Abstract
A subset of cytosolic proteins can be selectively degraded in lysosomes through chaperone-mediated autophagy. The lysosomal-membrane protein type 2A (LAMP-2A) acts as the receptor for the substrates of chaperone-mediated autophagy (CMA), which should undergo unfolding before crossing the lysosomal membrane and reaching the lumen for degradation. Translocation of substrates is assisted by chaperones on both sides of the membrane, but the actual steps involved in this process and the characteristics of the translocation complex were, for the most part, unknown. We have now found that rather than a stable translocon at the lysosomal membrane, CMA substrates bind to monomers of LAMP-2A driving the organization of this protein into a high molecular weight multimeric complex that mediates translocation. Assembly and disassembly of LAMP-2A into and from this complex is dynamic and it is regulated by hsc70 and hsp90, the two lysosomal chaperones related to CMA. This work thus unveils a unique mechanism of protein translocation across the lysosomal membrane, which involves only transient discontinuity of the membrane. The possible advantages of this transitory lysosomal translocon are discussed in light of the unique properties of the lysosomal compartment.
Keywords: autophagy, chaperones, membrane dynamics, membrane proteins, protein translocation
A subset of cytosolic proteins bearing a targeting motif in their amino acid sequence can be selectively targeted to lysosomes for degradation by chaperone-mediated autophagy (CMA) upon recognition of the targeting motif by a cytosolic chaperone.1,2 Hsc70, the constitutive member of the heat shock family of proteins of 70 kDa, interacts with the cytosolic substrates in an ATP-dependent manner and brings them to the surface of the lysosomes3 where they bind to a single-span membrane protein, the lysosome-associated membrane protein type 2 (LAMP-2A), which acts as receptor for CMA substrates.4
Delivery of CMA substrates into the lysosomal lumen takes place by a mechanism that is different from those described for macroautophagy or microautophagy. In fact, formation of autophagosomes, vesicular fusion events, or invaginations of the lysosomal membrane are not required for CMA-dependent substrate translocation. CMA is a saturable process,5,6 depends on binding to a receptor protein,4 and requires complete unfolding of substrate proteins before they can access the lysosomal lumen.7 These three characteristics make CMA resemble the direct translocation of proteins across membranes as described for other organelles such as the mitochondria, nucleus, peroxisomes and the endoplasmic reticulum. Each of these membrane transport systems presents unique characteristics. Our recent study of the translocation of cytosolic proteins into the lysosomal lumen via CMA has revealed yet another variation in the way in which proteins can cross membranes.8
In previous studies we found that the cytosolic proteins delivered to the lysosomal membrane by the hsc70 chaperone complex bind to the cytosolic tail of LAMP-2A.4,9 LAMP-2A is one of the three alternative spliced variants of a single gene, lamp2. LAMP-2A, B and C, share an identical lumenal region but differ in their trans-membrane and cytosolic tail10 which confer on each of them unique properties and functions. We found that only the cytosolic tail of LAMP-2A is recognized by the CMA substrates.11 In fact, RNAi against LAMP-2B and LAMP-2C did not affect CMA of cytosolic proteins (Zhang et al., in preparation).
To elucidate the mechanisms involved in the translocation of cytosolic proteins into the lysosomal lumen via CMA, we recently analyzed the dynamics of LAMP-2A at the lysosomal membrane. We hypothesized that since binding of CMA substrates to the cytosolic tail of LAMP-2A is required for their internalization, following the dynamics of substrate bound and unbound LAMP-2A could provide valuable information on the mechanism behind substrate translocation via CMA. We used three complementary approaches to analyze the organization of LAMP-2A at the lysosomal membrane: two types of native gel electrophoresis, molecular exclusion chromatography, and density gradient centrifugation.8 We found that LAMP-2A can be detected in different protein complexes at the lysosomal membrane and that the percentage of LAMP-2A present in each of these complexes varies with changes in CMA activity. In fact, in the presence of CMA substrates, LAMP-2A becomes enriched in a protein complex of 700 kDa. Mutations in the transmembrane region of LAMP-2A prevent its association with this protein complex and block CMA substrate translocation. These results support the idea that formation of the 700 kDa complex is necessary for substrates to cross the membrane. Substrate binding was, however, still preserved even when LAMP-2A was no longer incorporated into the complex, revealing that substrate proteins can bind to monomeric forms of LAMP-2A. In fact, we failed to detect binding of substrates to LAMP-2A once the protein was in the 700 kDa complex.
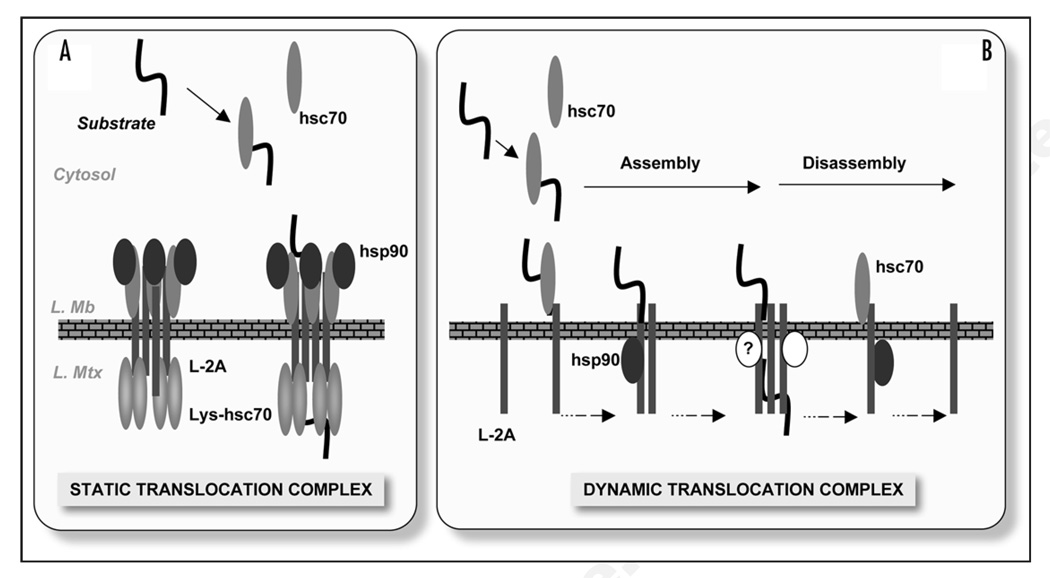
Previous studies demonstrate the interaction of LAMP-2A with substrate proteins and with a subset of chaperones and co-chaperones at the lysosomal membrane (hsc70, hsp90, hip, hop, bag-1 and hsp40).12 Those of us interested in this type of selective autophagy have traditionally drawn models of the CMA translocation complex that bear a strong resemblance to the mitochondria or ER trans-locons, where multimeric forms of LAMP-2A sit at the lysosomal membrane forming a stable complex with chaperones on both sides (Fig. 1, left). In these models, the substrate proteins would bind to the exposed tails of LAMP-2A proteins, and the associated chaperones would assist in substrate unfolding, translocation across the membrane and pulling the substrate into the lysosomal matrix. However, to our surprise, chaperones known to associate with LAMP-2A were not present in the 700 kDa complex required for translocation of substrates.8 Interaction between these proteins and LAMP-2A occurs in intermediate multimeric complexes but never in the 700 kDa complex. An added twist in our proposed model is the fact that the 700 kDa complex does not seem to remain as a stable complex at the lysosomal membrane, but instead there is a dynamic assembly and disassembly of LAMP-2A into and out of complexes.8
Figure 1.
CMA translocation at the lysosomal membrane. (A) Hypothetical model of the CMA translocation complex at the lysosomal membrane by analogy to the transport systems in other organelles. The translocation complex was conceived as a stable structure organized around multiple LAMP-2A (L-2A) molecules flanked by chaperones on both sides of the lysosomal membrane (L. Mb). Substrates bind to the exposed cytosolic tails, and chaperones facilitate substrate unfolding and translocation across the membrane and into the lysosomal matrix (L. Mtx). (B) Model of the CMA translocation complex proposed as a result of our recent studies.8 Substrate proteins bind to monomers of LAMP-2A, which progressively organize into a multimeric complex that facilitates delivery of substrate proteins to the lumen. Lysosomal chaperones, hsc70 and hsp90, interact with LAMP-2A when present in intermediate complexes formed during assembly and disassembly of the translocation complex. Other yet to be identified proteins (?) could also contribute to the formation of this translocation complex at the lysosomal membrane.
Both chaperones hsc70 and hsp90 play an active role in the dynamic association/dissociation of LAMP-2A to/from the CMA translocation complex.8 Added to the original function in delivery/ presentation of substrates to LAMP-2A, hsc70 fulfills a second function facilitating disassembly of LAMP-2A from the translocation complex. This dual function of hsc70 is modulated by the substrate proteins. In the presence of CMA substrates, hsc70 favors their binding to LAMP-2A, promoting the assembly of the translocation complex, whereas in the absence of substrates, addition of hsc70 facilitates dissociation of LAMP-2A into monomers.8 We also find that association of hsp90 with LAMP-2A is required to preserve LAMP-2A stability when transitioning from monomer to the multimeric complex or vice versa. Addition of hsp90 inhibitors remarkably decrease the amount of LAMP-2A at the lysosomal membrane. To our surprise, the hsp90 involved in this stabilizing function is not associated with the cytosolic side of the lysosomal membrane but instead corresponds to a population that is bound to its inner side. A study of the topology of the lysosome-associated hsp90 reveals that most of the chaperone (almost 85%) is bound to the lysosomal membrane, and that hsp90 distributes almost evenly between the cytosolic and the lumenal side of the membrane. The fact that the destabilizing effect on LAMP-2A of the hsp90 inhibitors, described above, is still observed after removing the hsp90 bound to the cytosolic side of the lysosomal membrane supports the finding that it is the hsp90 bound on the lumenal side of the lysosomal membrane that is the one that contributes to stabilization of LAMP-2A.8 Although most of the LAMP-2A molecule (from the N terminus to the transmembrane region) is continuously exposed to an environment with the highest content of proteases known inside the cell—the lysosomal matrix—the high level and complexity of glycosylation of this membrane protein has been proposed to protect its peptide backbone from proteolytic cleavage. It is conceivable that conformational changes within LAMP-2A required for its incorporation into the high molecular weight complex may expose regions of the protein, usually covered by the sugar residues, and that the interaction of hsp90 with those regions makes them inaccessible to the lumenal proteases. In fact, comparison of the susceptibility of LAMP-2A to exogenously added proteases in the presence or absence of hsp90, reveals higher resistance to cleavage in the presence of the chaperone. How the chaperone itself is protected from the lumenal proteases, what regulates binding/release of hsp90 from LAMP-2A and whether or not co-chaperones or other lysosomal membrane proteins modulate this interaction are all questions that remain to be elucidated.
Based on our recent study, we propose that assembly/disassembly of LAMP-2A into a translocation complex may be a continuous process occurring at the lysosomal membrane. But, why is this complex so dynamic in nature? Why not have a stable translocation unit at the membrane where substrate proteins bind, unfold, and cross the membrane, as we had initially predicted, similar to those of other organelle membranes such as the mitochondria or ER? Maybe the reason for the transient nature of this translocation complex is a direct consequence of the distinctive characteristics of the lysosomes, namely their acidic lumenal pH and their high concentration of enzymes able to hydrolyze any kind of cellular component. The ability to maintain the drastic difference of pH between both sides of the lysosomal membrane could be easily jeopardized by membrane discontinuities. Furthermore, if the membrane gap is big enough to allow proteins to go in, it might also be a possible avenue for the lumenal hydrolases to escape into the cytosol. In this respect, a very tightly regulated channel or pore may be the best option to preserve separation of intralysosomal and extralysosomal components. An alternative mechanism, which seems to be supported by our data, would be to make the discontinuity very transient. What are the possible advantages of a transient translocation complex versus a tightly regulated one? We speculate that dynamic assembly/disassembly avoids the possible risks associated with the loss of regulation of a stable complex. In addition, it may provide higher functional flexibility allowing the lysosomal system to rapidly accommodate to changes in the available cargo.
The unique characteristics of the CMA translocation complex now provide a new mechanism of regulation that could have important consequences for CMA pathophysiology. Lateral mobility of LAMP-2A in the lysosomal membrane and the previously described association of this receptor with lipid microdomains in this membrane13 become critical for maintenance of the dynamics of the translocation complex. Changes in the lipid composition of the lysosomal membrane or other modifications that affect its fluidity could be behind the functional dysregulation of CMA described in certain pathologies and in aging.14
Acknowledgements
This work was supported by NIH/NIA grants AG021904, AG025355 and DK041918 and by an Ellison Medical Foundation Award.
Footnotes
Addendum to: Bandyopadhyay U, Kaushik S, Vartikovski L, Cuervo AM. Dynamic organization of the receptor for chaperone-mediated autophagy at the lysosomal membrane.
References
- 1.Dice J. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 2.Massey A, Zhang C, Cuervo A. Chaperone-mediated autophagy in aging and disease. Curr Top Dev Biol. 2006;73:205–235. doi: 10.1016/S0070-2153(05)73007-6. [DOI] [PubMed] [Google Scholar]
- 3.Agarraberes F, Terlecky S, Dice J. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuervo A, Dice J. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 5.Cuervo A, Terlecky S, Dice J, Knecht E. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J Biol Chem. 1994;269:26374–26380. [PubMed] [Google Scholar]
- 6.Terlecky S, Dice J. Polypeptide import and degradation by isolated lysosomes. J Biol Chem. 1993;268:23490–23495. [PubMed] [Google Scholar]
- 7.Salvador N, Aguado C, Horst M, Knecht E. Import of a cytosolic protein into lysosomes by chaperone-mediated autophagy depends on its folding state. J Biol Chem. 2000;275:27447–27456. doi: 10.1074/jbc.M001394200. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay U, Kaushik S, Vartikovski L, Cuervo AM. Dynamic organization of the receptor for chaperone-mediated autophagy at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Nat Acad Sci USA. 2006;103:5905–5910. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskelinen E-L, et al. Unifying nomenclature for the isoforms of the lysosomal membrane protein LAMP-2. Traffic. 2005;6:1058–1061. doi: 10.1111/j.1600-0854.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 11.Cuervo A, Dice J. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000;113:4441–4450. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- 12.Agarraberes F, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. doi: 10.1242/jcs.114.13.2491. [DOI] [PubMed] [Google Scholar]
- 13.Kaushik S, Massey AC, Cuervo AM. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 2006;25:3921–3933. doi: 10.1038/sj.emboj.7601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiffin R, et al. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]