Abstract
The glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) are a group of peptides that have been implicated as important factors in inflammation, since they are released in increased amounts during inflammation and induce thermal hyperalgesia upon injection. Isolated sensory neurons in culture and freshly dissociated spinal cord slices were used to examine the enhancement in stimulated-release of the neuropeptide, calcitonin gene-related peptide (CGRP), as a measure of sensitization. Exposure of isolated sensory neurons in culture to GDNF, neurturin, and artemin enhanced the capsaicin-stimulated release of immunoreactive CGRP (iCGRP) two to three fold, but did not increase potassium-stimulated release of iCGRP. A similar profile of sensitization was observed in freshly dissociated spinal cord slices. Persephin, another member of the GFL family thought to be important in development, was unable to induce an enhancement in the release of iCGRP. These results demonstrate that specific GFLs are important mediators affecting sensory neuronal sensitivity, likely through modulation of the capsaicin receptor. The sensitization of sensory neurons during inflammation, and the pain and neurogenic inflammation resulting from this sensitization, may be due in part to the effects of these selected GFLs.
Keywords: artemin, dorsal root ganglia, inflammation, neurturin, sensitization
Inflammatory mediators, which are released in increased amounts in a number of diseases (Schaible et al., 2002; Yang et al., 2003), are implicated in hyperalgesia and sensory neuronal sensitization. Growth factors are one set of mediators found in higher concentrations during inflammation. Although growth factors previously were thought to be responsible only for the growth and maintenance of sensory neurons, they are postulated now to be responsible for inflammatory hyperalgesia (Mendell et al., 1999). Nerve growth factor (NGF) is one such mediator with an established role in inflammatory hyperalgesia and sensory neuronal sensitization (Lewin et al., 1993; McMahon, 1996; Shu and Mendell, 1999). Another set of molecules found in higher amounts during inflammation is the glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs). The levels of GFLs in the joint capsule and plasma of patients with osteoarthritis, Crohn's disease, and interstitial cystitis are greatly increased compared to people without these diseases (Okragly et al., 1999; De et al., 2004; von Boyen et al., 2006). Induction of inflammation by injection of complete Freund's adjuvant (CFA) or lipopolysaccharide (LPS) results in increased levels of the GFLs, GDNF, neurturin (NTN), and artemin (ART; Amaya et al., 2004; Hashimoto et al., 2005; Malin et al., 2006).
The GFLs are a group of small peptides in the TGFβ super-family of molecules. They exist naturally as homodimers and include GDNF, NTN, ART, and persephin (PSP; Eigenbrot and Gerber 1997; Wang et al., 2003). The actions of GFLs are initiated by binding to specific GDNF family receptor alpha subtypes (GFRα), glycolsyl phosphotidylinositol (GPI)-linked surface receptors found in lipid rafts. The GFL-GFRα. complex translocates to the receptor tyrosine kinase, Ret, to initiate intracellular signaling (reviewed by Saarma, 2001; Sariola and Saarma, 2003). Higher binding affinity is found for GDNF and GFRα1, NTN and GFRα2, ART and GFRα3 and PSP and GFRα4. Non-specific binding can occur between GDNF and GFRα2 or GFRα3 (Airaksinen and Saarma, 2002). Evidence also exists for non-specific binding of ART and NTN to GFRα1 (Airaksinen and Saarma, 2002).
There is a growing body of evidence that, through binding to and translocation of GFRα receptors, select GFLs (GDNF, NTN, and ART) play a role in the induction of hyperalgesia (Amaya et al., 2004; Malin et al., 2006; Vellani et al., 2006). Importantly, there is significant overlap in the expression of the GFRα1-3 receptors and the transient receptor potential vanilloid type 1 (TRPV1) receptor, a ligand-gated ion channel that is activated by noxious stimuli, including heat and acidic pH (Aoki et al., 2005; Malin et al., 2006). Application of GDNF to isolated sensory neurons increases their inward flux of calcium in response to capsaicin, a molecule used to activate TRPV1, and the number of TRPV1 channels present on the neurons (Anand et al., 2006). These same selected GFLs enhance both the peak calcium current and area under the curve of the calcium current in response to capsaicin in isolated sensory neurons, presumably via activation of the TRPV1 channel (Malin et al., 2006). Additionally, increased expression of acid sensing ion channels (ASICs) has been observed in the skin in response to GDNF exposure (Albers et al., 2006).
While there is evidence that select GFLs increase TRPV1-mediated calcium influx in sensory neurons, the cellular consequences of this change have not been established. The neuropeptide CGRP, found predominately in small diameter, nociceptive sensory neurons, has a high degree of co-localization with TRPV1 (Aoki et al., 2005). CGRP functions as a mediator of neurogenic inflammation in the periphery and has been associated with potentiation of the pain signal from primary sensory neurons to second order neurons in the spinal cord (Brain et al., 1985; Miletic and Tan, 1988; Ryu et al., 1988). To determine if GFLs alter the integrative activity of the primary sensory neuron, the ability of GFLs to alter the release of iCGRP from dorsal root ganglia (DRG) neurons was examined. Increase in the release of iCGRP from DRG neurons, indicating peripheral sensitization, could account in part for the hyperalgesia induced by GFLs.
Experimental Procedures
Materials
The mice used for all experiments, C57BL/6 mice, were purchased from Harlan Laboratories (Indianapolis, IN) and/or bred and housed in the Indiana University Laboratory Animal Research Center (LARC). Mice were housed in group cages in a light-controlled room at a constant temperature of 22° C. All mice were adults, between three and six months in age. Food and water were available at the convenience of the animals. Capsaicin was purchased from Sigma Chemical Company (St. Louis, MO) and was first dissolved in 1-methyl,2-pyrrolidinone (Aldrich Chemical Co., Milwaukee, WI) to a concentration of 10 mM. It was then serially diluted to a concentration of 50-500 nM in the appropriate release buffer as noted below. Horse serum, F-12 medium, L-glutamine, and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). NGF was purchased from Harlan Bioproducts for Science, Inc. (Indianapolis, IN, USA). Collagenase, poly-D-lysine, laminin, 5-fluoro-2-deoxyuridine, uridine and standard laboratory chemicals were from Sigma (St. Louis, MO, USA). Antibody to calcitonin gene-related peptide (CGRP) was generously provided by Michael R. Vasko (Indiana University School of Medicine, Indianapolis, IN, USA and originally produced by Michael J. Iadarola, NIH). The GFLs were purchased from Peprotech (Rocky Hills, NJ).
Preparation of dorsal root ganglia (DRG) cultures
Dorsal root ganglia (DRG) from adult mice were used to establish sensory neuronal cultures. Briefly, the DRG were removed from adult mice in a manner similar to that previously published (Hingtgen, et al., 2006). DRG were digested in 0.1% collagenase in two separate 30 minute incubations at 37 °C. Additionally, cells were digested in DNAse for one minute at room temperature. Lastly, the preparation was dissociated by mechanical agitation. Cells were plated in wells of 24-well Falcon culture dishes coated with poly-D-lysine and laminin at a density of 30,000–50,000 cells/well. Cultures were maintained at 37°C in a 5% CO2 atmosphere in F12 media supplemented with 2 mM glutamine, 50 μg/mL penicillin and streptomycin, 10% heat-inactivated horse serum and mitotic inhibitors (50 μM 5-fluoro-2-deoxyuridine and 150 μM uridine). NGF, at a concentration of 30 ng/mL, was added to this media. Growth medium was changed every 2–3 days, and the added NGF removed 48 hrs prior to all experiments.
Stimulated-Release of iCGRP
Measurement of stimulus-evoked release and content of immunoreactive CGRP (iCGRP) from isolated sensory neurons was accomplished as previously published (Hingtgen et al., 2006). After 5-7 days in culture, culture media was removed from the sensory neurons in culture and the basal or resting release of iCGRP measured from cells incubated for 10 minutes in HEPES buffer consisting of (in mM): 25 HEPES, 135 NaCl, 3.5 KCl, 2.5 CaCl2, 1 MgCl2, 3.3 dextrose, and 0.1% (w/v) bovine serum albumin, pH 7.4, and maintained at 37 °C. The cells were incubated in HEPES buffer containing stimulus (capsaicin or high potassium) for 10 minutes, and then incubated again with HEPES buffer alone to reestablish resting release levels. The concentrations of capsaicin and potassium were chosen because they lie on the low end of the highly sloped portion of the concentration response curve for iCGRP release (data not shown). The use of these concentrations allow for evaluation of enhancement in release of CGRP after exposure to sensitizing molecules. The amount of iCGRP released in each incubation was measured by radioimmunoassay (RIA). After the release protocol, the remaining peptide content in each well was determined by exposing the cells to 2 N acetic acid for 10 minutes. Aliquots of this incubation were diluted in HEPES and iCGRP was determined by RIA. The release of iCGRP during the 10 min incubation period is expressed as percent of the total content. GFLs were added in the basal incubation period (10 minutes) and in the stimulated incubation period (10 additional minutes). The neurons were exposed to GFLs for a total time of 20 minutes. A minimum of three different preparations were used for each condition, including growth factor application and stimulus.
Stimulus-evoked release and content of iCGRP from spinal cord slices was accomplished as previously published (Chen et al., 1996; Southall et al., 1998). Briefly, the entire spinal cord was removed from each animal. It was weighed and chopped into 300 μm cross-sections using a McIllwain Tissue Chopper. The chopped spinal cord from each animal was placed into its own individual chamber and perfused at a rate of 0.1 mL/minute for 20 minutes with HEPES buffer supplemented with 200 mM ascorbic acid, 100 μM Phe-Ala, and 20 μM bacitracin (all used as peptidase inhibitors to prevent the breakdown of CGRP during the process; Chen et al., 1996). The perfusion buffer was aerated with 95% O2/5% CO2 and maintained at a pH of 7.4. Serial 10 minute collections (1.0 mL of perfusate) were obtained from each spinal cord. Initially, the tissue was perfused with HEPES buffer alone or HEPES buffer containing 10 ng/mL growth factor for 30 minutes. The perfusate was changed to HEPES buffer containing 500 nM capsaicin ± 10 ng/mL growth factor for 30 minutes to measure stimulated release. For release experiments in spinal cord tissue, 500 nM capsaicin was used as a stimulus for peptide release. The higher concentration was used to ensure proper penetrance of the capsaicin into the tissue because of the lipophilic nature of capsaicin and the substantial density of the spinal cord tissue, and based on previous uses of this method (Chen et al., 1996; Southall et al., 1998). The tissue was perfused for 60 minutes with HEPES buffer after the stimulus exposure to allow a return to resting levels of peptide release. Aliquots from each 10 minute collection period were assayed for iCGRP using RIA. After the protocol was completed, the remaining iCGRP content of the tissue was determined by homogenizing the spinal cord tissue in 0.1 N HCl and serially diluting the supernatant with HEPES buffer and 1.0 M MES. The content was added to the amount of iCGRP released during the entire perfusion to obtain the total peptide content. The release of iCGRP during each 10 min period of perfusion is expressed as percent of the total iCGRP content for the tissue.
An RIA was used to quantify the amount of iCGRP released in the basal and stimulated conditions for both experiments with cells in culture and spinal cord tissue. The minimum amount of iCGRP detected by the RIA is 5 fmol with a 95% confidence interval (Chen et al., 1996). Additionally, the GFLs are peptides, which have the potential to affect the RIA. To that end, separate standard curves for the RIA were conducted using HEPES buffer containing each of the GFLs at the highest concentration used in experiments. None of the GFLs affected the sensitivity of the RIA (data not shown).
Statistical Analyses
Results, represented as percent total content of iCGRP, are expressed as the mean ± standard error of the mean (SEM). All differences in iCGRP release and total content were compared with analyses of variance (ANOVAs) and Dunnett's post hoc analysis or Student t-tests, as indicated. A p value of <0.05 was used to indicate statistical significance between treatment and non-treatment groups.
Results
GFLs enhance the capsaicin-stimulated release of iCGRP from isolated sensory neurons
The levels of the GFLs are increased during inflammation (Aloe et al., 1992) and treatment of isolated sensory neurons with GFLs increases intracellular calcium levels in response to capsaicin (Malin et al., 2006). While the local levels of the GFLs near the sensory neurons in intact animals have not been established, levels in whole brain (Kirik et al., 2000) and in plasma (Onodera et al., 1999) are in the high pg/mL and low ng/mL range. Additionally, the concentrations of the GFLs used in previous experiments on freshly dissociated sensory neuronal preparations and sensory neurons in culture are between 1 ng/mL and 100 ng/mL (Malin et al., 2006; Price et al., 2005). These concentrations correspond to 0.0667 nM to 6.67 nM for GDNF, 0.0847 nM to 8.47 nM for NTN, 0.0833 nM to 8.83 nM for ART, and 0.0971 nM to 9.71 nM for PSP. To determine if the actions of GFLs on the TRPV1 receptor result in increased functional output, such as enhanced transmitter release, the ability of different GFLs to modulate the stimulated-release of iCGRP from isolated sensory neurons was measured. Studies of the effects of the GFLs on sensory neuronal sensitization were conducted with concentrations of the GFLs between 0.1 ng/mL and 500 ng/mL to remain in the physiological range and to correspond to concentrations used in similar experiments.
Individual preparations of isolated sensory neurons in culture were exposed to different concentrations of GDNF for 10 minutes prior to and during a 10 minute capsaicin-stimulated period. In the absence of GDNF, basal release of iCGRP was 7.15 ± 1.25 fmol/well and capsaicin-stimulated release was 78.84 ± 6.21 fmol/well (mean ± SEM). When expressed as the percent of the total content of iCGRP in the well, these values correspond to 0.45 ± 0.11% in the basal condition and 4.19 ± 0.93% in the capsaicin-stimulated condition (Figure 1). When 1 or 10 ng/mL GDNF was added, capsaicin-stimulated release of iCGRP was significantly enhanced (No GFL: 4.19 ± 0.93%, 0.1 ng/mL GDNF: 6.74 ± 1.87%, 1.0 ng/mL GDNF: 8.45 ± 1.05%, 10 ng/mL GDNF: 8.35 ± 1.16%, 100 ng/mL GDNF: 6.34 ± 1.15%; Figure 1). There was no change in the basal release of iCGRP with exposure to GDNF. In addition, and as seen in figure 2, 10 ng/mL NTN and ART also significantly enhanced the capsaicin-stimulated release of iCGRP (No GLF: 4.30 ± 0.58%, NTN: 10.60 ± 1.70%, ART: 10.00 ± 1.57%). Unlike the other GFLs, PSP did not alter the capsaicin-stimulated release of iCGRP (PSP: 3.40 ± 0.52%). This may result from a lack of functional GFRα4 in adult DRG neurons, and the fact that PSP binds specifically to GFRα4 (Enokido et al., 1998; Paveliev et al., 2004). Even at concentrations as high as 500 ng/mL, PSP was unable to enhance the capsaicin-stimulated release of iCGRP (Table 1). Additionally, to ensure that the GFLs did not directly alter the resting release of iCGRP, sensory neurons were exposed to the GFLs for two consecutive 10 minute incubations in the absence of any stimulus. No enhancement in the release of iCGRP was observed with these treatments (Table 2). These data indicate that GDNF, NTN, and ART alter the sensitivity of sensory neurons to capsaicin stimulation, but do not directly evoke the release of CGRP.
Figure 1. GDNF enhances capsaicin-stimulated release of iCGRP from isolated sensory neurons.
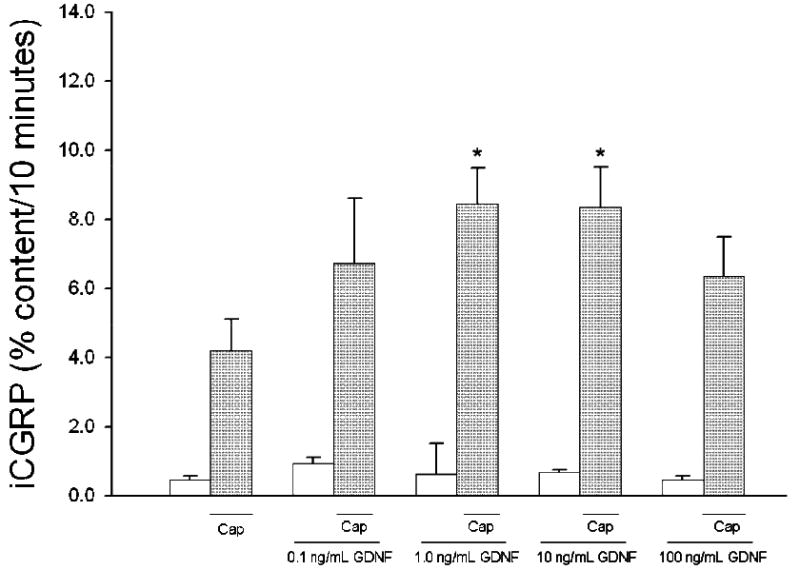
Peptide release elicited by a 10 minute exposure to HEPES buffer alone (open bars) or HEPES buffer containing 50 nM capsaicin (Cap; dark bars) is expressed as mean percent total peptide content of cells in each well ± SEM (n = 9 wells per condition). GDNF (at concentrations from 0.1 ng/mL to 100 ng/mL) was included in the 10 minutes prior to and throughout capsaicin exposure. Total growth factor exposure time was 20 minutes. Asterisks (*) indicate statistically significant differences in iCGRP release between treatment groups and the no GFL condition using an ANOVA with Dunnett's post-hoc test (p<0.05). In all cases, release stimulated by capsaicin was significantly higher than basal release.
Figure 2. GFLs enhance capsaicin-stimulated release of iCGRP from isolated sensory neurons.
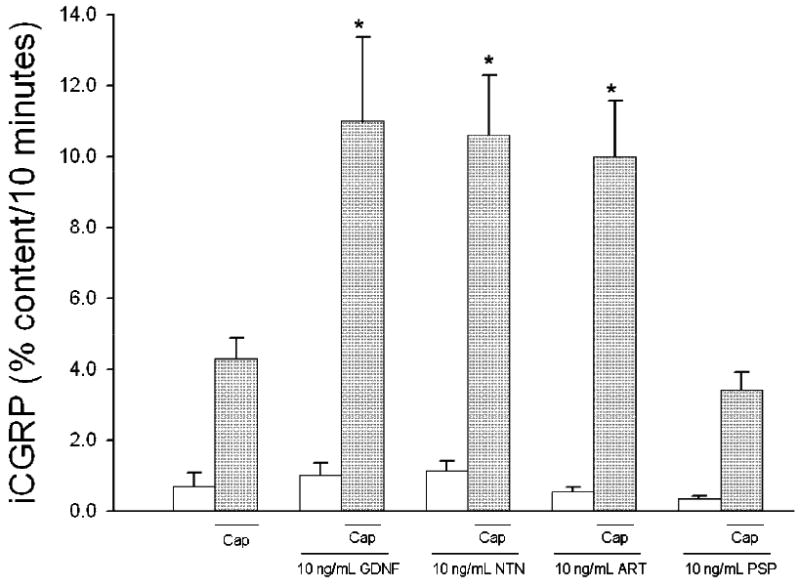
Peptide release elicited by a 10 minute exposure to HEPES buffer alone (open bars) or HEPES buffer containing 50 nM capsaicin (Cap; dark bars) is expressed as mean percent total peptide content of cells in each well ± SEM (n = 9-22 wells per condition). GDNF, neurturin (NTN), artemin (ART), or persephin (PSP), at 10 ng/mL, was included in the 10 minutes prior to and throughout capsaicin exposure. Total growth factor exposure time was 20 minutes. Asterisks (*) indicate statistically significant differences in iCGRP release between treatment groups and the no GFL condition using an ANOVA with Dunnett's post-hoc test (p<0.05). In all cases, release stimulated by capsaicin was significantly higher than basal release.
Table 1.
Persephin Does Not Enhance the Capsaicin-Stimulated Release of iCGRP
| Basal | 50 nM Capsaicin | |
|---|---|---|
| No GFL | 0.67 ± 0.13 | 5.28 ±0.46 |
| 10 ng/mL PSP | 0.68 ± 0.18 | 5.94 ± 0.88 |
| 100 ng/mL PSP | 0.68 ± 0.16 | 4.97 ± 0.41 |
| 500 ng/mL PSP | 1.23 ± 0.16 | 5.59 ± 0.64 |
All values are mean ± SEM % content iCGRP released, n = 9 wells per condition
Table 2.
20 Minute Exposure to GFLs Does Not Increase Release of iCGRP
| GFL | No GFL | 10 minute exposure to GFL | 20 minute exposure to GFL |
|---|---|---|---|
| No GFL | 0.55 ± 0.35 | 0.43 ± 0.11 | 0.76 ± 0.20 |
| 10 ng/mL GDNF | 0.9E ± 0.12 | 0.67 ± 0.14 | 0 50 ± 0 19 |
| 10 ng/mL NTN | 0.41 ± 0.06 | 0.44 ± 0.04 | 0.70 ± 0.15 |
| 10 ng/mL ART | 0.91 ± 0.30 | 0.68 ± 0.20 | 0.54 ± 0.16 |
All values are mean ± SEM % content iCGRP released, n = 9 wells per condition
When sensory neurons are exposed to the GFLs for several days, the levels of CGRP are increased (Ramer et al., 2003; Price et al., 2005). To ensure that the GFL-induced enhancement in capsaicin-stimulated release of iCGRP was not the result of an increase in the total content of iCGRP, we measured iCGRP content at the end of each experiment. There was no change in the total content of iCGRP after the 20 minute exposure to the GFLs (No GFL: 1487 ± 154 fmol/well, GDNF: 1322 ± 108 fmol/well, NTN: 1500 ± 128 fmol/well, ART: 1320 ± 102 fmol/well, and PSP: 1518 ± 177 fmol/well, n = 9-22 wells per condition).
NGF treatment of sensory neuronal cultures increases the expression of TRPV1 (Xue et al., 2007) and increases the amount of TRPV1 insertion into the plasma membrane (Stein et al., 2006). Additionally, sensory neuronal exposure to NGF in culture increases the expression of CGRP and other neuropeptides (MacLean et al., 1989; Sango et al., 1994). Therefore, there is a possibility that some of the media components, specifically the NGF, are affecting the responses of the sensory neurons to either or both the capsaicin and the GFLs. To address this concern, we conducted CGRP release studies on neurons grown in the presence or absence of NGF to determine whether this change in the media components would alter the capsaicin-stimulated release and the GFL-induced enhancement in this release. The presence or absence of NGF in the culture media did not change the magnitude of capsaicin-stimulated release of iCGRP or the GDNF-induced enhancement of peptide release (Figure 3). The absolute level of capsaicin-stimulated iCGRP released when NGF was omitted from the culture media was ∼25% less than when NGF was present (No added NGF: 97.39 ± 10.42 fmol/well, Growth in 30 ng/ml NGF: 127.83 ± 11.24 fmol/well). While the amount of capsaicin-evoked release was enhanced by 10 ng/mL GDNF in both conditions, the absolute level of iCGRP released was again ∼25% less in the cells that were not exposed to NGF (No added NGF: 162.54 ± 8.45, Growth in 30 ng/ml NGF: 219.27 ± 21.86 fmol/well). The reduction in absolute levels of iCGRP released from sensory neurons maintained in culture in the absence of added NGF, but an absence of change in the percent of the total content of iCGRP released is consistent with previous observations (Park et al., 2006).
Figure 3. NGF in culture media does not change the stimulated release of iCGRP and the GDNF-induced enhancement in release.
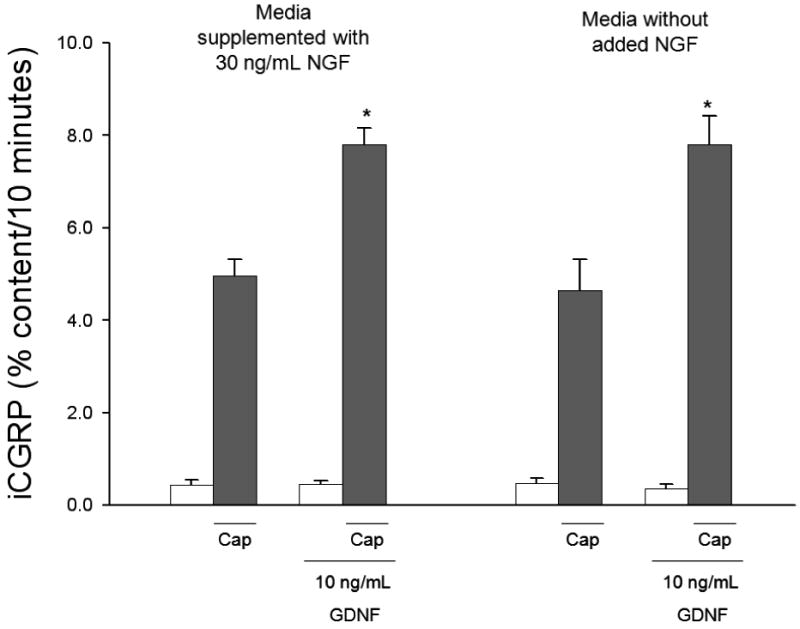
Peptide release elicited by a 10 minute exposure to HEPES buffer alone (open bars) or HEPES buffer containing 50 nM capsaicin (Cap; dark bars) is expressed as mean percent total peptide content of cells in each well ± SEM (n = 9 wells per condition). 10 ng/mL GDNF was included in the 10 minutes prior to and throughout capsaicin exposure. Total growth factor exposure time was 20 minutes. NGF was added in the culture media at a concentration of 30 ng/mL or omitted from the culture media. Asterisks (*) indicate statistically significant differences in iCGRP release between GDNF treatment group and the no GFL condition using an t-test (p<0.05). In all cases, release stimulated by capsaicin was significantly higher than basal release.
GFLs do not enhance the potassium-stimulated release of iCGRP from isolated sensory neurons
Previous studies have focused primarily on GFL-induced changes in response to capsaicin in isolated DRG neurons. To determine whether responses to stimuli other than capsaicin could be enhanced by GFLs, a general depolarizing stimulus, high extracellular potassium, was used. Exposure to HEPES buffer containing 50 mM high extracellular potassium (KCl) for 10 minutes caused a release of iCGRP of 6.99 ± 1.06%. Treatment with GDNF, NTN, or ART (10 ng/mL) 10 minutes prior to and throughout the stimulus period did not alter KCl-stimulated iCGRP release (Figure 4; No GFL: 6.99 ± 1.06%, GDNF: 6.95 ± 0.92%, NTN: 6.63 ± 0.61%, and ART: 6.74 ± 1.11%). In addition, treatment with 100 ng/mL GDNF was unable to enhance KCl-stimulated release of iCGRP (data not shown). PGE2 is a well established sensory neuronal sensitizing agent (Martin et al., 1987; Mense, 1981). It is known to sensitize sensory neurons to many stimuli, including high extracellular potassium (Southall and Vasko, 2000). Accordingly, PGE2 enhanced the potassium-stimulated release of iCGRP by nearly 2 fold (Figure 4; 14.3 ± 0.79%). These data suggest that GDNF, NTN, and ART, unlike PGE2, sensitize sensory neurons through an interaction with TRPV1 and not by mechanisms independent of the stimulus type.
Figure 4. GFLs do not enhance the potassium-stimulated release of iCGRP from isolated sensory neurons.
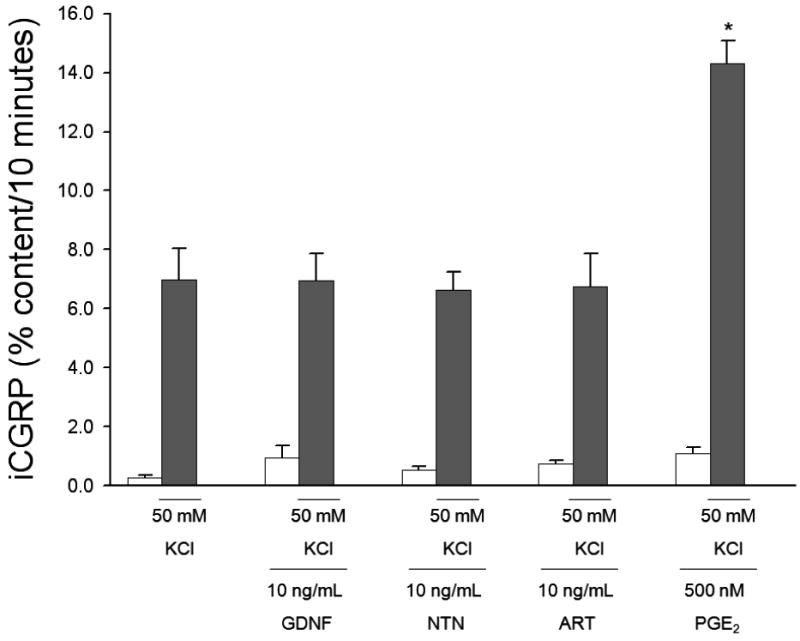
Peptide release elicited by a 10 minute exposure to HEPES buffer alone (open bars) or HEPES buffer containing 50 mM potassium (KCl; dark bars) is expressed as mean percent total peptide content of cells in each well ± SEM (n = 9-12 wells per condition). GDNF, neurturin (NTN), or artemin (ART) at 10 ng/mL, was included in the 10 minutes prior to and throughout potassium exposure. PGE2 was present in the basal and stimulated conditions at a concentration of 500 nM. Total growth factor and PGE2 exposure time was 20 minutes. There were no significant differences in iCGRP release between treatment groups and the no GFL condition. A significant enhancement in iCGRP release was observed with PGE2 using ANOVA with Dunnett's post-hoc test (p<0.05). In all cases, release stimulated by potassium was significantly higher than basal release.
GFLs enhance the capsaicin-stimulated release of iCGRP from spinal cord slices
Sensitization of the central terminals of primary sensory neurons, which synapse onto second order neurons in the spinal cord, is also important during inflammation and propagation of the pain signal. The GFRα receptors are present on the central terminal of primary afferent neurons (Josephson et al., 2001), and GFLs are released by astrocytes in the spinal cord (Nosrat et al., 1996;Nomura et al., 2002). In order to examine the actions of GFLs in sensitization of the central terminals of sensory neurons, iCGRP release from spinal cord slices was measured.
Figure 5A demonstrates a representative experiment comparing capsaicin-stimulated release of iCGRP in the absence or presence of 10 ng/mL GDNF. The fmol of iCGRP in each 10 minute collection fraction were normalized to the total iCGRP content in the spinal cord, as described in Experimental Procedures. In the three basal fractions, iCGRP release was similar for both treatments. The capsaicin-stimulated release of iCGRP was significantly enhanced by exposure to GDNF for 30 minutes prior to and throughout the stimulus period (No GFL: 0.94 ± 0.12%, 1.46 ± 0.18%, and 1.28 ± 0.15%; 10 ng/mL GDNF: 1.14 ± 0.09%, 2.29 ± 0.08%, and 1.96 ± 0.25%). The profiles of increased release of iCGRP were similar when the spinal cord slices were exposed to NTN and ART (data not shown). Evoked release was determined by subtracting the three basal fractions of iCGRP release from the three capsaicin-stimulated fractions. As demonstrated in Figure 5B, GDNF, NTN, and ART all were able to significantly enhance the capsaicin-evoked release of iCGRP from the spinal cord slices by two to three fold (No GFL: 2.50 ± 0.42%, GDNF 4.05 ± 0.43%, NTN: 6.18 ± 0.28%, ART: 5.88 ± 1.94%). The total content of iCGRP per mg of protein in the spinal cord slices was not changed by exposure to GFLs (No GFL: 298.41 ± 44.28 fmol/mg, GDNF: 275.46 ± 25.13 fmol/mg, NTN: 220.71 ± 84.84 fmol/mg, ART: 227.58 ± 98.63 fmol/mg). These data indicate that the GFLs are able to sensitize the central terminals of the sensory neurons to capsaicin stimulation.
Figure 5. GFLs enhance capsaicin-stimulated release of iCGRP from spinal cord slices.
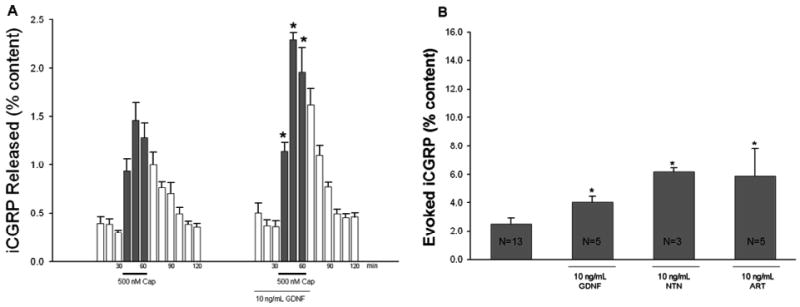
A) Peptide release from spinal cord slices stimulated by three 10 minute exposures to HEPES buffer alone (open bars) or HEPES buffer containing 500 nM capsaicin (dark bars) is expressed as mean percent total peptide content of iCGRP in the spinal cord slice ± SEM (n = 3-10 animals per condition). GDNF, neurturin (NTN), or artemin (ART), at 10 ng/mL, was included in the six 10 minute incubations indicated by lines with growth factor name below, for a total exposure time of 60 min. B) Evoked release, or release due to capsaicin stimulation alone, is compared between growth factor treatment and no GFL groups. The evoked release was obtained by subtracting peptide release during the three basal fractions from that during the three capsaicin-stimulated fractions in each treatment group. Asterisks (*) indicate statistically significant differences in iCGRP release between treatment groups and the no growth factor condition using an ANOVA with Dunnett's post-hoc test (p<0.05). In all cases, release stimulated by capsaicin was significantly higher than basal release.
Discussion
Previous studies have shown that the GFLs are potent modulators of the TRPV1 channel and that injection of these molecules induces hyperalgesia (Malin et al., 2006). However, whether this TRPV1 modulation results in a change in integrative functions of the sensory neurons, such as neurotransmitter release, and whether this change in function could be responsible for the GFL-induced hyperalgesia, is not clear. Here, direct evidence is provided, from both neuronal cultures and freshly dissociated neuronal tissues, that select GFLs sensitize sensory neurons, resulting in increased stimulated-release of iCGRP. This sensitization could be a contributing factor to the inflammatory hyperalgesia induced by GFLs.
Multiple GFLs were able to sensitize capsaicin-stimulated release of iCGRP. The selected GFLs were able to produce an enhancement in the capsaicin-stimulated release of iCGRP, not only in sensory neurons in culture but also in spinal cord slices. GFL-induced enhancement in the release of iCGRP from the spinal cord slices indicates that GFLs sensitize the central terminal of the primary sensory neurons, the terminal related to propagation of the nociceptive signal. GFRα1-3 are found throughout the central nervous system, and specifically in the dorsal horn of the spinal cord (Josephson et al., 2001; Quartu et al., 2007). It is possible that the GFLs are exerting their effects on the enhancement of release of iCGRP in sensory neurons through these GFRα receptors at this location during inflammation. There is direct evidence that CGRP is important in the propagation of the pain signal, since hyperalgesia due to both pancreatitis and inflammation induced by carrageenan is attenuated by a CGRP blocking antibody (Satoh et al., 1992; Wick et al., 2006). The GFRα receptors exist on motor neurons originating in the ventral horn of the spinal cord (Homma et al., 2003) and these neurons also can contain CGRP (Gibson et al., 1988;Kruger et al., 1988). Since the ventral half of the spinal cord is also present in the preparation used in these experiments, basal release levels of iCGRP in response to the GFLs could reflect release from both the dorsal and ventral neuronal terminals. However, motor neurons in the ventral horn are unlikely to significantly contribute to the stimulated release of iCGRP because motor neurons do not contain TRPV1 receptors (Lauria et al., 2006). Since CGRP from the central terminal of primary sensory neurons is important in propagation of the pain signal, modulation of release at this site may be a critically important component of pain processing and hyperalgesia.
These data add to the previous observation that GDNF, NTN, and ART are able to alter TRPV1 function directly (Malin et al, 2006). The modulation of TRPV1 channels has been observed with other sensitizers of sensory neurons, such as NGF (Shu and Mendell, 1999; Zhu and Oxford, 2007), which may provide some insight into the cellular mechanisms of GFL-induced sensitization. Specifically, the GFLs may sensitize sensory neurons by altering properties of TRPV1, perhaps by increasing the ion flow through the channel or rapidly increasing the membrane expression of TRPV1, similar to the mechanisms of NGF-induced sensitization (Zhang et al., 2005; Zhu and Oxford, 2007). Interestingly, at a concentration of 100 ng/mL, GDNF is unable to enhance the release of iCGRP. When the sensory neurons are exposed to this higher concentration of GDNF, compensatory pathways could be activated, which could alter its actions. This is not without precedence, since exposure of sensory neurons in culture to 10 ng/mL GDNF, but not 100 ng/mL GDNF, enhances the content and capsaicin-evoked release of CGRP (Price et al., 2005). Interestingly, the capsaicin concentration response curve is U-shaped (Kj°rsvik Bertelsen et al., 2003). Therefore, another possible mechanism for the inability of 100 ng/ml GDNF to induce sensitization is that the capsaicin concentration-response curve is shifted by GDNF. This could mean that 1 ng/mL and 10 ng/mL GDNF are at the peak of this curve, while 100 ng/mL is on the downward slopping portion of the curve.
Long term exposure to growth factors, specifically NGF, can change several properties of sensory neurons. NGF increases the amount of CGRP produced (MacLean et al., 1989;Sango et al., 1994) and the expression and membrane insertion of TRPV1 (Xue et al., 2007; Stein et al., 2006). These changes could affect the responses of sensory neurons to GFL-induced sensitization. However, while the absolute amount of iCGRP present and iCGRP released upon stimulation was reduced when the cultures lacked NGF, the sensitization profile of GFLs was unaffected (Figure 3). This observation indicates that the NGF present in the cultures did not alter the sensitization phenotype of the sensory neuron, which is in line with previous studies (Park et al., 2006). In addition, when spinal cord tissue was exposed to the GFLs, there was a similar profile of enhanced release of iCGRP to that seen with isolated sensory neurons in culture (Figure 5). Since this spinal cord tissue is freshly dissociated, and the responses of this tissue and the isolated sensory neurons in culture were identical, it is unlikely that the phenotype of the sensory neurons is changed by maintenance in culture.
While GDNF, NTN, and ART were able to sensitize capsaicin-stimulated release robustly, potassium-stimulated release was not enhanced by any of the GFLs. This is in contrast to previous observations in the trigeminal ganglia (TG) and neurons from transgenic mice (Price et al., 2005; Albers et al, 2006). The majority of previous studies have examined the role of GFLs in capsaicin-induced sensory neuronal changes. However, studies conducted on neurons from TG and transgenic mice have demonstrated that GDNF is able to alter the sensitivity of sensory neurons in ways other than through TRPV1. Potassium-stimulated release of CGRP from sensory neurons in the TG was sensitized by GDNF (Price et al., 2005). There could be critical physiological differences between the responses evoked in the TG and the DRG, which could be responsible for the observed differences in our studies. In fact, large differences exist between TG neurons and DRG neurons in the levels of CGRP, TRPV1, and isolectin B4 (IB4), as well as the co-localization of these proteins within the ganglia (Price and Flores, 2007). IB4 neurons are generally considered GDNF-responsive neurons (Kashiba et al., 2001). A much higher percentage of neurons in the DRG express both IB4 and TRPV1 than in the TG (Price and Flores, 2007), which may be one contributing factor to the differential responses seen with tissue from these two types of ganglia. Albers et al., 2006 used transgenic mice that over-expressed GDNF (GDNF-OE) in the skin and found that neurons from these mice exhibited enhanced electrical responses to mechanical stimuli. Mechanical hyperalgesia was also present in these GDNF-OE mice. The difference between the responses of the sensory neurons to the general stimulus (potassium) used in our studies and the results of studies conducted on the GDNF-OE mice described above could be the result of several factors. First, the amount of overlap between mechano-sensitive neurons and different TRP receptors is unclear (Lawson et al., 2008; Tender et al., 2008), which may mean that some of the mechanical hyperalgesia seen in these GDNF-OE mice is TRP-mediated. Since GDNF is necessary for the development of sensory neurons, the use of GDNF-OE mice may change certain properties of the neurons, thereby making them more likely to be sensitized to more types of stimuli by GFLs. What remains clear is that the GFLs sensitize sensory neurons through the TRPV1 channel. The mechanisms of this alteration in the TRPV1 channel, perhaps by changes in phosphorylation states of the channel, is not yet known and requires further investigation.
Unlike the other GFLs, PSP did not alter the capsaicin-stimulated release of iCGRP. Neurite outgrowth in adult dorsal root ganglia cultures, which can be efficiently induced by GDNF, NTN, and ART, is not caused by PSP (Lindahl et al., 2000; Paveliev et al., 2004). This inability of PSP to induce an enhancement in the capsaicin-stimulated release of iCGRP supports the current theory that adult mammalian sensory neurons are unresponsive to PSP. Even at concentrations as high as 500 ng/mL, PSP could not enhance the release of iCGRP. The use of these higher concentrations was necessary because the KD value of PSP for its receptor, GFRα4 is ∼6mM (Enokido et al., 1998) which is much higher than the other GFLs for their preferred GFRα receptors (0.6 mM and 1 mM; Baloh et al., 1997; Klein et al., 1997; Trupp et al., 1998). Since PSP is not able to induce sensory neuronal sensitization at these high concentrations, it is unlikely to have significant binding or actions on the other GFRα receptors in our system.
We have demonstrated that the GFLs, GDNF, NTN, and ART, are sensitizers of neuropeptide release from sensory neurons. The level of enhancement of iCGRP release by the GFLs is similar to that produced in DRGs and trigeminal ganglia by NGF, both in CGRP levels normalized to percent content and absolute levels of CGRP released (Hingtgen et al., 2006; Price et al., 2005). Additionally, inflammation, induced by an injection of a mixture of inflammatory mediators, noxious heat, and acidic shifts in pH all enhanced the release of CGRP to similar levels as the GFLs (Eberhardt et al., 2008). The actions of the GFLs are specific to activation with capsaicin suggesting that the GFLs may induce sensitization through TRPV1-specific mechanisms. Since the levels of GFLs are greatly enhanced during inflammation, directly alter TRPV1 channel properties, and induce thermal hyperalgesia upon injection, they are clearly important mediators of sensory neuronal function. The cellular mechanisms responsible for this enhancement in release are not known. A number of candidate pathways exist, including the MAPK and PI-3K pathways, since these pathways are activated by GFLs (Bron et al., 2003) and are known to be associated with sensory neuronal sensitization (Zhuang et al., 2004). Additionally, Ret-independent pathways may play a role in this sensitization. The Ret-independent pathways, initiated by integrin β1 and/or NCAM, are a novel set of pathways induced by GFLs in sensory neurons (Cao et al., 2008a; Cao et al., 2008b). It may be important to evaluate the role of these Ret-dependent and Ret-independent pathways in the GFL-induced sensory neuronal sensitization, as well as to determine how these pathways are specifically modulating TRPV1 channel function. With the GFL-induced increases in peptide release demonstrated here, we have provided a connection between the modulation of TRPV1 by GFLs and the hyperalgesia associated with the release of GFLs during inflammation.
Acknowledgments
Funded in part by NINDS R01 NS051668 (CMH) and a Young Investigator's Award from the Children's Tumor Foundation (BSS). The authors wish to thank Michael Vasko for CGRP antibody and RIA supplies and Neilia Gracias for technical assistance with the spinal cord slice release technique.
List of Abbreviation
- ART
Artemin
- Cap
Capsaicin
- CFA
Complete Freund's Adjuvant
- CGRP
Calcitonin gene-related peptide
- DRG
Dorsal root ganglia
- GDNF
Glial cell line-derived neurotrophic factor
- GFL
Glial cell line-derived neurotrophic factor family ligands
- GFRα
GDNF family receptor alpha
- KCl
Potassium chloride
- IB4
Isolectin B4
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- NCAM
Neural cell adhesion molecule
- NGF
Nerve growth factor
- NTN
Neurturin
- PI-3K
Phosphoinositide-3 Kinase
- PSP
Persephin
- RIA
Radioimmunoassay
- SEM
Stardard error of the mean
- TRPV1
Transient receptor potential vanilloid 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Tuveri MA, Levi-Montalcini R. Studies on carrageenan-induced arthritis in adult rats: presence of nerve growth factor and role of sympathetic innervation. Rheumatol Int. 1992;12:213–216. doi: 10.1007/BF00302155. [DOI] [PubMed] [Google Scholar]
- Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, Anand P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett. 2006;399:51–56. doi: 10.1016/j.neulet.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22:118–132. doi: 10.1016/s1044-7431(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Cao JP, Wang HJ, Yu JK, Yang H, Xiao CH, Gao DS. Involvement of NCAM in the effects of GDNF on the neurite outgrowth in the dopamine neurons. Neurosci Res. 2008a;61:390–397. doi: 10.1016/j.neures.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Cao JP, Yu JK, Li C, Sun Y, Yuan HH, Wang HJ, Gao DS. Integrin beta1 is involved in the signaling of glial cell line-derived neurotrophic factor. J Comp Neurol. 2008b;509:203–210. doi: 10.1002/cne.21739. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Barber LA, Dymshitz J, Vasko MR. Peptidase inhibitors improve recovery of substance P and calcitonin gene-related peptide release from rat spinal cord slices. Peptides. 1996;17:31–37. doi: 10.1016/0196-9781(95)02091-8. [DOI] [PubMed] [Google Scholar]
- De CF, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323:960–969. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- Eberhardt M, Hoffmann T, Sauer SK, Messlinger K, Reeh PW, Fischer MJ. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides. 2008;42:311–317. doi: 10.1016/j.npep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C, Gerber N. X-ray structure of glial cell-derived neurotrophic factor at 1.9 A resolution and implications for receptor binding. Nat Struct Biol. 1997;4:435–438. doi: 10.1038/nsb0697-435. [DOI] [PubMed] [Google Scholar]
- Enokido Y, de SF, Hongo JA, Ninkina N, Rosenthal A, Buchman VL, Davies AM. GFR alpha-4 and the tyrosine kinase Ret form a functional receptor complex for persephin. Curr Biol. 1998;8:1019–1022. doi: 10.1016/s0960-9822(07)00422-8. [DOI] [PubMed] [Google Scholar]
- Gavazzi I, Kumar RD, McMahon SB, Cohen J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur J Neurosci. 1999;11:3405–3414. doi: 10.1046/j.1460-9568.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Giaid A, Hamid QA, Kar S, Jones PM, Denny P, Legon S, Amara SG, Craig RK. Calcitonin gene-related peptide messenger RNA is expressed in sensory neurones of the dorsal root ganglia and also in spinal motoneurones in man and rat. Neurosci Lett. 1988;91:283–288. doi: 10.1016/0304-3940(88)90694-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ito T, Fukumitsu H, Nomoto H, Furukawa Y, Furukawa S. Stimulation of production of glial cell line-derived neurotrophic factor and nitric oxide by lipopolysaccharide with different dose-responsiveness in cultured rat macrophages. Biomed Res. 2005;26:223–229. doi: 10.2220/biomedres.26.223. [DOI] [PubMed] [Google Scholar]
- Hingtgen CM, Roy SL, Clapp DW. Stimulus-evoked release of neuropeptides is enhanced in sensory neurons from mice with a heterozygous mutation of the Nf1 gene. Neuroscience. 2006;137:637–645. doi: 10.1016/j.neuroscience.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Homma S, Yaginuma H, Vinsant S, Seino M, Kawata M, Gould T, Shimada T, Kobayashi N, Oppenheim RW. Differential expression of the GDNF family receptors RET and GFRalpha1, 2, and 4 in subsets of motoneurons: a relationship between motoneuron birthdate and receptor expression. J Comp Neurol. 2003;456:245–259. doi: 10.1002/cne.10529. [DOI] [PubMed] [Google Scholar]
- Horie S, Michael GJ, Priestley JV. Co-localization of TRPV1-expressing nerve fibers with calcitonin-gene-related peptide and substance P in fundus of rat stomach. Inflammopharmacology. 2005;13:127–137. doi: 10.1163/156856005774423854. [DOI] [PubMed] [Google Scholar]
- Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, Delaney J, Schultz H, Zhou R, Fox GM. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J Biol Chem. 1997;272:33111–33117. doi: 10.1074/jbc.272.52.33111. [DOI] [PubMed] [Google Scholar]
- Josephson A, Widenfalk J, Trifunovski A, Widmer HR, Olson L, Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. J Comp Neurol. 2001;440:204–217. doi: 10.1002/cne.1380. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sensory ganglia. Brain Res Mol Brain Res. 2001;95:18–26. doi: 10.1016/s0169-328x(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, Vandlen R, Simmons L, Gu Q, Hongo JA, Devaux B, Poulsen K, Armanini M, Nozaki C, Asai N, Goddard A, Phillips H, Henderson CE, Takahashi M, Rosenthal A. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997;387:717–721. doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- Kj°rsvik Bertelsen A, Warsame A, Gustafsson H, Tj°lsen A, Hole K, Stiller CO. Stimulation of spinal 5-HT2A/2C receptors potentiates the capsaicin-induced in vivo release of substance P-like immunoreactivity in the rat dorsal horn. Brain Research. 2003;987:10–16. doi: 10.1016/s0006-8993(03)03216-5. [DOI] [PubMed] [Google Scholar]
- Kruger L, Mantyh PW, Sternini C, Brecha NC, Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: patterns of immunoreactivity and receptor binding sites. Brain Res. 1988;463:223–244. doi: 10.1016/0006-8993(88)90395-2. [DOI] [PubMed] [Google Scholar]
- Lauria G, Morbin M, Lombardi R, Capobianco R, Camozzi F, Pareyson D, Manconi M, Geppetti P. Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J Peripher Nerv Syst. 2006;11:262–271. doi: 10.1111/j.1529-8027.2006.0097.x. [DOI] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Timmusk T, Rossi J, Saarma M, Airaksinen MS. Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol Cell Neurosci. 2000;15:522–533. doi: 10.1006/mcne.2000.0845. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Martling CR, Saria A, Fischer JA, Hokfelt T, Lundberg JM. Calcitonin gene-related peptide and the lung: neuronal coexistence with substance P, release by capsaicin and vasodilatory effect. Regul Pept. 1988;20:125–139. doi: 10.1016/0167-0115(88)90046-8. [DOI] [PubMed] [Google Scholar]
- MacLean DB, Bennett B, Morris M, Wheeler FB. Differential regulation of calcitonin gene-related peptide and substance P in cultured neonatal rat vagal sensory neurons. Brain Res. 1989;478:349–355. doi: 10.1016/0006-8993(89)91515-1. [DOI] [PubMed] [Google Scholar]
- McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–261. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E2. Brain Res. 1981;225:95–105. doi: 10.1016/0006-8993(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Miletic V, Tan H. Iontophoretic application of calcitonin gene-related peptide produces a slow and prolonged excitation of neurons in the cat lumbar dorsal horn. Brain Res. 1988;446:169–172. doi: 10.1016/0006-8993(88)91310-8. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Snider WD. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol. 1997;381:428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Nomura H, Furuta A, Iwaki T. Dorsal root rupture injury induces extension of astrocytic processes into the peripheral nervous system and expression of GDNF in astrocytes. Brain Res. 2002;950:21–30. doi: 10.1016/s0006-8993(02)02982-7. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286:191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–441. [PubMed] [Google Scholar]
- Onodera H, Nagata T, Kanazawa M, Taguma Y, Itoyama Y. Increased plasma GDNF levels in patients with chronic renal diseases. Nephrol Dial Transplant. 1999;14:1604–1605. doi: 10.1093/ndt/14.6.1604. [DOI] [PubMed] [Google Scholar]
- Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Park KA, Thompson EL, Richter JA, Vasko MR. Program No 245.25/O8. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. The Ras/MEK/ERK cascade mediates nerve growth factor-induced increases in expression of calcitonin-gene related peptide in sensory neurons. Online. [Google Scholar]
- Paveliev M, Airaksinen MS, Saarma M. GDNF family ligands activate multiple events during axonal growth in mature sensory neurons. Mol Cell Neurosci. 2004;25:453–459. doi: 10.1016/j.mcn.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, Diogenes A, Trott AA, Hargreaves KM, Flores CM. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartu M, Serra MP, Boi M, Ferretti MT, Lai ML, Del FM. Tissue distribution of Ret, GFRalpha-1, GFRalpha-2 and GFRalpha-3 receptors in the human brainstem at fetal, neonatal and adult age. Brain Res. 2007;1173:36–52. doi: 10.1016/j.brainres.2007.07.064. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bradbury EJ, Michael GJ, Lever IJ, McMahon SB. Glial cell line-derived neurotrophic factor increases calcitonin gene-related peptide immunoreactivity in sensory and motoneurons in vivo. Eur J Neurosci. 2003;18:2713–2721. doi: 10.1111/j.1460-9568.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Ryu PD, Gerber G, Murase K, Randic M. Actions of calcitonin gene-related peptide on rat spinal dorsal horn neurons. Brain Res. 1988;441:357–361. doi: 10.1016/0006-8993(88)91414-x. [DOI] [PubMed] [Google Scholar]
- Sango K, Verdes JM, Hikawa N, Horie H, Tanaka S, Inoue S, Sotelo JR, Takenaka T. Nerve growth factor (NGF) restores depletions of calcitonin gene-related peptide and substance P in sensory neurons from diabetic mice in vitro. J Neurol Sci. 1994;126:1–5. doi: 10.1016/0022-510x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Sanicola M, Hession C, Worley D, Carmillo P, Ehrenfels C, Walus L, Robinson S, Jaworski G, Wei H, Tizard R, Whitty A, Pepinsky RB, Cate RL. Glial cell line-derived neurotrophic factor-dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc Natl Acad Sci U S A. 1997;94:6238–6243. doi: 10.1073/pnas.94.12.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Kuraishi Y, Kawamura M. Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain. 1992;49:273–278. doi: 10.1016/0304-3959(92)90151-Z. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Southall MD, Michael RL, Vasko MR. Intrathecal NSAIDS attenuate inflammation-induced neuropeptide release from rat spinal cord slices. Pain. 1998;78:39–48. doi: 10.1016/S0304-3959(98)00113-4. [DOI] [PubMed] [Google Scholar]
- Southall MD, Vasko MR. Prostaglandin E(2)-mediated sensitization of rat sensory neurons is not altered by nerve growth factor. Neurosci Lett. 2000;287:33–36. doi: 10.1016/s0304-3940(00)01158-7. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tender GC, Li YY, Cui JG. Vanilloid receptor 1-positive neurons mediate thermal hyperalgesia and tactile allodynia. Spine J. 2008;8:351–358. doi: 10.1016/j.spinee.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Trupp M, Raynoschek C, Belluardo N, Ibanez CF. Multiple GPI-anchored receptors control GDNF-dependent and independent activation of the c-Ret receptor tyrosine kinase. Mol Cell Neurosci. 1998;11:47–63. doi: 10.1006/mcne.1998.0667. [DOI] [PubMed] [Google Scholar]
- Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Geerling I, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn's disease. Inflamm Bowel Dis. 2006;12:346–354. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- Wick EC, Pikios S, Grady EF, Kirkwood KS. Calcitonin gene-related peptide partially mediates nociception in acute experimental pancreatitis. Surgery. 2006;139:197–201. doi: 10.1016/j.surg.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat TRPV1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem. 2007;101:212–222. doi: 10.1111/j.1471-4159.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Hooper WC, Phillips DJ, Tondella ML, Talkington DF. Induction of proinflammatory cytokines in human lung epithelial cells during Chlamydia pneumoniae infection. Infect Immun. 2003;71:614–620. doi: 10.1128/IAI.71.2.614-620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


