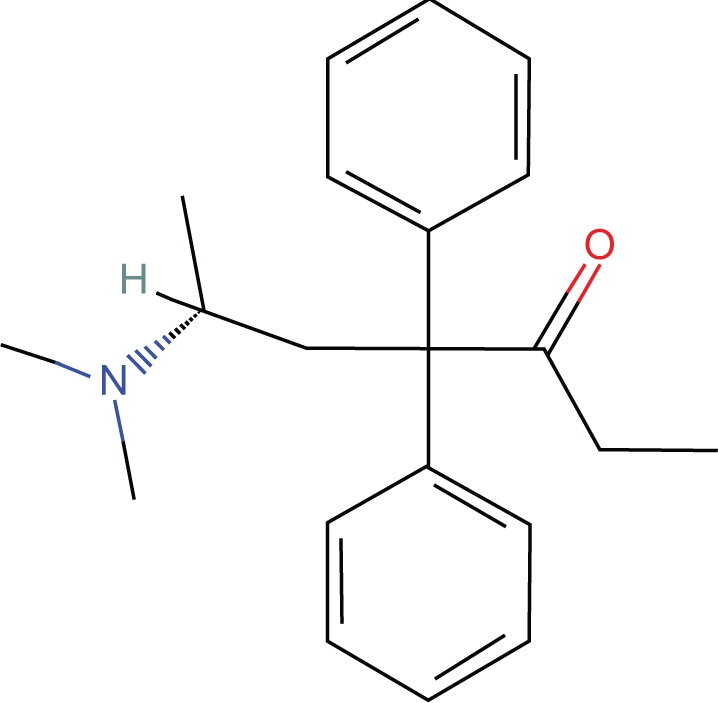
I-Methadone.
Figure 8.
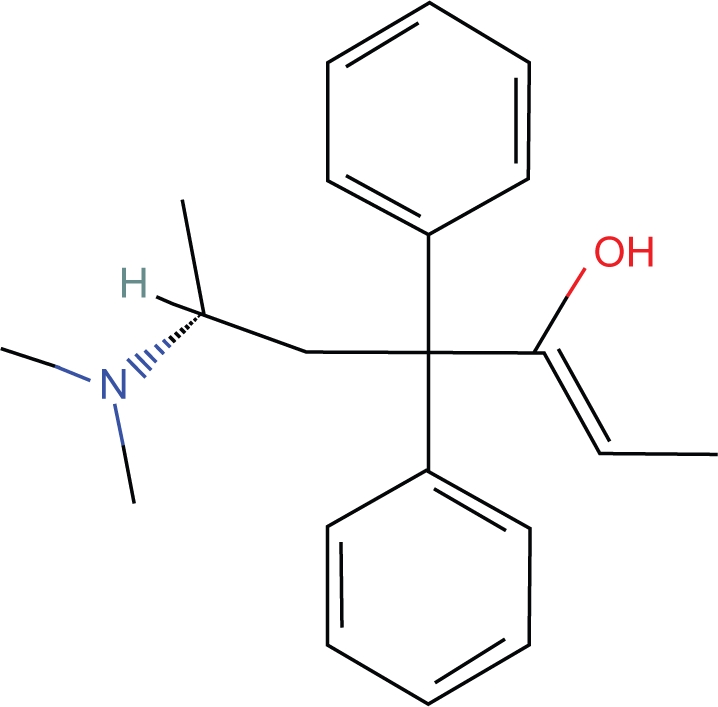
Enol I-Methadone.
Figure 9.
Virtual Ring d-Methadone.
Figure 10.
Virtual Ring I-Methadone.
Figure 11.
+ Demonstration of steric effects from methyl groups in racemic (d,1) methadone.
Proposition #4 The piperidine ring of fentanyl can assume the morphine position under conditions of nitrogen inversion
A rigid tertiary amine moiety of fentanyl limits conformational changes which would allow the piperidine ring to assume a position similar to that found in morphine unless nitrogen inversion exists (Fig. 12).
The nitrogen of this amine is chiral, yet a racemic mixture of fentanyl which would have stereospecific activity is not known to exist. Nitrogen inversions are probable low energy conformational changes at body temperature which can change the length and angles of the tertiary amine and allow a conformation to exist in which the aromatic and the piperidine rings of fentanyl coincide with those of morphine.
Substitution of the tertiary nitrogen with carbon results in an inactive molecule which strongly suggests that a tetrahedral structure is not the active structure.6
Most congeners of fentanyl in which the nitrogen of the tertiary amine is cyclically restrained are inactive presumably because the inversion is restrained.6
Klein et al studied two restrained analogues of 3-methyl fentanyl and reported three times greater activity with the cis isomer but also activity in the trans molecule. During nitrogen inversion, the trans conformation may hinder the piperidine ring more than the cis conformation but not enough to produce antagonism.16
Figure 12.
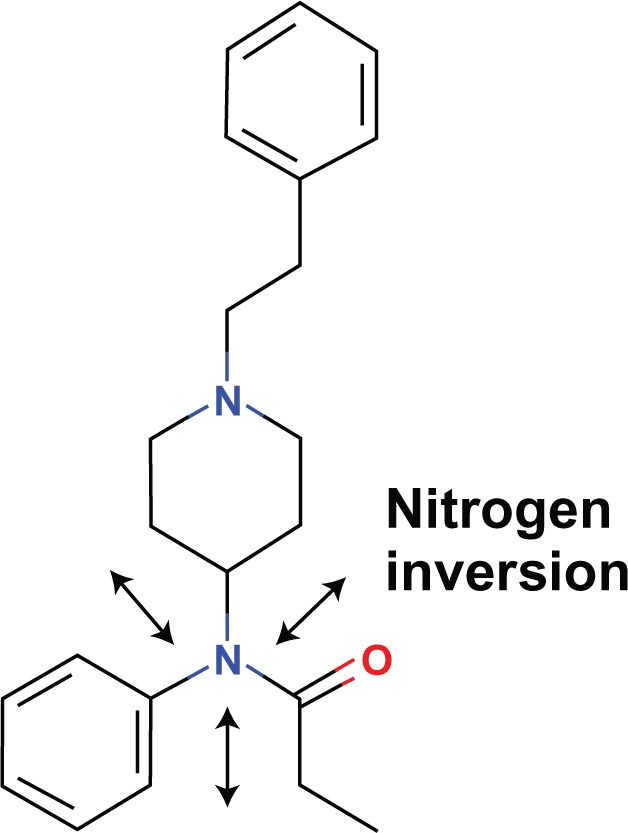
Fentanyl.
Proposition #5 The first 3 amino acid sequences of beta endorphin (l-try-gly-gly) and the active opioid dipeptide, l-tyr-pro, (as a result of a peptide turn and zwitterion bonding) form a virtual piperazine-like ring which is similar in size, shape and location to the heterocyclic rings of morphine, meperidine, and methadone
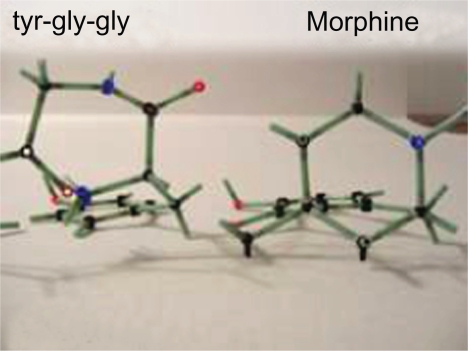
1. Tyr-gly-gly- is the N terminus amino acid sequence of beta endorphin (Fig. 13). However, many apparently dissimilar, di, tri, tetra, penta, and polypeptides are known to have opioid activity, the smallest being l-Tyr-pro (Fig. 14). There are at least 68 peptides defined as opioid peptides cited in the literature. 17 These compounds display in vitro and in vivo activity in a number of mammalian species. Forty nine of 68 of the compounds contain Tyr as the initial amino acid residue. Of the 49, 34 of these compounds have a second amino acid residue as gly. Of the 34, 32 have the initial 3 residues as tyr-gly-gly. Tyrosine d-alanine is present in 5/68 peptides. Ten of 68 peptides are found in humans. Five of 10 have the sequence tyr-gly-gly. Most importantly, these observations suggest that there is some structural commonality among dissimilar opioid peptides in addition to being comprised of an initial l-tyrosine aromatic ring and peptide bonds.
- 2. Three groups of investigators provided cystallographic evidence that a ring structure is present along with a peptide turn in opioid peptides.
- Vass et al describe two types of beta turns in N-glycated leu-enkephalin. Their beta turn includes a 7 member ring.18
- Ishida et al described a G-G Type II beta turn as the most stable conformer of met-enkephalin in the zwitterionic state which is consistent with a 7 member virtual 6 member piperazine ring.19
- Bloomberg et al described conformational changes of a beta turn mimetic incorporated in leu-enkephalin. They proposed a 10 member ring formation supported by NMR and crystallography studies of leuenkephalin. 20 While the 10 member ring cannot be superimposed on the piperidine of morphine an intermediate virtual 7 member ring may exist.
-
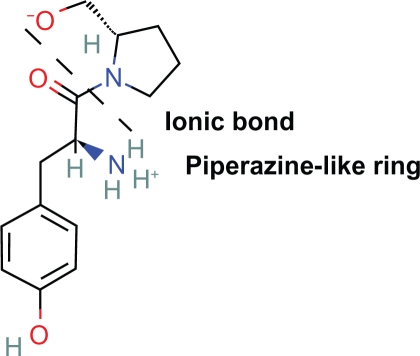
3. Within the peptide turn of tyr-gly-gly an ionic bond and virtual heterocyclic ring exists formed by the intramolecular zwitterion attraction of the negative carbonyl oxygen to the positively charged nitrogen of tyrosine (Fig. 15).
Demonstration of the similar conformations of the piperazine-like ring of tyr-gly-gly and piperidine ring of morphine (Fig. 16).
4. When viewed from the initial l tyrosine moiety, these 7 member rings exist in a plane similar to the piperidine ring of morphine. The geometry conforms to a 6 member piperazine-like ring. Formation of this ring will cause the first two amino acids joined by a peptide bond to conform to the rarer but active cis conformation which has been shown to be critical for analgesia. Not intuitively obvious, it is not possible to form the ring with the first and second amino acids in the trans conformation without disturbing rigid and planar peptide bonds.
5. Another observation supporting the virtual ring is that d-tyr-gly-gly is inactive as well as other peptides beginning with d-tyr and this stereochemistry changes the conformation of the virtual heterocyclic ring in such a manner that it cannot be congruent with the piperidine ring of morphine when the phenyl group of tyrosine is superimposed on the phenyl group of morphine.21
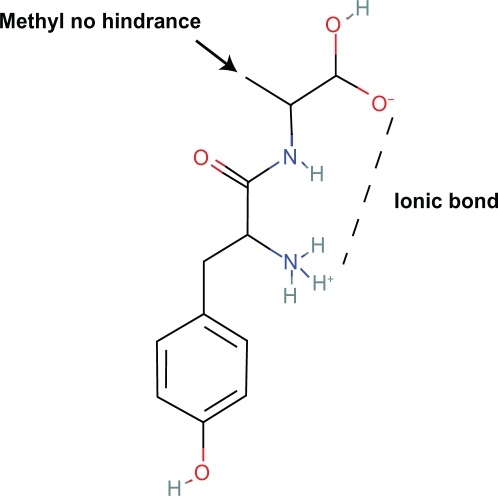
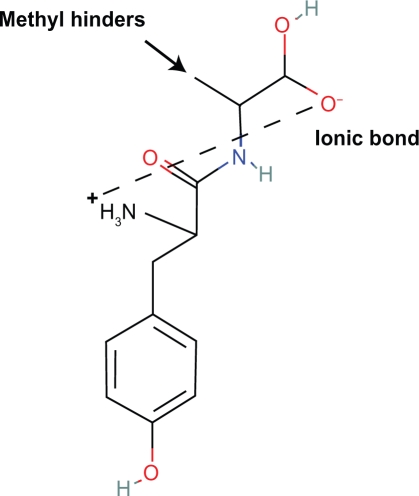
6. Amino acids with the l conformation are exclusive in first class opioid peptides. D-alanine can be substituted for glycine as in DAMGO with high potency and minimal steric effects on the virtual ring. However, peptides beginning with l-tyrosine-l-alanine are inactive.22 In this conformation the methyl group of alanine hinders the virtual ring (Figs. 17, 18).
7. Synthetic isomers of beta endorphin with substitutions of d-phe, or d-met are inactive because of steric hindrance of the virtual ring. In such conformations, the amino acid R group hinders the ring. However, more distal substitutions of d amino acids may not be associated with steric hindrance of the virtual ring and may active. Also, it is imaginable that in the larger more potent opioid peptides like beta endorphin, met-enkephalin, and leu-enkephalin the l-amino acid residues exert influences on the size, shape and location of the virtual ring by changes in the peptide tertiary structure which increase analgesia.
8. Tyr-pro is the minimal length peptide which has been shown by two laboratories to possess opioid activity.23,24 Tyr-pro can form a hydrogen bonded virtual ring and peptide turn which is consistent with this proposition (Fig. 19).
9. The second amino acids (glycine, alanine or proline) which are bonded to l-tyrosine and found in opioid peptides are known to be associated with peptide turns which supports the existence of virtual ring formations.
Figure 13.
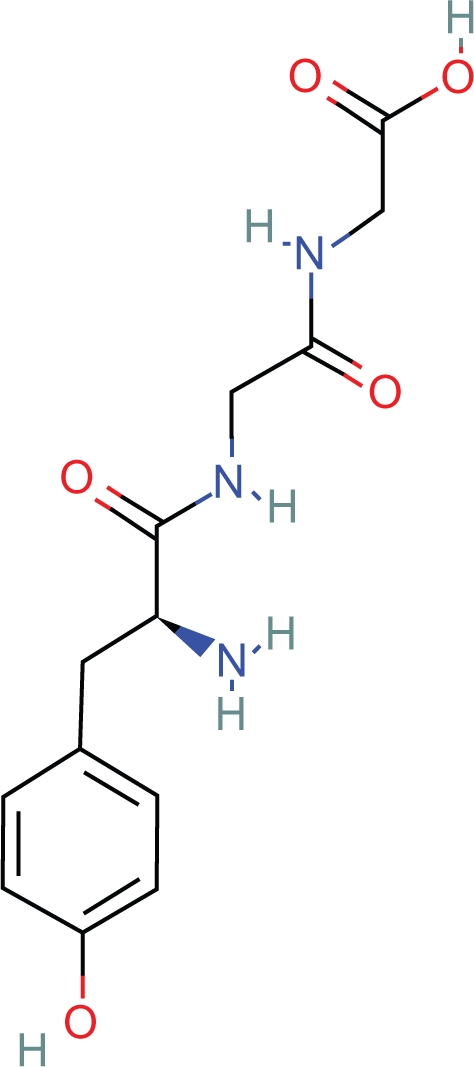
I-Tyr-Gly-Gly.
Figure 14.
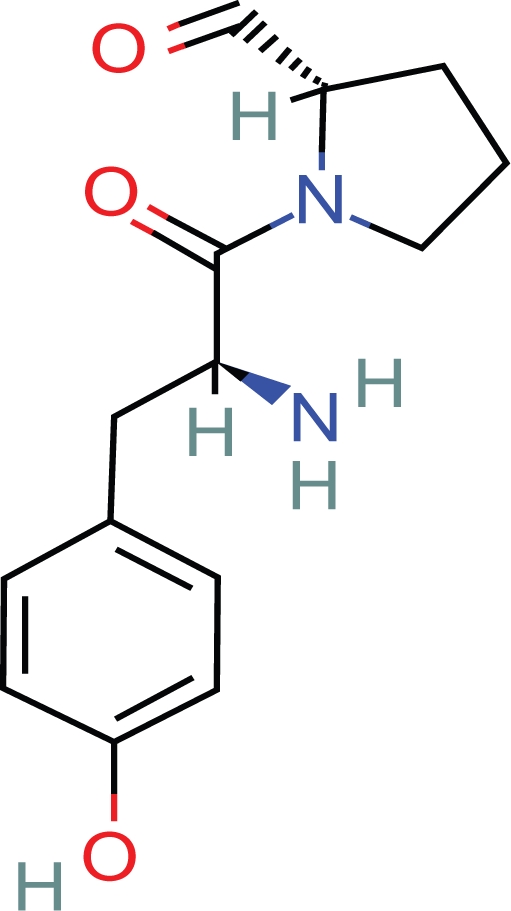
I-Tyr-Pro.
Figure 15.
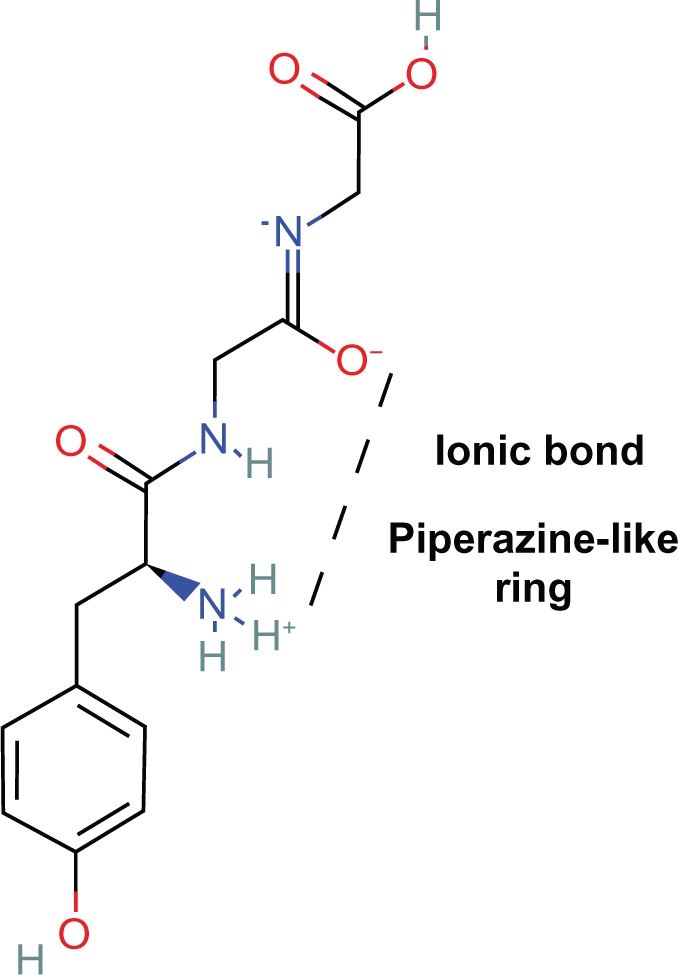
Zwitterion I-Tyr-Gly-Gly.
Figure 16.
Figure 17.
I-Tyr-d-Ala.
Figure 18.
I-Tyr-d-Ala.
Figure 19.
Zwitterion I-Tyr-Pro.
Discussion
In first class opioids and opioid peptides, a heterocyclic ring exists which occupies a similar plane and space as the piperidine ring of morphine. The location and degree of steric hindrance of the ring predicts analgesic activity. Formation of the heterocyclic ring produces stereoisomers of these analgesics, where various enantiomers produce changes in activity. In methadone, these assumptions are based on the enol tautomer of methadone and intramolecular hydrogen bonding producing a stable virtual ring conformation. Supporting the presence of a virtual ring are observations of ring hindrance defining the activity of d and l isomers of methadone and d and l isomers of alanine in tyr-ala. Thus, the stereochemical changes which occur because of the virtual heterocyclic ring and steric hindrance explain many of the pharmacologic activities of the respective isomers.
In l-tyr-gly-gly- and l-tyr-pro ionic attractions between the zwitterions and a peptide turn forms a seven member ring with properties of a six member piperazine-like ring favoring the cis conformation of the peptide bond between the initial first (tyr) and second amino acid which has analgesic activity. Further support of the opioid peptide proposition (#5) is that the inactive peptides beginning with d-tyrosine, positions the ring in a much less favorable position when compared to the piperidine ring of morphine. Thus, first class opioids and opioid peptides have a conformation where heterocyclic rings are approximate to that of morphine and the plane of the heterocyclic ring is defined by two vectors originating from the plane of the aromatic ring and the distance between two points on each plane. This theory, in its simplicity, is presented as an alternative to present theories which describe more complex ligand receptor relationships or do not address the structure activity relationships of both opioids and opioid peptides. Furthermore, the analgesic activities of the small peptides tyr-pro and tyr-gly-gly support this theory but does not exclude that larger more potent opioid peptides may have amino acid residues that slightly favorably change the aromatic-heterocyclic relationship.
It has been demonstrated that more potent opioids (define by receptor binding) are associated with less tolerance.25 The better the “fit” to the receptor, the better the pharmacodynamic response. Although drug tolerance is not the prevailing problem in treating most diseases (if it were, physicians would be constantly readjusting dosage even when there is no disease progression), improved opioid analgesics hybridizing some properties of opioid peptides with opiates may enhance opioid receptor fit and decrease the tolerance that can be associated with morphine and related drugs. Development of a simple stable opioid, opioid peptide or “hybridized” opioid with the essential aromatic-heterocyclic relationships discussed in this writing and with the capability of crossing the blood brain barrier would further substantiate this theory.
Possible Flaws in This Theory
This theory was conceived to answer a basic question: Is there a commonality of structure among first class opioids? Instead of trying to recreate the opioid pharmacophore and explore ligand docking, or perform computational and in silico analysis, this theory attempts to explain structure activity relationships of selected first class opioids primarily on the basis of stereochemistry, physical properties and the structure of morphine as the prototype.26,27 But this theory could be erroneous and may not be accurate because energy minimization, molecular and quantum mechanics, thermodynamics and the message-address concept of opioid binding have not been addressed.28–30 Other more recent developments in opioid pharmacology such as the identification of opioid ligand binding to the toll-like receptor (TLR4), the structure activity relationships of the opioid dipeptide (kyotorphin), or the recently released tapentadol, an opioid/norepinephrine agonist, have not been examined in the context of this theory.31–33 More specifically, there is minimal recent computational evidence to support an active piperazine like ring in opioid peptides or support coexistance of nitrogen inversion with multiple docking sites in the fentanyl series of molecules.34,35 This theory may be overly simplistic and is surely not representative of present mainstream investigations in medicinal chemistry. On the other hand, Louis Pasteur and Emil Fischer made some incredible discoveries based on stereochemistry.
Excluding advances in analgesic bioavailability and modifications of the fentanyl molecule, it has been more than 50 years since a new pure opioid agonist has been made available for clinical use, despite the discovery of the opioid receptors in the 1970s. In addition, the opioid pharmacology citations in PubMed continue to grow at a rapid rate especially in the discipline of computational medicinal chemistry. However, morphine (circa 1804) and methadone (circa 1937) remain the long acting opioids most prescribed in pain centers. With all the investigations being conducted, one would anticipate that new and improved pure opioid agonists should be clinically available and it is hoped that this theory will prove useful even if flaws do exist.
Conclusion
A unified theory based on the stereochemistry of a common aromatic-heterocyclic relationship in opioid and opioid peptides is presented. This theory is supported by five propositions which include experimental data derived from the literature and stereochemical observations from the author’s perspective. Some of the support for the propositions explains new relationships about steric hindrance and optical activity of opioids. This theory could be important for future analgesic drug design.
Acknowledgments
The author would like to thank Julie Rosato of Duke University for preparation of the illustrations. Also Craig L Jackson of Eaton Corporation and Kenneth Goldberg of Durham Veterans Administration Medical Center for assistance in molecular modeling measurements.
The author would like to acknowledge The Pub-Chem Project for the chemical structures used in this text.
Biography
Biography: Dr. Goldberg received his undergraduate degree in chemistry magna cum laude from Duke University as well as his medical degree from Duke. He completed his residency in anesthesiology at the Massachusetts General Hospital and held an appointment as clinical fellow in anesthesiology at Harvard Medical School. Since 1988, Dr. Goldberg has served as Director of the Pain Clinic and Chairman of the Pharmacy and Therapeutics Committee of the Durham Veterans Affairs Medical Center and is presently Associate Professor of Anesthesiology at Duke University School of Medicine. His medical practice is exclusively limited to providing care to United States veterans who suffer from chronic pain. Dr. Goldberg’s research activities include exploring and developing topics in theoretical medicine.

Footnotes
Conflicts of Interest
The author has no conflicts of interest to report. This manuscript was not supported by any funding.
References
- 1.Fürst S, Hosztafi S. The chemical and pharmacological importance of morphine analogues. Acta Physiol Hung. 2008 Mar;95(1):3–44. doi: 10.1556/APhysiol.95.2008.1.1. Review. [DOI] [PubMed] [Google Scholar]
- 2.Janecka A, Perlikowska R, Gach K, Wyrębska A, Fichna J. Development of Opioid Peptide Analogs for Pain Relief. Curr Pharm Des. 2009 Dec 23; doi: 10.2174/138161210790963869. [DOI] [PubMed] [Google Scholar]
- 3.Beckett AH, Casy AF. Stereochemistry of certain analgesics. Nature. 1954 Jun 26;173(4417):1231–2. doi: 10.1038/1731231a0. [DOI] [PubMed] [Google Scholar]
- 4.Beckett AH, Casy AF. Synthetic analgesics: stereochemical considerations. J Pharm Pharmacol. 1954 Dec;6(12):986–1001. doi: 10.1111/j.2042-7158.1954.tb11033.x. [DOI] [PubMed] [Google Scholar]
- 5.Kane BE, Svensson B, Ferguson DM. Molecular recognition of opioid receptor ligands peridyl)-N-phenylpropanamide and N-(3-methyl-1-(1-methyl-2-phenylethyl)-4-piperidyl)-N-phenylpropanamide. J Med Chem. 1974 Oct;17(10):1047–51. doi: 10.1021/jm00256a003. [DOI] [PubMed] [Google Scholar]
- 6.Cometta-Morini C, Maguire PA, Loew GH. Molecular determinants of mu receptor recognition for the fentanyl class of compounds. Mol Pharmacol. 1992 Jan;41(1):185–96. [PubMed] [Google Scholar]
- 7.Patrick, Graham L. An introduction to medicinal chemistry. Oxford University Press; 1995. pp. 264–5. [Google Scholar]
- 8.Takemori AE, Portoghese PS. Comparative antagonism by naltrexone and naloxone of mu, kappa, and delta agonists. Eur J Pharmacol. 1984 Sep 3;104(1–2):101–4. doi: 10.1016/0014-2999(84)90374-1. [DOI] [PubMed] [Google Scholar]
- 9.Gero A. Steric Considerations on the Chemical Structure and Physiological Activity of Methadone and Related Compounds. Science. 1954 Jan 22;119(3082):112–4. doi: 10.1126/science.119.3082.112. [DOI] [PubMed] [Google Scholar]
- 10.Pauling Linus. The nature of the chemical bond. Cornell University Press; 1960. [Google Scholar]
- 11.Sullivan HR, Due SL, McMahon RE. The difference in activity between (+)- and (−)-methadone is intrinsic and not due to a difference in metabolism. J Pharm Pharmacol. 1975 Oct;27(10):728–32. doi: 10.1111/j.2042-7158.1975.tb09391.x. [DOI] [PubMed] [Google Scholar]
- 12.Duax WL, Smith GD, Griffin JF, Portoghese PS. Methadone conformation and opioid activity. Science. 1983 Apr 22;220(4595):417–8. doi: 10.1126/science.6301007. [DOI] [PubMed] [Google Scholar]
- 13.Loew GH, Berkowitz DS, Newth RC. Quantum chemical studies of methadone. J Med Chem. 1976 Jul;19(7):863–9. doi: 10.1021/jm00229a001. [DOI] [PubMed] [Google Scholar]
- 14.Henkel JG, Bell KH, Portoghese PS. Stereochemical studies on medicinal agents. 16. Conformational studies of methadone and isomethadone utilizing circular dichroism and proton magnetic resonance. J Med Chem. 1974 Jan;17(1):124–9. doi: 10.1021/jm00247a023. [DOI] [PubMed] [Google Scholar]
- 15.Martin J, Andrews P. Conformation-activity relationships of opiate analgesics. J Comput Aided Mol Des. 1987 Apr;1(1):53–72. doi: 10.1007/BF01680557. [DOI] [PubMed] [Google Scholar]
- 16.Klein CL, Stevens ED, Fifer EK, Borne RF. Molecular structure of two conformationally restrained fentanyl analogues: cis-and trans-isomers of N-(3-methyl-1-[2-(1,2,3,4-tetrahydro)naphthyl]-4-piperidinyl)-N-phenylpropanamide. J Pharm Sci. 1985 Nov;74(11):1147–51. doi: 10.1002/jps.2600741103. [DOI] [PubMed] [Google Scholar]
- 17.Peptides with opioid activity. www.lycaeum.org/drugs/Peptides/opioidpeptides.html
- 18.Vass E, Hollósi M, Kveder M, Kojić-Prodić B, Cudić MB, Horvat S. Spectroscopic evidence of beta-turn in N-glycated peptidomimetics related to leucine-enkephalin. Spectrochim Acta A Mol Biomol Spectrosc. 2000 Nov 1;56A(12):2479–89. doi: 10.1016/s1386-1425(00)00336-x. [DOI] [PubMed] [Google Scholar]
- 19.Ishida T, Kenmotsu M, Mino Y, et al. X-ray diffraction studies of enkephalins. Crystal structure of [(4’-bromo)Phe4,Leu5]enkephalin. Biochem J. 1984 Mar 15;218(3):677–89. doi: 10.1042/bj2180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomberg D, Hedenström M, Kreye P, Sethson I, Brickmann K, Kihlberg J. Synthesis and conformational studies of a beta-turn mimetic incorporated in Leu-enkephalin. J Org Chem. 2004 May 14;69(10):3500–8. doi: 10.1021/jo0356863. [DOI] [PubMed] [Google Scholar]
- 21.Choh Hao Li, United States Patent 4,317,770, D-amino acid analogs of beta-endorphin, March 2, 1982.
- 22.Loew GH, Burt SK. Energy conformation study of Met-enkephalin and its D-Ala2 analogue and their resemblance to rigid opiates. Proc Natl Acad Sci U S A. 1978 Jan;75(1):7–11. doi: 10.1073/pnas.75.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzevatykh LS, Voronina TA, Emel’ianova TG, et al. [Analgesic activity of dipeptide Tyr-Pro] [Article in Russian] Izv Akad Nauk Ser Biol. 2008 Jan–Feb;:61–7. [PubMed] [Google Scholar]
- 24.Jaba IM, Vasincu D, Manolidis G, Haulică I, Mungiu OC. Experimental data regarding the implications of certain minimum structure enkephalinlike peptides in nociceptive processing. Rom J Physiol. 2004 Jan–Jun;41(1–2):119–26. [PubMed] [Google Scholar]
- 25.Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol. 2007 Jun 1;563(1–3):92–101. doi: 10.1016/j.ejphar.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senderowitz H, Marantz Y. G Protein-Coupled Receptors: target-based in silico screening. Curr Pharm Des. 2009;15(35):4049–68. doi: 10.2174/138161209789824821. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Chase AR, Slivka PF, Baggett CT, Zhao TX, Yin H. Design, synthesis, and evaluation of biotinylated opioid derivatives as novel probes to study opioid pharmacology. Bioconjug Chem. 2008 Dec;19(12):2585–9. doi: 10.1021/bc8003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou T, Huang D, Caflisch A. Quantum Mechanical Methods for Drug Design. Curr Top Med Chem. 2009 Nov 23; doi: 10.2174/156802610790232242. [DOI] [PubMed] [Google Scholar]
- 29.Dailey MM, Hait C, Holt PA, et al. Structure-based drug design: from nucleic acid to membrane protein targets. Exp Mol Pathol. 2009 Jun;86(3):141–50. doi: 10.1016/j.yexmp.2009.01.011. Epub 2009 Jan 31. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pogozheva ID, Przydzial MJ, Mosberg HI. Homology modeling of opioid receptor-ligand complexes using experimental constraints. AAPS J. 2005 Oct 5;7(2):E434–48. doi: 10.1208/aapsj070243. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010 Jan;24(1):83–95. doi: 10.1016/j.bbi.2009.08.004. Epub 2009 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes SC, Soares CM, Baptista AM, Goormaghtigh E, Cabral BJ, Castanho MA. Conformational and orientational guidance of the analgesic dipeptide kyotorphin induced by lipidic membranes: putative correlation toward receptor docking. J Phys Chem B. 2006 Feb 23;110(7):3385–94. doi: 10.1021/jp053651w. [DOI] [PubMed] [Google Scholar]
- 33.Tzschentke TM, Jahnel U, Kogel B, et al. Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc) 2009 Jul;45(7):483–96. doi: 10.1358/dot.2009.45.7.1395291. Review. [DOI] [PubMed] [Google Scholar]
- 34.Borics A, Tóth G. Structural comparison of mu-opioid receptor selective peptides confirmed four parameters of bioactivity. J Mol Graph Model. 2009 Dec 3; doi: 10.1016/j.jmgm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Vucković S, Prostran M, Ivanović M, et al. Fentanyl analogs: structure-activity-relationship study. Curr Med Chem. 2009;16(19):2468–74. doi: 10.2174/092986709788682074. Review. [DOI] [PubMed] [Google Scholar]