Abstract
The 5-HT4 partial agonist tegaserod is effective in the treatment of chronic constipation and constipation predominant irritable bowel syndrome. 5-HT4 receptors are located on presynaptic terminals in the enteric nervous system. Stimulation of 5-HT4 receptors enhances the release of acetylcholine and calcitonin gene related peptide from stimulated nerve terminals. This action strengthens neurotransmission in prokinetic pathways, enhancing gastrointestinal motility. The knockout of 5-HT4 receptors in mice not only slows gastrointestinal activity but also, after 1 month of age, increases the age-related loss of enteric neurons and decreases the size of neurons that survive. 5-HT4 receptor agonists, tegaserod and RS67506, increase numbers of enteric neurons developing from precursor cells and/or surviving in culture; they also increase neurite outgrowth and decrease apoptosis. The 5-HT4 receptor antagonist, GR113808, blocks all of these effects, which are thus specific and 5-HT4-mediated. 5-HT4 receptor agonists, therefore, are neuroprotective and neurotrophic for enteric neurons. Because the age-related decline in numbers of enteric neurons may contribute to the dysmotilities of the elderly, the possibility that the neuroprotective actions of 5-HT agonists can be utilized to prevent the occurrence or worsening of these conditions should be investigated.
Keywords: 5-HT4 receptors, constipation, enteric nervous system, gastrointestinal motility, lubiprostone, tegaserod
Enteric Nervous System Development is Incomplete at Birth
Although the bowel and the enteric nervous system (ENS) of a newborn mammal must be functioning at the time of birth to cope with oral feeding, both the gut and the ENS enlarge as a function of postnatal growth. Enlargement of the ENS is not just a matter of the hypertrophy of existing cells. New neurons must also be generated. As a result, the ENS of a mature mammal contains a larger number of neurons than that of a newborn.1,2 In mice, the birth of new neurons has been demonstrated to occur at least through postnatal day 21 (P21).3 The corresponding age has not been ascertained for humans, but extrapolation on the basis of relative life span would suggest that it is ∼3 years. Very little is known about the postnatal generation of new enteric neurons; nevertheless, the postnatal gut has recently been demonstrated to contain stem cells,4,5 which are a likely source of neurons added to the ENS during postnatal life. It is thus possible that neurons continue to be added to the ENS in adult life, but if so, this addition would have to occur at a rate that is too slow to be detected by the conventional techniques that have thus far been utilized to look for it.
Neurons are Lost from the Mature Ens as a Function of Age
The number of neurons in the bowel increases in postnatal life, reaches a peak and then stabilizes.2 The age at which the peak number of neurons is achieved in humans is unknown. Ageing, however, is associated with a postpeak decline so that the senescent gut has fewer neurons than does the young mature bowel.6–9 An age-related loss of neurons occurs in humans as well as in animals.10–12 In most mammals about 40-60% of neurons are lost during senescence.6,9 In rats, slowing of intestinal motility with increasing age has also been documented and related to a high caloric diet.7,13 Such a diet, in turn, has been demonstrated to accelerate the loss of enteric neurons in aged rats by increasing the frequency of neuronal cell death.7,8 It follows that a similar occurrence of neurodegeneration in humans may be a significant cause of the increasing incidence of intestinal dysmotility in the aged.14–22 In humans, the effects of age are reflected mainly in a slowing of colonic transit because gastric emptying and small bowel motility appear to be more age-resistant than motility of the colon.23 Still, even if age-related neurodegeneration was to be primarily a colon-specific phenomenon in humans, it is likely that it would be useful if drug therapy could be employed to counteract ENS neurodegeneration. Such treatment might help to prevent or ameliorate enteric motility disturbances of ageing.14 Certainly, drug therapy for this purpose is likely to be more popular than subjecting a human population to long-term calorie deprivation.
Tegaserod is Effective for the Treatment of Chronic Constipation
Although tegaserod has demonstrated efficacy in the treatment of constipation-associated irritable bowel syndrome (IBS-C)24 and chronic constipation,24,25 it has recently been withdrawn from the market at the request of the Food and Drug Administration (FDA) because a safety analysis revealed the possibility that it increased the chance of a myocardial infarct, stroke, or worsening of cardiac chest pain (see FDA Advisory, March 30, 2007; http://www.fda.gov/cder/drug/advisory/tegaserod.htm). This action was taken as a result of the analysis of 29 clinical studies in which 13/11 614 (0.1%) patients given tegaserod and 1/7031 (0.01%) patients given placebo experienced serious adverse events. Tegaserod is a partial agonist at 5-HT4 receptors.26 These receptors are presynaptic in the ENS (Fig. 1).27 Their action in the gut is to enhance the release of acetylcholine (Fig. 2) and calcitonin gene related peptide (CGRP) from nerve terminals between submucosal intrinsic primary afferent neurons (IPANs) and their follower neurons, between myenteric interneurons, and between motor neurons and their effectors, which may be smooth muscle, interstitial cells of Cajal, or glands24,27–34 (Fig. 3). When stimulated by an agonist, such as tegaserod, therefore, 5-HT4 receptors increase the strength of neurotransmission in prokinetic pathways. This mechanism of action adds to the gastrointestinal safety of tegaserod. The drug does not directly initiate peristaltic reflexes, which might induce excessive propulsion and thus diarrhoea, but strengthens these reflexes once natural stimuli gets them started. Although 5-HT4 receptors are expressed in the human heart,35 it is not clear how their stimulation might lead to myocardial infarction or stroke. Tegaserod is also an antagonist at 5-HT2B receptors.36 This effect does not appear to contribute to tegaserod's stimulation of colonic motility or to its ability to inhibit visceral hypersensitivity.37,38 5-HT2B receptors are expressed in the cardiovascular system, and their stimulation can thicken heart valves, enlarge the heart, and release atrial natriuetic peptide; however, these effects are prevented by a 5-HT2B antagonist. Again, therefore, it is not clear how 5-HT2B antagonism by tegaserod leads to the adverse events that caused it to be withdrawn. Further work and analysis, therefore, is needed to analyse the safety of 5-HT4 receptor agonists in medical treatment. The adverse events associated with tegaserod administration might not be related to 5-HT4 agonism. It is also conceivable that stimulation of 5-HT4 receptors on enteric neurons might be sufficiently beneficial that administration of a 5-HT4 receptor agonist might outweigh the perceived risk.
Figure 1.
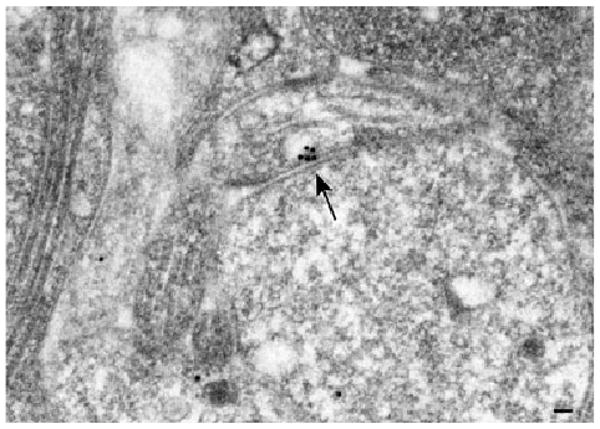
An electron micrograph of an axo-dendritic synapse in the myenteric plexus of a mouse. 5-HT4 receptors were demonstrated by electron microscopic immuncytochemistry using an antibody to the 5-HT4a receptor (the most abundant isoform in the ENS). Postembedding immunostaining was used with colloidal gold. A cluster of gold particles (arrow) in the presynaptic membrane shows the location of the receptors. Bar = 100 nm.
Figure 2.
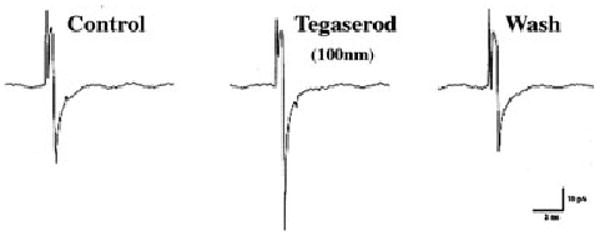
Whole cell patch clamp recordings were obtained from a guinea pig myenteric neuron that has been cultured overnight. A series of three fast excitatory postsynaptic currents (EPSCs) are shown. These responses are cholinergic. Tegaserod reversibly increases the amplitude of the EPSCs, indicating that it enhances the synaptic release of acetylcholine.
Figure 3.
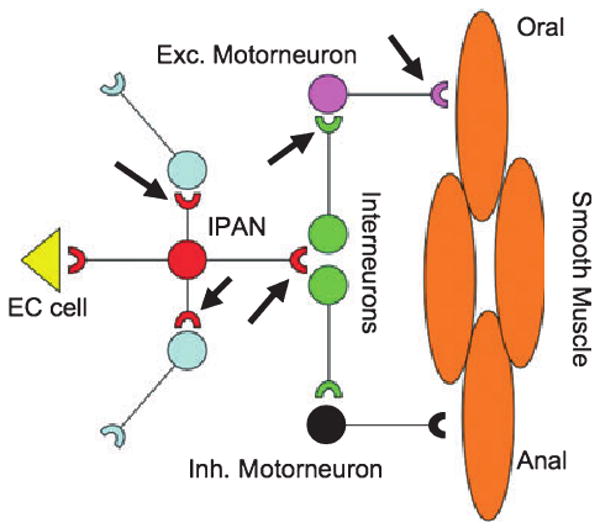
A minimal enteric nervous system circuit, leading to a peristaltic reflex is diagrammed. Pressure or chemical stimuli release 5-HT from an EC cell. 5-HT activates an intrinsic primary afferent neuron in the submucosal plexus (IPAN). This neuron activates other submucosal neurons, but also projects to interneurons in the myenteric plexus. Ascending interneurons activate excitatory motor neurons, which in turn secrete acetylcholine to cause oral contraction of the smooth muscle. Descending interneurons activate inhibitory motor neurons, which in turn secrete nitric oxide, VIP and/or ATP to cause anal relaxation of the smooth muscle. Presynaptic 5-HT4 receptors (arrows) increase the strength of neurotransmission in prokinetic pathways. These events occur between IPANs and follower neurons, interneurons within the myenteric plexus, and at the cholinergic (muscarinic) excitatory neuromuscular junction.
Neuronal Numbers Decrease in the Ens of Mice Lacking 5-Ht4 Receptors
Considerable evidence supports the hypothesis that the direct action of 5-HT4 receptor stimulation of neurons positively affects the ability of these cells to survive. 5-HT4 receptor stimulation, for example, is able to inhibit secretion of β-amyloid peptides by cultured cortical neurons from transgenic mice that express human amyloid precursor protein and enhances neuronal survival.39 Recent work from our laboratory has investigated the role of 5-HT4 receptors in enteric neuronal survival and gastrointestinal motor function by studying mice lacking the 5-HT4 receptor (KO). Aspects of this work have been published in abstract form.40,41 We found, by immunostaining preparations to demonstrate the neuronal markers Hu or PGP9.5, that the numbers of enteric neurons are reduced in both plexuses of KO mice. This reduction, however, is age-dependent. At 1 month of age, the number of neurons in the colons of KO and wild-type (WT) littermates did not differ significantly; however, by 4 months, the number of neurons in the colonic myenteric plexus of KO mice was significantly less than in the colonic myenteric plexus of WT littermates (P < 0.001). Between 4 and 12 months the numbers of neurons in the colonic myenteric plexus declined, even in WT mice. Still, significantly more neurons were present in the WT than in KO colonic myenteric plexus at 12 months of age (P < 0.5). Neuronal size and the proportion of myenteric neuronal nitric oxide synthase (nNOS)-immunoreactive neurons were also significantly decreased in KO mice at 12 months, but not at younger ages.
The largest diameter of myenteric neurons (Feret's diameter) was measured with computer-assistance (OpenLab software from Improvision, Coventry, UK) to estimate cell size. To do so, neurons were identified microscopically, imaged, selected and outlined. The measuring tool was then applied to obtain Feret's diameter. nNos was studied as an example of one well-defined subset of enteric neurons. Antibodies to Hu were employed to label all enteric neurons and thus to ascertain the total number present. The numbers of nNOS-immunoreactive neurons were normalized to the number demonstrated with antibodies to Hu. Feret's diameter was 21.5 ± 0.3 μm in KO and 23.2 ± 0.4 μm in WT mice (n = 100, P < 0.01); the nNOS/Hu ratio was 31.4 ± 2.1% in KO animals and 39.2 ± 1.8% in WT (n = 15, P < 0.01).
We previously reported in abstract form that colonic motility slows in KO mice (in comparison to WT littermates).40,41 It is possible therefore, that the correlation between the loss of neurons from the senescing bowel and the slowing of motility is the result of a causal relationship between the two; that is, colonic motility slows because colonic neurons are lost. It is, of course, also possible that neurons are lost because motility slows. One can envision a number of indirect effects of decreased colonic motility that would cause neurons to be lost, including enhanced infection of a bowel that cannot expel pathogens as effectively as a normal gut. Slowing of motility in KO mice could also reflect the absence of 5-HT4-mediated strengthening of neurotransmission.
Stimulation of 5-Ht4 Receptors are Neuroprotective and Neurotrophic
Because of the evidence that 5-HT4 receptors might protect neurons from death, we tested the hypothesis that their stimulation exerts a neuroprotective or neurotrophic action. Both the prevention of neuronal death and the maintenance of plasticity through axonal re-growth or remodelling would be predicted to be beneficial. To study trophism and/or neuroprotection directly, the 5-HT4 agonists, tegaserod and RS67506, and the 5-HT4 receptor antagonist, GR113808, were applied to enteric neurons developing in vitro from immunoselected neural crest-derived precursors. These cells were obtained from the E12 fetal bowel.42 The gut was dissociated with collagenase and the single cell suspension was treated with antibodies to p75NTR, which is a marker for crest-derived cells in the fetal gut.43,44 The cell suspension was then exposed to secondary antibodies coupled to magnetic beads. The cells were finally passed through a column in a magnetic field. Crest-derived cells, which are decorated by beads, are retained in the field while non-crest-derived cells pass through. The crest-derived cells are then eluted from the column by removing the magnetic field. The isolated and purified crest-derived neuronal precursors were cultured and allowed to differentiate into neurons in serum-free medium.
Both tegaserod and RS67506 concentration-dependently increased neuronal numbers and length of neurites; these effects were blocked by GR113808, which exerted no effects of its own. A substantial portion of a population of neurons plated for growth in culture dies. Finding that 5-HT4 agonists increase numbers of neurons in a cultured population, therefore, can either be due to enhanced survival (decreased cell death) or increased differentiation of neurons from precursors, or both. Tegaserod and RS67506 decreased apoptosis, assessed by the TUNEL method; these decreases in apoptosis were blocked by GR113808. 5-HT4 agonists thus oppose neuronal cell death. These compounds may also affect the differentiation of neurons from precursors; whether or not that happens, however, remains to be determined. Because the length of neurites was increased by 5-HT4 receptor stimulation, it is clear that, in addition to their neuroprotective properties, 5-HT4 agonists enhance the potential of enteric neurons to exhibit plasticity through re-growth or remodelling of projections.
Our observations are consistent with the idea that 5-HT4 receptor stimulation is neuroprotective (decreasing cell death in vitro) and trophic (increasing neurite outgrowth) for enteric neurons. Experiments are now underway to determine whether or not 5-HT4 receptor stimulation can prevent the age-related decline in neuronal numbers in vivo. They might do so by decreasing or even stopping the age-related loss of neurons, which may or may not be a natural phenomenon, but would appear in either case to be detrimental. Conceivable mechanisms of action, which need not be mutually exclusive, include the inhibition of neuronal cell death and the recruitment of stem cells to generate new neurons. The ability of 5-HT4 agonists to decrease apoptosis in enteric neurons stressed by growth in culture supports the hope that such compounds might exert a similar action in vivo to save enteric neurons stressed by the ravages of ageing or disease. The further ability of 5-HT4 agonists to promote enteric neurogenesis from precursors in vitro supports the more speculative hypothesis that 5-HT4 agonists might also promote the generation of new enteric neurons from the stem cells that have been demonstrated to be present in the adult gut. Of course, the promotion of neurogenesis from precursors isolated from fetal bowel is very different from promoting neurogenesis from adult stem cells in vivo. Still, if this could be done, and the effect does not desensitize, then 5-HT4 agonists might be useful, not only to provide relief from constipation and IBS-C, but also to arrest and even reverse a potential worsening of these conditions due to neuronal cell loss. Such a denouement merits further research on the neuroprotective effects of 5-HT4 receptors.
Acknowledgments
This work was supported by grants from the NIH, NS12969 and NS15547 and by Novartis. Work on neuroprotection in the authors' laboratory was begun with Dr Elena Fiorica-Howells.
Footnotes
Conflict of Interest Statement: MDG has received research funding from Novartis. M.-T.L. has declared no conflicts.
References
- 1.Gabella G. Development and ageing of intestinal musculature and nerves: the guinea-pig taenia coli. J Neurocytol. 2001;30:733–66. doi: 10.1023/a:1019660519961. [DOI] [PubMed] [Google Scholar]
- 2.Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–77. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- 3.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system. J Comp Neurol. 1991;314:789–98. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 4.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–69. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–56. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 6.Santer RM, Baker DM. Enteric neuron numbers and sizes in Auerbach's plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988;25:59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- 7.Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–60. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade PR, Cowen T. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol Motil. 2004;16(Suppl 1):19–23. doi: 10.1111/j.1743-3150.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 9.Wade PR, Hornby PJA. Age-related neurodegenerative changes and how they affect the gut. Sci Aging Knowledge Environ. 2005;2005:pe8. doi: 10.1126/sageke.2005.12.pe8. [DOI] [PubMed] [Google Scholar]
- 10.de Souza RR, Moratelli HB, Borges N, Liberti EA. Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology. 1993;39:183–8. doi: 10.1159/000213532. [DOI] [PubMed] [Google Scholar]
- 11.Hanani M, Fellig Y, Udassin R, Freund HR. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113:71–8. doi: 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Wade PR. Aging and neural control of the GI tract. I. Age-related changes in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;283:G489–95. doi: 10.1152/ajpgi.00091.2002. [DOI] [PubMed] [Google Scholar]
- 13.Metugriachuk Y, Marotta F, Pavasuthipaisit K, et al. The aging gut motility decay: may symbiotics be acting as “implantable” biologic pace-makers? Rejuvenation Res. 2006;9:342–5. doi: 10.1089/rej.2006.9.342. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M, Lee JS, Viramontes B, Bharucha AE, Tangalos EG. Insights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older people. J Am Geriatr Soc. 2000;48:1142–50. doi: 10.1111/j.1532-5415.2000.tb04793.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Lissner S. General geriatrics and gastroenterology: constipation and faecal incontinence. Best Pract Res Clin Gastroenterol. 2002;16:115–33. doi: 10.1053/bega.2002.0269. [DOI] [PubMed] [Google Scholar]
- 16.Schiller LR. Constipation and fecal incontinence in the elderly. Gastroenterol Clin North Am. 2001;30:497–515. doi: 10.1016/s0889-8553(05)70192-8. [DOI] [PubMed] [Google Scholar]
- 17.Orr WC, Chen CL. Aging and neural control of the GI tract: IV. Clinical and physiological aspects of gastrointestinal motility and aging. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1226–31. doi: 10.1152/ajpgi.00276.2002. [DOI] [PubMed] [Google Scholar]
- 18.Watson R. Assessing the gastrointestinal (GI) tract in older people: 2. The lower GI tract. Nurs Older People. 2001;13:27–8. doi: 10.7748/nop.13.1.27.s13. [DOI] [PubMed] [Google Scholar]
- 19.Hall KE. Aging and neural control of the GI tract. II. Neural control of the aging gut: can an old dog learn new tricks? Am J Physiol Gastrointest Liver Physiol. 2002;283:G827–32. doi: 10.1152/ajpgi.00162.2002. [DOI] [PubMed] [Google Scholar]
- 20.Rumiantsev VG, Bondarenko E. Chronic constipations in elderly people. Eksp Klin Gastroenterol. 2004;109:48–54. [PubMed] [Google Scholar]
- 21.Saffrey MJ. Ageing of the enteric nervous system. Mech Ageing Dev. 2004;125:899–906. doi: 10.1016/j.mad.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Terrell KM, Heard K, Miller DK. Prescribing to older ED patients. Am J Emerg Med. 2006;24:468–78. doi: 10.1016/j.ajem.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Madsen JL, Graff J. Effects of ageing on gastrointestinal motor function. Age Ageing. 2004;33:154–9. doi: 10.1093/ageing/afh040. [DOI] [PubMed] [Google Scholar]
- 24.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Norman P. Tegaserod (Novartis) IDrugs. 2002;5:171–9. [PubMed] [Google Scholar]
- 26.Scott LJ, Perry CM. Tegaserod. Drugs. 1999;58:491–6. doi: 10.2165/00003495-199958030-00013. discussion 497-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–63. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 28.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol. 1996;270(5 Pt 1):G778–82. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 29.Grider JR, Foxx-Orenstein AE, Ji-Guang J. 5-Hydroxytryp-tamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–80. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 30.Grider JR. Desensitization of the peristaltic reflex induced by mucosal stimulation with the selective 5-HT4 agonist tegaserod. Am J Physiol Gastrointest Liver Physiol. 2006;290:G319–27. doi: 10.1152/ajpgi.00326.2005. [DOI] [PubMed] [Google Scholar]
- 31.Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol. 1994;266:G230–8. doi: 10.1152/ajpgi.1994.266.2.G230. [DOI] [PubMed] [Google Scholar]
- 32.Galligan JJ, Pan H, Messori E. Signalling mechanism coupled to 5-hydroxytryptamine4 receptor-mediated facilitation of fast synaptic transmission in the guinea-pig ileum myenteric plexus. Neurogastroenterol Motil. 2003;15:523–9. doi: 10.1046/j.1365-2982.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 33.Clarke DE, Craig DA, Fozard JR. The 5-HT4 receptor: naughty but nice. Trends Pharmacol Sci. 1989;10:385–6. doi: 10.1016/0165-6147(89)90177-6. [DOI] [PubMed] [Google Scholar]
- 34.Craig DA, Clarke DE. Pharmacological characterization of a neuronal receptor for 5-hydroxytryptamine in guinea pig ileum with properties similar to the 5-hydroxytryptamine4 receptor. J Pharmacol Exp Ther. 1990;252:1378–86. [PubMed] [Google Scholar]
- 35.Bach T, Syversveen T, Kvingedal AM, et al. 5HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:146–60. doi: 10.1007/s002100000299. [DOI] [PubMed] [Google Scholar]
- 36.Beattie DT, Smith JA, Marquess D, et al. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol. 2004;143:549–60. doi: 10.1038/sj.bjp.0705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwood-Van Meerveld B, Campbell-Dittmeyer K, Johnson AC, Hicks GA. 5-HT2B receptors do not modulate sensitivity to colonic distension in rats with acute colorectal hypersensitivity. Neurogastroenterol Motil. 2006;18:343–5. doi: 10.1111/j.1365-2982.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 38.McCullough JL, Armstrong SR, Hegde SS, Beattie DT. The 5-HT2B antagonist and 5-HT4 agonist activities of tegaserod in the anaesthetized rat. Pharmacol Res. 2006;53:353–8. doi: 10.1016/j.phrs.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Cho S, Hu Y. Activation of 5-HT4 receptors inhibits secretion of beta-amyloid peptides and increases neuronal survival. Exp Neurol. 2007;203:274–8. doi: 10.1016/j.expneurol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 40.Fiorica-Howells E, Liu MT, Ponimaskin EG, et al. Distribution of 5-HT4 receptors in wild-type mice and anaylsis of intestinal molity in 5-HT4 knockout mice. Gastroenterology. 2003;124:A342. [Google Scholar]
- 41.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39(5 Suppl):S184–93. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 42.Chalazonitis A, D'Autreaux F, Guha U, et al. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–82. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Dev Biol. 1989;132:189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- 44.Baetge G, Pintar JE, Gershon MD. Transiently catecholaminergic (TC) cells in the bowel of fetal rats and mice: precursors of non-catecholaminergic enteric neurons. Dev Biol. 1990;141:353–80. doi: 10.1016/0012-1606(90)90391-u. [DOI] [PubMed] [Google Scholar]


