Abstract
Vagal sensory axons navigate to specific sites in the bowel during fetal life. Netrin/deleted in colorectal cancer (DCC) were found to mediate the attraction of vagal sensory axons to the fetal mouse gut. We tested the hypothesis that laminin-111 can reverse the chemoattractive effects of netrin and act as a stop signal for vagal sensory axons. Laminin-111-expressing cells were located in the E12 and E16 mouse bowel by in situ hybridization. At E12, these cells extended centrifugally from the endoderm; by E16, laminin-111 expressing cells were found in the mucosa and outer gut mesenchyme. A similar pattern was seen in preparations of E13 and E15 mouse gut labeled with antibodies to laminin. Application of DiI to nodose ganglia identified vagal sensory axons growing into the fetal bowel. These terminals were found to avoid concentrations of laminin or to terminate at laminin-delimited boundaries. Soluble laminin inhibited the preferential growth of nodose neurites toward netrin-secreting cells (p < 0.01). This effect was mimicked by a peptide, YIGSR, a sequence within the β1 chain of laminin-111 (p < 0.004) and antagonized by a peptide, IKVAV, a sequence within the α1 chain of laminin-111. Antibodies to β1-integrins were also able to reverse the inhibitive effects of laminin and restore the attraction of nodose neurites towards netrin-1-secreting cells. Soluble laminin inhibited the preferential growth of nodose neurites toward a cocultured explant of foregut. These findings suggest that laminin terminates the attraction of vagal sensory axons towards sources of netrin in the developing bowel.
Keywords: vagus nerve, enteric nervous system, laminin, axon guidance, gastrointestinal tract
INTRODUCTION
The vagus nerve is the major conduit of information between the brain and the bowel and, because it contains both sensory and motor fibers, the vagus conducts information bidirectionally (Aziz and Thompson, 1998). The perikaya of vagal sensory neurons are found in the jugular (superior) and nodose ganglia (Powley and Phillips, 2002). The pathway that must be navigated by developing visceral sensory neurons ganglia to innervate the bowel is long and complex (Berthoud and Neuhuber, 2000; Powley and Phillips, 2002). Once in the bowel, moreover, vagal sensory axons are not randomly distributed. They are found in intramuscular arrays (IMAs) within longitudinal and circular smooth muscle layers, in intraganglionic laminar endings on the sheaths of myenteric ganglia (IGLEs), and in the lamina propria (Powley and Phillips, 2002; Berthoud et al., 2004). During development, therefore, vagal sensory axons have to find their way to correct destinations while avoiding regions that are inappropriate.
Relatively little is known about the guidance of vagal sensory axons to and within the developing bowel. The long-range guidance molecule, netrin, has recently been shown to attract vagal sensory axons to the fetal mouse gut by acting on the receptor, deleted in colorectal cancer (DCC), which extending vagal sensory axons express (Ratcliffe et al., 2006). The netrin/DCC interaction also participates in the guidance of migrating neural crest-derived cells to the submucosal and pancreatic plexuses (Jiang et al., 2003). In vitro, neurites extending from explanted nodose ganglia grow preferentially towards cocultured netrin-1-secreting cells. Antibodies to DCC block this preferential growth, which thus is DCC-mediated (Ratcliffe et al., 2006). Although vagal sensory axons have, thus far, only been shown to respond to an attractive signal, it is also likely that repellent signals exist because these axons fail to enter many regions of the bowel wall.
Laminins are adhesion molecules of the extracellular matrix, but they also play roles in cell signaling (Colognato and Yurchenco, 2000). Laminins are heterotrimers with three genetically distinct chains (α, β, and γ), which exist in a variety of isoforms that are categorized by their chain composition (Aumailley et al., 2005); laminin-111 (formerly known as laminin-1) is composed of α1, β1, and γ1 chains. Adhesive and signaling functions of laminin have been traced to particular domains and can be mimicked by peptides with corresponding sequences. For example, the sequence, YIGSR, of the β1 chain has been of interest for its putative roles in adhesion (Graf et al., 1987), tumor metastases (Iwamoto et al., 1987), cell signaling (Bushkin-Harav and Littauer, 1998), and neural crest migration (Bilozur and Hay, 1988). The peptide sequence, IKVAV, located on the α1 chain of laminin-111, has been implicated in angiogenesis, tumor growth, collagenase IV production (Grant et al., 1989; Kanemoto et al., 1990; Kibbey et al., 1992), adhesion, cell migration, neurite outgrowth (Sephel et al., 1989; Tashiro et al., 1989; Nomizu et al., 1995), and differentiation of enteric neurons (Chalazonitis et al., 1997).
Laminin-111 is known to be present in the developing gut (Simon-Assmann et al., 1998) and, when the terminal bowel becomes aganglionic because of defective endothelin-3 (Edn3) signaling, laminin-111 accumulates in the aganglionic region (Rothman et al., 1996). Laminin-111, moreover, promotes the differentiation of migrating crest-derived cells into enteric neurons (Chalazonitis et al., 1997). The abilities of laminin-111 to promote enteric neuronal differentiation, as well as its accumulation in a zone that becomes aganglionic, suggest that laminin-111 may play roles during enteric neurogenesis and/ganglio-genesis. The known effects of laminin-111 are also consistent with the hypothesis that it functions in the guidance of migrating crest-derived cells and/or the extrinsic nerves that innervate the bowel. This hypothesis has not previously been tested.
Previous studies have shown that soluble laminin-111 converts the netrin-mediated attraction of retinal axons to repulsion by decreasing levels of cAMP in their growth cones (Höpker et al., 1999). This effect can be mimicked by YIGSR, but not IKVAV. Although netrins are secreted by the endodermal epithelium of the bowel, that epithelium is neither entered nor contacted by the crest-derived cells (Jiang et al., 2003), nor the vagal sensory axons (Ratcliffe et al., 2006) that are attracted by netrins. In addition to netrins, the endodermal epithelium secretes laminin-111 (Simon-Assmann et al., 1998); therefore, if enteric crest-derived cells and/or vagal sensory axons were to respond to laminin-111 in the same way as retinal axons, laminin-111 would prevent netrin-attracted cells or fibers from reaching the source of netrin in the epithelium. Additional vagal sensory axons terminate in the outer gut mesenchyme. Myenteric ganglia and the muscularis externa arise in this region and both appear to be sources of netrins (Ratcliffe et al., 2007). Laminin, moreover, is found in the sheaths that surround myenteric ganglia (Mawe and Gershon, 1989), which are the sites of termination of the vagal sensory axons that form IGLEs, and in the basal laminae of smooth muscle cells, which abut on the vagal sensory axons that form IMAs. We thus tested the idea that enteric laminin acts as a stop signal for vagal sensory axons by reversing the chemoattractive effects of netrins. Our data are compatible with the idea that netrin/DCC-mediated attraction is terminated when extending vagal sensory axons encounter locally high concentrations of laminin in the developing gut.
METHODS
Animals
Timed pregnant mice (CD-1) were obtained from Charles River (Waltham, MA). Gestation was dated from the day a vaginal plug was discovered, which was considered E0. When used for experiments, animals were killed by exposure to CO2 gas. The Animal Care and Use Committee of Columbia University has approved this procedure.
Immunohistochemistry and Histochemistry
Vagal sensory fibers were identified in the developing gut by applying the lipophilic dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) to the nodose ganglia of fetal mice, as previously described (Ratcliffe et al., 2006). Fetuses were fixed for 24 h at 4°C in a solution containing 4% phosphate-buffered formaldehyde (freshly prepared from paraformaldehye; pH 7.4) and subsequently incubated in phosphate buffered saline, pH 7.4 (PBS). Fetuses were cryoprotected with 30% sucrose, embedded in Neg-50 Frozen Section Medium (Richard-Allan Scientific, Kalamazoo, MI), frozen in liquid nitrogen, and sectioned at 10 μm. Sections were thaw-mounted onto Colorfrost/Plus microscope slides (Fisher Scientific Company L.L.C, Pittsburg, PA) and mounted in glycerol containing 2.7 mg/ml 1,4 diazabicyclo (2,2,2) octane (Sigma-Aldrich, St. Louis, MO) as an agent to counteract fading.
For immunocytochemistry, tissues were permeabilized and blocked by incubation in PBS containing 0.4% Triton X-100 and 4% normal goat serum. For double-labeling with DiI, tissues were permeabilized with 0.3% Tween 20 (Lukas et al., 1998). Primary antibodies to laminin were raised in rabbit against laminin isolated from the basement membrane of Englebreth-Holm-Swarm mouse sarcoma (diluted 1:100; Sigma; L9393). Antibodies were applied overnight in a humidified chamber; sites of antibody binding were detected by incubation for 3 h at room temperature with goat anti-rabbit antibodies labeled with Alexa 488 (diluted 1:200; Molecular Probes). The primary antibodies were omitted in control sections. DNA was stained with bisbenzimide (1 μg/ml in PBS; Sigma).
Photomicrographs were obtained with a Q Imaging digital camera attached to a Leica DMRXA2 microscope and an Apple computer with Openlab software (Improvision, Lexington, MA). An image editing software program, Adobe Photoshop 10.0 (Adobe Systems, San Jose, CA) was used to merge images and to adjust brightness and contrast.
In Situ Hybridization
Probes for in situ hybridization were subcloned into the expression vectors pCR®II (Invitrogen, Carlsbad, CA) or pGEM® (Promega Corporation, Madison, WI). The probe for the laminin α1 chain contained cDNA that spanned nucleotides 6346-6452 (Sasaki et al., 1988). The probe for the laminin β1 chain was supplied by Dr. Y. Yamada (NIH, Bethesda, MD) and contained cDNA that spanned nucleotides 566–5126 (Sasaki et al., 1987). Sense and anti-sense probes were synthesized by “run-off” transcription in the presence of DIG-UTP according to the manufacturer’s instructions (DIG RNA Labeling Kit, Roche Applied Science, Indianapolis, IN).
In situ hybridization was carried out by incubating sections with sense (control) and anti-sense probes (0.1–0.2 ng/μl) in a moist chamber at 60°C for 20 h. The hybridization buffer contained 50% formamide, 5× saline sodium citrate buffer (SSC; pH 4.5), 1% sodium dodecyl sulfate (SDS), 250 μg/ml yeast tRNA and 100 μg/ml heparin. Following hybridization, preparations were washed once in 2× SSC (pH 7.0)/50% formamide at 55°C and once in 2× SSC (pH 7.0) at 55°C. The preparations that had been incubated with sense and anti-sense probes for laminin β1 were further washed in 0.2× SSC (pH 7.0) at 55°C. Bound digoxigenin was detected with antibodies coupled to alkaline phosphatase (diluted 1:2000; Roche Applied Science). Alkaline phosphatase activity was demonstrated and visualized with nitroblue tetrazolium chloride (NBT; Roche Applied Science) and 5-bromo-4-chloro-indolyl-phosphate (BCIP; Roche Applied Science) in a buffer containing 0.1M Tris buffer (pH 9.5), 0.1M NaCl, 0.05M MgCl2, and 0.1% Triton X-100. Tissues were incubated with 6 mg/ml levamisole (Sigma) to inhibit endogenous alkaline phosphatase activity.
Tissue Culture
Explants
Nodose ganglia and distal foregut were dissected from E14 mice and placed into 35 mm Petri dishes in iced Dulbecco’s Minimal Essential Media with F12 (DMEM/F12; GIBCO, Invitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal bovine serum (GIBCO), 0.6% d-glucose, and 1% penicillin-streptomycin (GIBCO). The ganglia and foregut were then transferred to 4 well culture dishes (Nalge Nunc International, Rochester, NY) for culture in a 3D collagen gel. Each well contained a small base of collagen (20 μl) prepolymerized with bicarbonate from a solution of rat tail collagen (BD Biosciences, Bedford, MA) in 10× Minimal Essential Media (GIBCO). Two layers of collagen, first 2 μl and then 4 μl were sequentially applied over the explants. The collagen was allowed to polymerize for 10 min at room temperature after each layer was applied; growth medium was then added and the cultures were incubated at 37°C in an atmosphere containing 5% CO2. The explanted nodose ganglia were cultured with either foregut or aggregrates of cells (below). All explants were maintained in culture for 48 h and fixed overnight as described earlier.
Netrin-1-secreting Cells
Control 293-EBNA cells (Invitrogen) and 293-EBNA cells stably transfected with a construct encoding chick netrin-1 protein tagged at the C-terminus with a c-Myc epitope (supplied by Dr. M. Tessier-Lavigne, Genentech, South San Francisco) (Keino-Masu et al., 1996) were maintained as cell aggregates, prepared as previously described (Ratcliffe et al., 2006). A block of netrin-secreting and a block of control 293-EBNA cells were placed on either side of a freshly explanted nodose ganglion on a base of polymerized collagen. The assembled coculture was then layered with collagen as described earlier so that both the nodose and the two blocks of 293-EBNA cells were embedded in the same 3D collagen gel. The cocultures were then incubated and fixed as described earlier.
Reagents
Soluble ultrapure mouse laminin (extracted from Engelbreth-Holm-Swarm mouse sarcoma, majority laminin-111; BD Biosciences, San Diego, CA) was added to the media in which nodose ganglia were cocultured with cell aggregates or with distal foregut. The following peptides were added to the culture media of cocultures of nodose ganglia and cell aggregates: YIGSR (Tyr-Ile-Gly-Ser-Arg; Sigma), RSGIY (Arg-Ser-Gly-Ile-Tyr; custom made, Proteintech Group Incorporated, Chicago, Il) and the peptide containing IKVAV (Cys-Ser-Arg-Ala-Arg-Lys-Gln-Ala-Ala-Ser-Ile-Lys-Val-Ala-Val-Ser-Ala-Asp-Arg; Sigma). Low endotoxin-containing, purified hamster antibodies to rat CD29 (integrin β1 chain BD Biosciences; clone Ha2/5) were added to the culture media to determine their ability to block the effects of laminin on the growth of nodose axons toward netrin-1 secreting cells.
Quantitation
Cocultures of nodose ganglia with netrin-1-secreting and control 293-EBNA cells were photographed through an inverted microscope using Hoffman modulation contrast optics. The images were digitized and visualized with an image editing software program, Adobe Photoshop 10.0 (Adobe Systems). The relative densities of nodose neurite outgrowth towards either the netrin-1-secreting or control cells were determined by superimposing a regular grid over the images (Gershon et al., 1994). A schematic representation is shown in Figure 1. Measurement fields of equal area (0.32 mm2) were defined on opposite sides of the nodose ganglion to include, respectively, neurites growing toward the netrin-1-secreting or toward the control cells. The relative neurite density was calculated using the super-imposed grid; the number of intersections of the lines covered by neurites was counted and normalized to the total number of intersections in the measurement area. The relative neurite density in the measurement field positioned on the side of netrin-1-secreting cells was compared to that in the measurement field positioned on the side of the control cells by using a paired t test. For each coculture, the relative density of nodose neurites growing towards control cells was subtracted from that of neurites growing toward netrin-1-secreting cells. A mean difference was then calculated for each condition; a mean difference >0 signifies attraction and <0 signifies that the attraction has been blocked. The mean differences between groups were compared using unpaired t tests.
Figure 1.
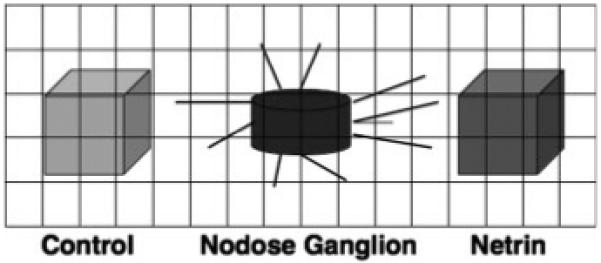
Schematic representation of an explanted nodose ganglion with aggregates of netrin-1-secreting (Netrin) and control (Control) 293-EBNA cells arranged on either side, at 180° apart. A sample superimposed grid is illustrated. Neurite density is measured in defined fields by counting the number of grid line intersections covered by neurites, normalized to the total number of intersections.
Cocultures of nodose ganglia and explanted foregut were, like the cocultures of nodose ganglia and cell aggregates, photographed. Images were again digitized and a regular grid was superimposed over them. Measurement fields (0.24 mm2) were defined in 2 areas on either side of the nodose ganglia. The measurement field that was positioned between the explanted nodose ganglion and the explanted bowel was defined as “toward;” the field on the opposite side of the nodose ganglion was defined as “away.” The relative neurite density in the measurement field defined as “toward” was compared to the field “away” by using a paired t test. The “toward” to “away” difference was calculated by subtracting the mean neurite density “away” from the mean neurite density “toward” the explanted foregut.
RESULTS
Laminin-111 is Expressed in the Fetal Gut
Sites of laminin-111 expression in the developing mouse gut were located by in situ hybridization. Labeling was considered specific if it was demonstrable with an antisense [Fig. 2(A,C,E)] but not a sense [Fig. 2(B,D,F)] probe. Transcripts encoding laminin β1 were found to be expressed in the endoderm and surrounding mesenchyme of bowel isolated from E12 mice [Fig. 2(A)]. Mesenchymal laminin β1 expression was most intense nearest the epithelium and decreased in a centrifugal gradient so that little mRNA encoding laminin β1 was detected in cells of the outer mesenchyme [Fig. 2(A)]. An identical pattern was observed for the localization of transcripts encoding laminin α1 (not illustrated). By E14, the pattern changed and laminin β1 expression became evident in the outer mesenchyme [Fig. 2(C)]. Two distinct zones of laminin-expressing cells thus become evident. This pattern is repeated at E16 for mRNA encoding laminin α1 [Fig. 2(E)].
Figure 2.
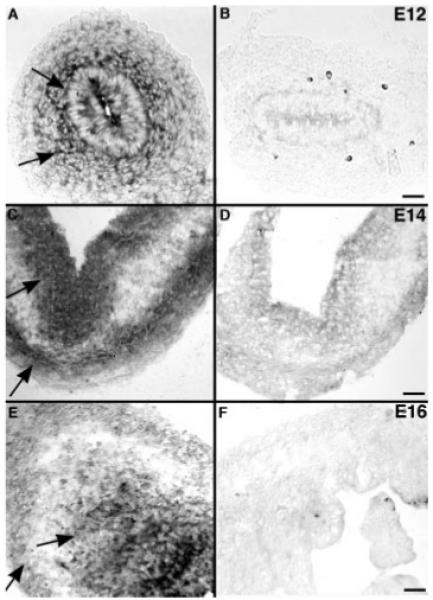
Transcripts encoding laminin-111 chains in the fetal gut are located by in situ hybridization. (A) Gut (E12). Transcripts encoding laminin β1 are detected in the endoderm and surrounding mesenchyme (arrows). The expression of laminin β1 is most intense closest to the epithelium and decreases in a centrifugal concentration gradient; few cells in the outer gut mesenchyme express laminin β1. (B) Gut (E12). No labeling is detected with the sense probe. (C) Gut (E14). Transcripts encoding laminin β1 are found in two distinct zones, the endoderm and the outer gut mesenchyme (arrows), without an intervening concentration gradient. (D) Gut (E14). No labeling is detected with the sense probe. (E). Gut (E16). Transcripts encoding laminin α1 are found in distribution similar to that of transcripts endoding β1 at E14; cells expressing mRNA encoding laminin α1 (arrows) are found in both the endoderm and the outer gut mesenchyme. (F) Gut (E16). No labeling is detected with the sense probe. Scale bars = 50 μm in A, B; 25 μm in C–F.
Laminin immunoreactivity was located in sections of murine fetal bowel. At E13, laminin was concentrated in basal laminae underlying the mucosal and serosal epithelia. Concentrations of laminin were also found outlining cells of the mesenchyme [Fig. 3(A,B)]. This laminin immunoreactivity was most abundant close to the mucosa in a distribution that corresponded well with that of the laminin-expressing cells found by in situ hybridization [compare Fig. 3(A,B) with Fig. 2(A)]. By E15, laminin was still found to be concentrated in mucosal and serosal basal laminae and in the subendodemal mesenchyme, but was now also abundant around cells of the outer gut mesenchyme [Fig. 3(C,D)]. The ganglia of the primordial myenteric plexus were now evident in the outer gut mesenchyme as islands of cells that were outlined as a group, but not individually, by laminin immunoreactivity [Fig. 3(C,D)]. The ganglia thus stood out in negative image because of their internal exclusion of laminin. In control sections, no staining was seen when the primary antibodies were omitted (data not shown).
Figure 3.
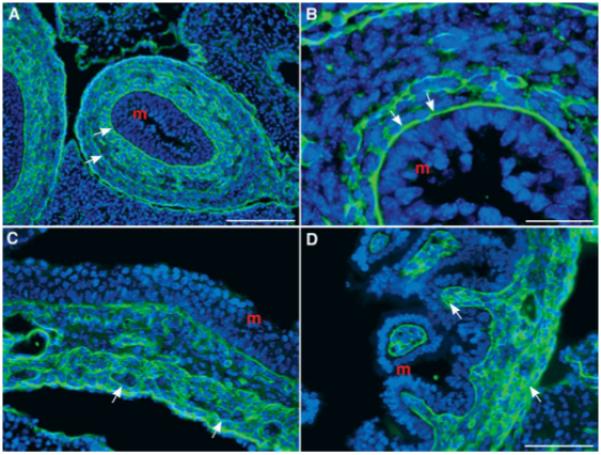
Laminin immunoreactivity is found in the fetal mouse gut. (A) Colon (E13). Laminin immunoreactivity is concentrated in the basal laminae and is also found outlining cells of the mesenchyme (arrows; m = mucosa). The pattern of immunoreactivity reveals a concentration gradient that is greatest near the mucosa and is similar to that seen with in situ hybridization [see Fig. 2(A)]. (B) Colon (E13). The same section as A at higher magnification. The concentration of laminin immunoreactivity is clearly seen in the basal laminae (arrows; m = mucosa). (C) Stomach (E15). Laminin is still concentrated near the mucosa, but is now also detected in the outer gut mesenchyme. The ganglia of the myenteric plexus are outlined by laminin and can be detected by their negative image because of the absence of intraganglionic laminin immunoreactivity (arrows; m = mucosa). (D) Small bowel (E15). Laminin immunoreactivity is abundant in the gut wall and, as in C, is concentrated near the mucosa and in the outer gut mesenchyme (arrows; m = mucosa). Scale bars = 100 μm in A; 25 μm in B; 50 μm in C (applied to D).
Vagal Sensory Axons Avoid Laminin-Rich Regions in the Fetal Gut
To identify descending vagal sensory axons in the E16 fetal gut, DiI was applied bilaterally to the developing nodose ganglia. DiI has been demonstrated to move distally in the plasma membrane to reach the terminals of these axons, which can then be positively identified by the fluorescence of DiI (Ratcliffe et al., 2006). Laminin immunoreactivity was located simultaneously in the DiI-labeled preparations to study the relationship between vagal sensory fibers and laminin-rich zones of the bowel [Fig. 4(A-C)]. DiI-labeled vagal sensory terminals were often found in close proximity, or even in apposition to regions rich in laminin, but the vagal sensory axons were never observed to cross laminin-rich zones [Fig. 4(B,C)]. Sensory nerve-laminin appositions were seen in the sheaths of developing ganglia in both the stomach [Fig. 4(B)] and small intestine [Fig. 4(C)]. These observations are consistent with the hypothesis that sites of laminin concentration or primordial basal laminae repel or act as stop signals for vagal sensory axons extending through the fetal bowel.
Figure 4.
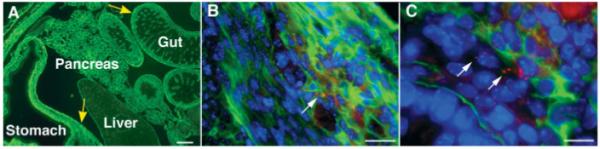
Bilateral application of DiI to the nodose ganglia at E16 identifies vagal sensory terminals in the gut. Sections are double labeled with antibodies to laminin. (A) Coronal section of abdomen. Laminin is seen most prominently in the bowel and pancreas. The locations corresponding to panels B and C are identified (yellow arrows). (B) Stomach. DiI-labeled punctate terminals of vagal sensory axons are seen in the myenteric plexus, in close proximity to a ganglion, possibly forming an IGLE. The DiI-labeled sensory terminals are very close to, but do not cross the areas where laminin immunoreactivity is highly concentrated. (C) Small bowel. DiI-labeled nerve terminals extend into the outer mesenchyme of the bowel wall, but are located between areas of concentrated laminin. Scale bars = 200 μm in A; 25 μm in B, 10 μm in C.
Soluble Laminin Inhibits the Netrin-Attracted Growth of Nodose Axons
In vitro experiments were carried out to determine whether laminin could interfere with the netrin-attracted growth of vagal sensory axons. Nodose ganglia were explanted from E14 fetal mice and cocultured in 3D collagen gels with aggregates of control and stably transfected netrin-1-secreting 293-EBNA cells. Aggregates of control and netrin-1-secreting cells were positioned on either side of an explanted nodose ganglion, so as to offer neurites extending from the ganglion a choice of cell type to which to grow. Preferential growth could then be determined by determining the relative densities of neurites extending to the control or to the netrin-1-secreting cells, which were 180° apart. Cultures were photographed through an inverted microscope, images were digitized, and the relative numbers of neurites extending toward the netrin-1-transfected or control cells were quantified as described in the methods section.
In the absence of added laminin, as previously observed (Ratcliffe et al., 2006), neurites were found to grow preferentially towards netrin-1-secreting cells (Fig. 5; p < 0.02; n = 25). Soluble mouse laminin (20 μg/ml) was added to ambient culture media at the time nodose ganglion explants and cell aggregates were plated. After 24 h, soluble laminin (10 μg/ml) was again added to offset degradation of the protein. The addition of soluble laminin abolished the preferential growth of neurites toward the netrin-1-secreting cells (Fig. 5; p < 0.01; n = 10). To determine which laminin chain mediates the repulsion caused by laminin, peptide with the sequence YIGSR (50 μg/ml; Sigma), which is located within the β1 chain of laminin, was added to ambient culture media at the time nodose ganglion explants and cell aggregates were plated. The YIGSR peptide (25 μg/ml) was again added after 24 h to offset degradation. Addition of the YIGSR peptide, like that of laminin, blocked the preferential growth of nodose axons toward netrin-1-secreting cells (Fig. 5; p < 0.004; n = 21). Surprisingly, the similar addition of a peptide with the reverse sequence, RSGIY, (n = 7) also interfered with the preferential growth of nodose axons toward netrin-1-secreting cells. The effect of YIGSR appeared to be greater than that of RSGIY in that in the presence of YIGSR (p < 0.05), but not RSGIY, axons tended to grow away from the netrin-secreting and toward the control cells. The mean netrin-1 to control difference, which was +7.3 in the absence of laminin, was −6.9 in the presence of laminin, −6.0 in the presence of YIGSR, and −1.6 in the presence of RSGIY.
Figure 5.
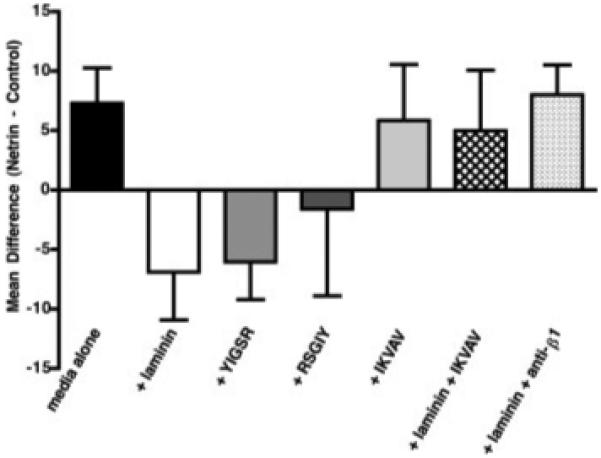
The preferential outgrowth of neurites from explants of E14 nodose ganglia toward cocultured netrin-1-secreting cells is blocked by soluble laminin. Aggregates of stably transfected netrin-1-secreting and control cells were positioned on either side of cocultured nodose ganglia. The difference in neurite density in defined regions of outgrowth either toward the netrin-secreting or control cells were calculated. The mean of the differences was used to compare conditions; a mean difference greater than 0 signifies attraction. The selective extension of nodose neurites towards netrin-1-secreting cells is significantly blocked by soluble laminin (p < 0.01) and by the peptide sequence YIGSR (p < 0.004). Addition of the reverse peptide sequence, RSGIY, to the culture media also reduces the mean difference to <0. The peptide sequence IKVAV, on its own, does not affect the ability of neurites from nodose ganglia to be attracted to netrin-1-secreting cells, but can block the action of laminin when both IKVAV and soluble laminin are added to the culture media and restore the mean difference to >0. Similarly, the simultaneous addition of antibodies to β1-integrins (anti-β1) and soluble laminin reverses the repellent effects of lamimin and significantly restores the ability of neurites from nodose ganglia to be attracted to netrin-1-secreting cells (p < 0.03). Error bars = standard error of the mean.
Experiments similar to those with peptides of the β1 chain were carried out to investigate the IKVAV sequence of the α1 of laminin-111. A 19-amino acid peptide within the α1 chain of laminin, which contains the IKVAV sequence (50 μg/ml), was added to ambient culture media at the time nodose ganglion explants and cell aggregates were plated. The IKVAV-peptide (25 μg/ml) was added again after 24 h. By itself, IKVAV did not alter the baseline preference of nodose axons to grow toward netrin-1-secreting cells (Fig. 5; n = 5); however, at high concentration (100 μg/ml at plating and 50 μg/ml after 24 h), the IKVAV-peptide inhibited the ability of laminin to reverse the attractive effects of netrin-1 (Fig. 5; n = 4).
Laminin Acts on a β1-Integrin Receptor to Reverse the Effects of Netrin-1
The β1-family of integrins have previously been demonstrated to be involved in mediating the migration of crest-derived cells during development (Lallier and Bronner-Fraser, 1991; Bronner-Fraser, 1994; Stepp et al., 1994), and α1β1 has been found to be the primary integrin involved in laminin recognition in cells of neural crest origin (Lallier et al., 1994). Neurite extension on laminin is also integrin-dependent and can be blocked by antibodies to β1-integrins (Skubitz et al., 1991; Engvall et al., 1992; Tomaselli et al., 1993). We tested the hypothesis that a β1-integrin receptor mediates the effects of laminin on the response of nodose neurites to netrin-1. Antibodies to β1-integrin abolish the soluble laminin-mediated reversal of the attractive effects of netrin-1 on retinal axons (Höpker et al., 1999). Accordingly, we determined whether antibodies to β1-integrins could prevent the reversal by soluble laminin of the preferential growth of nodose axons toward a source of netrin-1. Aggregates of control and netrin-1-secreting cells were again positioned on either side of an explanted nodose ganglion and the cultures were maintained in laminin-containing media (as described earlier). Antibodies to β1-integrin (10 μg/ml) were also added to the culture media. In this case, fresh antibodies were added every 12 h, which was previously found to be necessary to maintain the function-blocking properties of antibodies in culture media (Ratcliffe et al., 2006). Antibodies to β1-integrin abolished the laminin-mediated reversal of the attractive effect of netrin-1 on nodose axons (see Fig. 5). In the presence of antibodies to β1-integrin, significantly more axons again grew toward than away from netrin-1-secreting cells (p < 0.03; n = 5), despite the simultaneous presence of soluble laminin. The mean netrin-1 to control difference, which was −6.9 in the presence of laminin alone, was +8 with the addition of antibodies to β1-integrin (p < 0.03). The netrin-1 to control difference in the presence of soluble laminin + antibodies to β1-integrin was thus not significantly different from that observed under basal conditions with no additives to the media (+7.3; see above).
Soluble Laminin Blocks the Preferential Growth of Nodose Axons Toward Explants of Gut
We have previously reported that neurites extending from nodose ganglia explanted from E14 fetal mice grow preferentially toward cocultured explants of primordial stomach and proximal duodenum (Ratcliffe et al., 2006). If, as postulated, this effect, which is antagonized by antibodies to DCC, is mediated by netrin-1, then it would be expected also to be blocked by soluble laminin. To test this hypothesis, nodose ganglia and distal foregut (distal stomach and proximal duodenum) were explanted from E14 fetal mice and cocultured in 3D collagen gels. The relative densities of nodose neurites growing “toward” or “away” from the enteric explants were measured, as described in the methods, in the presence or absence of soluble laminin. In the absence of soluble laminin, the density of nodose axons in the measurement field “toward” was significantly greater than those in the measurement fields “away” from the cocultured explants of foregut (p < 0.01; n = 20; Fig. 6). The mean “toward” to “away” difference was +14.5. This preferential growth toward the gut was significantly reversed by the addition of soluble laminin (20 μg/ml at the time of plating and 10 μg/ml after 24 h). In the presence of soluble laminin, the mean ” toward” to ”away” difference was −3.9 (p < 0.05 vs no laminin; n = 12).
Figure 6.
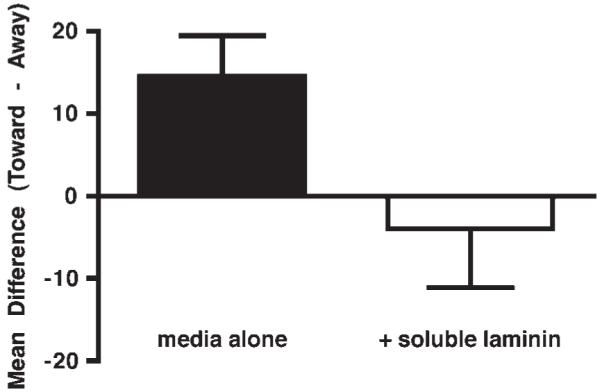
The preferential outgrowth of neurites from explants of E14 nodose ganglia toward a cocultured explant of distal foregut is blocked by soluble laminin. The difference in neurite density in defined regions of outgrowth either toward the cocultured gut or 180° away from it was calculated. The mean of the differences was used to compare conditions; a mean difference greater than 0 signifies attraction. The selective extension of nodose neurites towards the cocultured foregut is blocked significantly by soluble laminin (p < 0.05). Error bars = standard error of the mean.
DISCUSSION
We tested the hypothesis that, by opposing the netrin/DCC-mediated attraction of vagal sensory axons, laminin can act as a stop signal for these fibers as they extend toward their targets in the fetal gut. Observations were consistent with this hypothesis. Prior data had indicated that mucosal epithelial cells express netrins; nevertheless, despite the ability of netrins to attract crest-derived ENS precursors and vagal sensory axons, neither actually contact the mucosal epithelium (Jiang et al., 2003; Ratcliffe et al., 2006). The mucosal epithelium and underlying mesenchyme express laminin and laminin concentrates in the mesenchyme immediately adjacent to the epithelium. During their colonization of the bowel, subsets of DCC-expressing crest-derived émigrés turn perpendicularly from a predominantly proximo-distal path of migration to approach the mucosa (Jiang et al., 2003). These cells, however, stop short of the epithelium and give rise to the submucosal plexus. Vagal sensory axons also approach the mucosa and enter the subepithelial mesenchyme, but they too stop short of the epithelium (Powley and Phillips, 2002; Berthoud et al., 2004; Ratcliffe et al., 2006). Contact with laminin, secreted by the epithelium and associated mesenchyme could be responsible for stopping the epithelium-directed movement of both the crest-derived cells and vagal sensory axons by converting the attractive action of mucosally secreted netrins to a repulsive effect. This possibility is supported by the current observations that epithelial and subjacent mesenchymal cells express α1 and β1 chains of laminin and that laminin immunoreactivity is highly concentrated under the epithelium of the fetal gut. Laminin (probably laminin-111) is thus expressed and concentrated in locations that are suitable for stopping migrating cells or extending axons that are following an epithelial-derived netrin gradient. This hypothesis thus depends on netrins acting as long-range factors while laminin acts as a local stop signal.
The hypothesis that laminin acts as a stop signal was further supported by the observation that laminin did, in fact, have the ability to oppose the preferential growth of vagal axons to sources of netrin-1. The attractive effect of netrin-1 was analyzed in vitro. Neurites extending from nodose ganglia embedded in a 3D collagen gel grew preferentially toward stably transfected 293-EBNA cells that secrete netrin-1. This preferential growth was reversed by the addition of soluble laminin (predominantly laminin-111). The effect of laminin was mimicked by a peptide with the sequence, YIGSR, which is found in the β1 chain of laminin-111, and blocked by a peptide that includes the sequence, IKVAV, which is found in the α1 chain of laminin-111. The YIGSR-peptide, moreover, was able to convert the attractive effect of netrin-1 to repulsion, so that, in the presence of the YIGSR-peptide, vagal sensory axons preferentially grew away from the netrin-1-secreting cells. Although a peptide with the reverse sequence, RSGIY, interfered with the preferential growth of axons toward netrin-1-secreting cells, it lacked the ability of a YIGSR-peptide to convert the netrin-1 effect to repulsion. In addition to the IKVAV-containing peptide, antibodies to β1 integrins antagonized the effects of laminin. These observations confirm that netrins attract growing vagal sensory axons and they indicate that this effect can be reversed by laminin. The specificity of the reversal of netrin-mediated attraction by soluble laminin-111 is supported by the observations that laminin reversal is mimicked and antagonized, respectively, by peptides found in the β1 and α1 chains of laminin-111, and that the effect is dependent on a β1-integrin.
Netrins are expressed in the developing gut, not only in the endoderm, but also in the outer region of the bowel (Jiang et al., 2003). The targets of netrin in the outer gut wall have not been established, but vagal sensory axons migrate into this region of the bowel and are likely to respond to the attractive effects of netrins because they express DCC (Ratcliffe et al., 2006). In the outer wall of the adult bowel, vagal axons are found in the form of IGLEs, which are distinctive, leaf-like endings that ramify over the surfaces of myenteric ganglia (Zagorodnyuk et al., 2001), and IMAs, which run parallel to muscle fibers (Berthoud et al., 2004). Vagal sensory axons thus appear to be attracted to forming myenteric ganglia and smooth muscle, which is not surprising because both developing myenteric neurons and mesenchymal smooth muscle precursors express netrin-1 (Ratcliffe et al., 2007). Myenteric ganglia characteristically lack connective tissue but are enclosed by a sheath that is rich in laminin (Mawe and Gershon, 1989). The ability of laminin to reverse the attractive effects of netrins on the DCC-expressing vagal sensory terminals could, during development, modulate the ganglionic concentration of IGLEs and contribute to their eventual distribution. Laminin in the basal laminae of smooth muscle could do the same for IMAs. Again, in both cases, netrins would be envisioned to act as long-range attractive signals and laminin to act as a localized stop signal.
Experiments in which developing nodose ganglia were cocultured with distal foregut provided evidence in favor both of the long range attractive effect of netrins and of the ability of laminin to oppose them. As previously observed (Ratcliffe et al., 2006), nodose axons grew preferentially toward explants of bowel. This directionally selective outgrowth of neurites is netrin-mediated because antibodies to the netrin receptor, DCC, block it. It seems likely that neurons and mesenchymal cells of the outer wall of the gut (Ratcliffe et al., 2007) are the sources of the netrin to which cocultured nodose neurites respond in growing selectively toward the bowel. This preferential growth, like that of nodose axons toward stably transfected netrin-expressing cells, was reversed by soluble laminin. In situ, within the outer mesenchyme of the developing gut, laminin is not a circulating soluble protein, as in the artificial situation of coculture experiments. Instead, laminin is locally concentrated. During early ontogeny, electron microscopic studies have shown that laminin is found in amorphous puffs of electron-dense material located close to developing ganglia (Pomeranz et al., 1991). Later in ontogeny, the concentrations of laminin in the outer wall of the bowel shift to periganglionic and smooth muscle basal laminae. Growth cones of vagal sensory axons, repelled by an encounter with laminin in either early puffs within the mesenchyme or later in basal laminae, could thus cease to follow the netrin-mediated attraction, stop, and be remodeled as IGLEs or IMAs, depending on where migration ceases. The current observation that DiI-labeled vagal sensory axons did not cross local concentrations of laminin immunoreactivity in the developing bowel is consistent with the idea that encounters with laminin stop the extension of these axons. The presence of laminin in periganglionic sheaths could also serve to insulate the crest-derived precursors of neurons and glia from the attractive effects they might otherwise feel of the netrins secreted by the non-neuronal cells of the mesenchyme outside of ganglia. A laminin-containing periganglionic sheath that opposed outward migration of neural precursors might contribute to the maintenance of ganglionic integrity by rein-forcing the intraganglionic adhesive or attractive forces that act on developing neurons (Faure et al., 2007).
Laminin-111 is highly abundant during early development and is essential for the formation of basal laminae (Yurchenco and Wadsworth, 2004). The N-terminal and epidermal growth factor-like domains of the short arms of laminin-111 and other laminins share structural homologies with all of the netrins (Schneiders et al., 2007). Laminins utilize these short arm domains in their self-assembly into complex networks. Netrin-4, but not other netrins, is a component of basal laminae that becomes integrated into laminin polymers by interacting with laminin γ1 short arms. Netrin-4 binds to laminin-111 but netrin-1 does not. Netrin-1 is expressed in the developing murine bowel (Jiang et al., 2003; Ratcliffe et al., 2006). Truncated and full-length netrin-4 can disrupt the self-assembly and self-interactions of laminin-111 (Schneiders et al., 2007). Because, in contrast to netrin-4, netrin-1 does not interact with laminin short arms to become incorporated into laminin complexes, they will not be substrate bound in the developing bowel and thus able to diffuse beyond laminin barriers. Axons in the enteric mesenchyme can then follow these gradients until laminin is encountered and their response to netrins is reversed. This pattern for the bowel is similar to that previously reported for the retina in which ganglion cell axons follow netrin gradients to the optic nerve head and are repelled from other regions of the retinal periphery where laminin and netrins are coexpressed (Höpker et al., 1999). The ability of the peptide sequences, YIGSR and IKVAV to mimic and antagonize respectively the effects of laminin in the bowel, suggest that the laminin β1 and α1 chains are both involved in the interaction with netrins. The ability of antibodies to β1-integrin to abolish laminin-induced reversal of netrin-mediated attraction in both gut and retina is consistent with the idea in both organs that the effect of laminin requires the interaction of an integrin with DCC (Nikolopoulos and Giancotti, 2005).
Abnormal accumulations of laminin have been described in the bowel of animals with a congenital aganglionosis (Rothman et al., 1996; Gershon, 1999) and of humans with Hirschsprung’s disease (Parikh et al., 1992; Alpy et al., 2005). The lack of β1-integrins in crest-derived cells has also been reported to cause an incomplete colonization of the gut and an abnormal organization of enteric ganglia (Breau et al., 2006). It is possible that abnormalities in extracellular matrix molecules not only perturb the development of the enteric nervous system, but that of the extrinsic innervation as well. The terminal aganglionic bowel of mice lacking endothelin-3, in which increased expression of laminin-111 has been identified, is extrinsically innervated by thick, fasciculated nerve trunks (Rothman et al., 1996). A similar process occurs in humans with short-segment Hirschsprung’s disease (Wedel et al., 1999), moreover, differences in the expression pattern of laminin isoforms have been described in the presumed peripheral nerves found in aganglionic specimens from humans with Hirschsprung’s disease (Alpy et al., 2005). Further experiments will be required to characterize the ability of laminins to influence the development of the extrinsic innervation of the lower gut, and how this influence is related to human disease.
Acknowledgments
The authors thank Alcmène Chalazonitis for advice and Tandi Mohammed and Monique Anderson for excellent technical assistance.
Contract grant sponsors: Foundation for Digestive Health and Nutrition, Children’s Digestive Health and Nutrition Foundation.
Contract grant sponsor: NIH; contract grant numbers: NS-15547, NS-12969.
REFERENCES
- Alpy F, Ritie L, Jaubert F, Becmeur F, Mechine-Neuville A, Lefebvre O, Arnold C, et al. The expression pattern of laminin isoforms in Hirschsprung disease reveals a distal peripheral nerve differentiation. Hum Pathol. 2005;36:1055–1065. doi: 10.1016/j.humpath.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;1(16 Suppl):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bilozur ME, Hay ED. Neural crest migration in 3D extracellular matrix utilizes laminin, fibronectin, or collagen. Dev Biol. 1988;125:19–33. doi: 10.1016/0012-1606(88)90055-3. [DOI] [PubMed] [Google Scholar]
- Breau MA, Pietri T, Eder O, Blanche M, Brakebusch C, Fassler R, Thiery JP, et al. Lack of β1 integrins in enteric neural crest cells leads to a Hirschsprung-like phenotype. Development. 2006;133:1725–1734. doi: 10.1242/dev.02346. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Neural crest cell formation and migration in the developing embryo. FASEB J. 1994;8:699–706. doi: 10.1096/fasebj.8.10.8050668. [DOI] [PubMed] [Google Scholar]
- Bushkin-Harav I, Littauer UZ. Involvement of the YIGSR sequence of laminin in protein tyrosine phosphorylation. FEBS Lett. 1998;424:243–247. doi: 10.1016/s0014-5793(98)00180-x. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Tennyson VM, Kibbey MC, Rothman TP, Gershon MD. The α-1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immuno-selected from the fetal mouse gut. J Neurobiol. 1997;33:118–138. doi: 10.1002/(sici)1097-4695(199708)33:2<118::aid-neu2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: The laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Engvall E, Earwicker D, Day A, Muir D, Manthorpe M, Paulsson M. Merosin promotes cell attachment and neurite outgrowth and is a component of the neurite-promoting factor of RN22 schwannoma cells. Exp Cell Res. 1992;198:115–123. doi: 10.1016/0014-4827(92)90156-3. [DOI] [PubMed] [Google Scholar]
- Faure C, Chalazonitis A, Rheaume C, Bouchard G, Sampathkumar SG, Yarema KJ, Gershon MD. Ganglio-genesis in the enteric nervous system: Roles of the polysialylation of the neural cell adhesion molecule and its regulation by bone morphogenetic protein-4. Dev Dyn. 2007;236:44–59. doi: 10.1002/dvdy.20943. [DOI] [PubMed] [Google Scholar]
- Gershon A, Zhu Z, Sherman DL, Gabel CA, Ambron RT, Gershon MD. Intracellular transport of newly synthesized varicella-zoster virus: Final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Endothelin and the development of the enteric nervous system. Clin Exp Pharmacol Physiol. 1999;26:985–988. doi: 10.1046/j.1440-1681.1999.03176.x. [DOI] [PubMed] [Google Scholar]
- Graf J, Ogle RC, Robey FA, Sasaki M, Martin GR, Yamada Y, Kleinman HK. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26:6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Höpker V, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu M, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Kanemoto T, Reich R, Royce L, Greatorex D, Adler SH, Shiraishi N, Martin GR, et al. Identification of an amino acid sequence from the laminin A chain that stimulates metastasis and collagenase IV production. Proc Natl Acad Sci USA. 1990;87:2279–2283. doi: 10.1073/pnas.87.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SSY, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: An in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Lallier T, Bronner-Fraser M. Avian neural crest cell attachment to laminin: Involvement of divalent cation dependent and independent integrins. Development. 1991;113:1069–1084. doi: 10.1242/dev.113.4.1069. [DOI] [PubMed] [Google Scholar]
- Lallier T, Deutzmann R, Perris R, Bronner-Fraser M. Neural crest cell interactions with laminin: Structural requirements and localization of the binding site for α1β1 integrin. Dev Biol. 1994;162:451–464. doi: 10.1006/dbio.1994.1101. [DOI] [PubMed] [Google Scholar]
- Lukas JR, Aigner M, Denk M, Heinzl H, Burian M, Mayr R. Carbocyanine postmortem neuronal tracing. Influence of different parameters on tracing distance and combination with immunocytochemistry. J Histochem Cytochem. 1998;46:901–910. doi: 10.1177/002215549804600805. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Gershon MD. Structure, afferent innervation, and transmitter content of ganglia of the guinea pig gallbladder: Relationship to the enteric nervous system. J Comp Neurol. 1989;283:374–390. doi: 10.1002/cne.902830306. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Giancotti FG. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle. 2005;4:e131–135. [PubMed] [Google Scholar]
- Nomizu M, Weeks BS, Weston CA, Kim WH, Kleinman HK, Yamada Y. Structure-activity study of a laminin α1 chain active peptide segment Ile-Lys-Val-Ala-Val (IKVAV) FEBS Lett. 1995;365:227–231. doi: 10.1016/0014-5793(95)00475-o. [DOI] [PubMed] [Google Scholar]
- Parikh DH, Tam PKH, VanVelzen D, Edgar D. Abnormalities in the distribution of laminin and collagen type IV in Hirschsprung’s disease. Gastroenterology. 1992;102:1236–1241. [PubMed] [Google Scholar]
- Pomeranz HD, Sherman DL, Smalheiser NR, Tennyson VM, Gershon MD. Expression of a neurally related laminin binding protein by neural crest-derived cells that colonize the gut: Relationship to the formation of enteric ganglia. J Comp Neurol. 1991;313:625–642. doi: 10.1002/cne.903130408. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: What’s new in our understanding of vago-vagal refexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–G1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Ratcliffe EM, Anderson M, Chalazonitis A, Gershon MD. Netrin biosynthesis by enteric neurons: Roles in normal guidance of vagal sensory axons and their mislocation in aganglionic gut. Gastroenterology. 2007;132:A81. [Google Scholar]
- Ratcliffe EM, Setru SU, Chen JJ, Li ZS, D’Autreaux F, Gershon MD. Netrin/DCC-mediated attraction of vagal sensory axons to the fetal mouse gut. J Comp Neurol. 2006;498:567–580. doi: 10.1002/cne.21027. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Chen J, Howard MJ, Costantini FD, Pachnis V, Gershon MD. Increased expression of laminin-1 and collagen (IV) subunits in the aganglionic bowel of ls/ls, but not c-ret−/- mice. Dev Biol. 1996;178:498–513. doi: 10.1006/dbio.1996.0234. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Kato S, Kohno K, Martin GR, Yamada Y. Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci USA. 1987;84:935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kleinman HK, Huber H, Deutzmann R, Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988;263:16536–16544. [PubMed] [Google Scholar]
- Schneiders FI, Maertens B, Bose K, Li Y, Brunken WJ, Paulsson M, Smyth N, et al. Binding of Netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem. 2007;282:23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- Sephel GC, Tashiro K, Kleinman HK, Sasaki M, Yamada Y, Martin GR. A laminin A chain synthetic peptide with neurite outgrowth activity. Biochem Biophys Res Commun. 1989;162:821–829. doi: 10.1016/0006-291x(89)92384-x. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P, Lefebvre O, Bellissent-Waydelich A, Olsen J, Orian-Rousseau V, De Arcangelis A. The laminins: Role in intestinal morphogenesis and differentiation. Ann N Y Acad Sci. 1998;859:46–64. doi: 10.1111/j.1749-6632.1998.tb11110.x. [DOI] [PubMed] [Google Scholar]
- Skubitz AP, Letourneau PC, Wayner E, Furcht LT. Synthetic peptides from the carboxy-terminal globular domain of the A chain of laminin: Their ability to promote cell adhesion and neurite outgrowth, and interact with heparin and the β 1 integrin subunit. J Cell Biol. 1991;115:1137–1148. doi: 10.1083/jcb.115.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Urry LA, Hynes RO. Expression of α 4 integrin mRNA and protein and fibronectin in the early chicken embryo. Cell Adhes Commun. 1994;2:359–375. doi: 10.3109/15419069409014210. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Sephel GC, Weeks B, Sasaki M, Kleinman HK, Martin GR, Yamada Y. A synthetic peptide containing the IKVAV sequence in the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264:16174–16182. [PubMed] [Google Scholar]
- Tomaselli KJ, Doherty P, Emmett CJ, Damsky CH, Walsh FS, Reichardt LF. Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel T, Holschneider AM, Krammer HJ. Ultrastructural features of nerve fascicles and basal lamina abnormalities in Hirschsprung’s disease. Eur J Pediatr Surg. 1999;9:75–82. doi: 10.1055/s-2008-1072217. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


