Abstract
Summary: The maintenance of appropriate intracellular concentrations of alkali metal cations, principally K+ and Na+, is of utmost importance for living cells, since they determine cell volume, intracellular pH, and potential across the plasma membrane, among other important cellular parameters. Yeasts have developed a number of strategies to adapt to large variations in the concentrations of these cations in the environment, basically by controlling transport processes. Plasma membrane high-affinity K+ transporters allow intracellular accumulation of this cation even when it is scarce in the environment. Exposure to high concentrations of Na+ can be tolerated due to the existence of an Na+, K+-ATPase and an Na+, K+/H+-antiporter, which contribute to the potassium balance as well. Cations can also be sequestered through various antiporters into intracellular organelles, such as the vacuole. Although some uncertainties still persist, the nature of the major structural components responsible for alkali metal cation fluxes across yeast membranes has been defined within the last 20 years. In contrast, the regulatory components and their interactions are, in many cases, still unclear. Conserved signaling pathways (e.g., calcineurin and HOG) are known to participate in the regulation of influx and efflux processes at the plasma membrane level, even though the molecular details are obscure. Similarly, very little is known about the regulation of organellar transport and homeostasis of alkali metal cations. The aim of this review is to provide a comprehensive and up-to-date vision of the mechanisms responsible for alkali metal cation transport and their regulation in the model yeast Saccharomyces cerevisiae and to establish, when possible, comparisons with other yeasts and higher plants.
INTRODUCTION
The relevance of alkali metal cations to cell physiology, including their transport into and out of the cell, aroused scientists' interest as early as 100 years ago. It became obvious very soon that, whereas potassium is highly accumulated in different types of living cells and is indispensable for many physiological functions, sodium is toxic and at higher concentrations becomes lethal. Among other cell types, yeast cells were developed into an ideal model to study alkali metal cation transport and homeostasis more than 50 years ago, and the pioneering work in the field has already been brilliantly summarized (65, 235).
The reasons for using Saccharomyces cerevisiae as a model to study alkali metal cation homeostasis in eukaryotic cells are the availability of the complete annotated genome sequence (the first one among eukaryotic cells) (95), a comprehensive in silico prediction of all transporters (190), and its fast growth and genetic tools to prepare mutants. Later, a comparative analysis of sequenced genomes from different yeast species showed that approximately 10% of a Hemiascomecete yeast genome corresponds to membrane transporters (56), and a detailed analysis of genes encoding putative transporters revealed that the systems ensuring efficient uptake and efflux of potassium and/or sodium have been highly conserved (56).
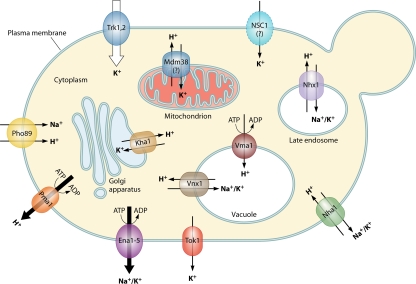
Although most yeast do not normally encounter high concentrations of sodium in their natural habitats, they may be confronted with situations in which larger amounts of toxic sodium than of requisite potassium cations are present in the environment. In these circumstances, cells spend a lot of energy accumulating sufficiently large amounts of intracellular K+ and maintaining low cytosolic Na+. Potassium is required for many physiological functions, such as regulation of cell volume and intracellular pH, maintenance of stable potential across the plasma membrane, compensation of negative charges in many macromolecules, protein synthesis, and enzyme activation. However, S. cerevisiae cells are able to grow in the presence of a broad range of external concentrations of K+ (10 μM to 2.5 M) and Na+ (<1.5 M), and some nonconventional halotolerant yeast species can grow in almost-saturated salt solutions. To maintain an optimum intracellular concentration of potassium ions and a stable and high intracellular K+/Na+ ratio, yeast cells employ three different approaches: (i) strict discrimination among alkali metal cations at the level of influx (transporters displaying higher affinity for potassium than for sodium [235]), (ii) efficient efflux of toxic or surplus cations from cells, and (iii) selective sequestration (compartmentalization) of cations in organelles. In yeast cells, transport systems exist at both the plasma and organellar membranes, which mediate alkali metal cation fluxes with different substrate specificities and using diverse mechanisms (e.g., primary active ATPases, secondary active symporters and antiporters, and passive channels) (Fig. 1). At least 10 different alkali metal cation-specific transporters in S. cerevisiae have been characterized so far (Table 1), and strong evidence exists indicating that at least a few additional transporters will be defined at the molecular level (see below).
FIG. 1.
The major plasma membrane and intracellular cation transporters in the yeast S. cerevisiae.
TABLE 1.
Alkali metal cation transporters in S. cerevisiae
| Name | ORF | YETI no.a | Localizationb | Transporter type | Length (aa) | No. of TMS | Substrate specificityc | Main function | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Trk1 | YJL129c | 2.A.38.2.1 | PM | Uniporter | 1,235 | 12 | K+, Rb+ | K+ uptake | 82 |
| Trk2 | YKR050w | 2.A.38.2.1 | PM | Uniporter | 889 | 12 | K+, Rb+ | K+ uptake | 128 |
| Tok1 | YJL093c | 1.A.1.7.1 | PM | Channel | 691 | 10 | K+ | K+ efflux | 116 |
| Ena1 | YDR040c | 3.A.3.9.1 | PM | ATPase | 1,091 | 10 | Na+, Li+, K+, Rb+ | Detoxification | 102 |
| Nha1 | YLR138w | 2.A.36.4.1 | PM | Antiporter | 985 | 12 | K+, Na+, Li+, Rb+ | K+ efflux | 219 |
| Pho89 | YBR296c | 2.A.20.2.2 | PM | Symporter | 574 | 10 | Na+, Pi | Pi uptake | 156 |
| NSC1 | Not known | PM | Channel | Not known | Not known | Monovalent + bivalent cations | Not known | 30 | |
| Kha1 | YJL094c | 2.A.37.4.1 | GA | Antiporter | 873 | 12 | K+, Li+, Na+ | Detoxification | 153 |
| Nhx1 | YDR456w | 2.A.36.2.1 | LE | Antiporter | 633 | 8 (?) | Na+, Rb+, K+, Li+ | Detoxification, vesicle trafficking | 185 |
| Vnx1 | YNL321w | 2.A.19.Y1.Z1 | VAC | Antiporter | 908 | 13 | Na+, K+ | Detoxification | 37 |
Plasma membrane transporters serve to (i) provide cells with sufficient amounts of potassium; (ii) maintain the potassium homeostasis; (iii) eliminate toxic sodium (lithium) cations; (iv) preserve the membrane potential; (v) regulate the intracellular pH; (vi) keep a positive turgor inside the cell, which is necessary for plasma membrane/cell wall expansion and cell division, and (vii) cope with osmotic stress. There are six different well-characterized transporters at the plasma membrane. They comprise the potassium uptake systems Trk1 and Trk2, the potassium channel Tok1, the Pi-Na+ symporter Pho89, and the efflux systems Ena Na+-ATPases and Nha1 Na+/H+ antiporter. Besides these six specific alkali metal cation transporters, there is a cationic channel whose existence has been experimentally proven but whose gene has not been identified yet (NSC1) and several other (nonspecific) proteins/transporters marginally involved in potassium and/or sodium fluxes across the plasma membrane (e.g., Pmp3 and Qdr2).
Intracellular transporters have been identified and characterized only recently. They comprise mainly alkali metal cation/H+ antiporters of vacuolar (Vnx1), endosomal (Nhx1), and Golgi apparatus (Kha1) membranes. Very efficient and physiologically crucial exchange of K+ for H+ by an antiport mechanism also exists across the mitochondrial membrane, but the transporter gene has not been identified yet. Similarly to the plasma membrane transporters, organellar systems serve to (i) regulate cytosolic cation homeostasis (e.g., detoxification of sodium by sequestration in the vacuole), (ii) maintain and regulate intraorganellar potassium homeostasis and pH, and (iii) modulate protein trafficking through the endosomal and/or secretory pathway.
Role of H+-ATPases in Alkali Metal Cation Homeostasis
Most of the proteins listed above mediate transport by an antiport mechanism, exchanging alkali metal cations for protons. Therefore, a proton motive force across membranes serves as a source of energy to pump alkali metal cations against their gradients. Nevertheless, in some cases (e.g., sudden alkalinization of cytosol at high pHout), cells can use the outward gradient of potassium across the plasma membrane as a driving force to transport protons against their gradient into the cells (17). Under normal physiological conditions, the secondary active antiporters localized in the plasma and organellar membranes depend on the activity of primary active transporters that build up the necessary gradient of protons. In yeast cells, this function is fulfilled by the plasma membrane and vacuolar ATPases.
The plasma membrane H+-ATPase, encoded by the PMA1 gene, is the essential and main primary yeast transporter belonging to the widely distributed family of P2-type ATPases (7). In yeast cells, it is the most abundant plasma membrane protein, which is very stable and consumes at least 20% of cellular ATP (177). Its electrogenic activity creates the electrochemical gradient of protons across the plasma membrane, which is in turn indispensable for all secondary active symporters and antiporters. The activity of Pma1 is tightly regulated according to the metabolic activity and physiological conditions of cells; e.g., external glucose is a powerful stimulus leading to a rapid and strong activation (254). Its activity is also positively regulated in response to decreased intracellular pH or increased potassium uptake (258). S. cerevisiae encodes a second plasma membrane H+-ATPase, Pma2, which is 89% identical to Pma1 (252). Pma2 is able to export protons, although its enzymatic properties differ from those of Pma1 (267). The expression of PMA2 under standard growth conditions is much lower than that of PMA1, which explains the nonessential nature of the former and its minor impact on cation homeostasis.
In contrast to the plasma membrane H+-ATPase, the yeast vacuolar ATPase is not a single polypeptide but has a complex structure consisting of two main domains, peripheral V1 and membrane-bound Vo. Each of the two domains is composed of several proteins, and the whole structure is related to the mitochondrial F1Fo-ATP synthase (114, 115). This ATPase not only plays a crucial role in the acidification of the vacuolar lumen but is also indispensable for proper functioning of other organelles (251). In yeast cells, the analysis of its activity in organelles along the secretory pathway confirmed its importance but simultaneously suggested that this ATPase may exist in organelles in distinct forms and under selective regulation (250). It is very likely that its activity creates the gradient of protons across organellar membranes which, in turn, mediates the function of organellar alkali metal cation/H+ antiporters. The plasma membrane and vacuolar H+-ATPases most probably collaborate in maintaining cytosolic pH homeostasis, and there is evidence that they are functionally interdependent and coordinately regulated on multiple levels (158).
General Aspects of Alkali Metal Cation Homeostasis
The high potassium content in yeast cells corresponds to the steady state between simultaneous influx and efflux across the plasma membrane, and this continuous circulation is believed to be necessary for basic physiological functions (136, 198). The coordination of potassium uptake and efflux systems is necessary for the maintenance of the potential across the plasma membrane. Whereas the absence of potassium uptake systems leads to plasma membrane hyperpolarization (148), the deletion of efflux systems results in depolarization (122, 154).
In the presence of relatively low extracellular concentrations of sodium, the uptake of potassium via efficient transporters dominates the sodium influx. In K+-starved cells, even though the concentration of external sodium is 100 times higher than that of K+, the intracellular concentration of Na+ remains relatively low (see below). When the extracellular Na+/K+ ratio exceeds approximately 700:1 (238), significant amounts of sodium enter, and as a consequence, equivalent amounts of K+ must leave to maintain the electroneutrality inside the cells. The intracellular concentration of potassium cations in S. cerevisiae cells under normal growth conditions (i.e., in standard laboratory media which contain negligible amounts of sodium salts) ranges from 200 to 300 mM (depending on the strain and medium) and decreases with the influx of sodium. As soon as the intracellular concentration of Na+ approaches that of K+, the growth of cells is inhibited, and with increasing Na+ concentrations, cells die. The effect of sodium combines two components: first, the toxicity of sodium cations, inhibiting many enzymatic functions, and second, the considerable osmotic stress caused by the high concentrations of external sodium salts. The precise cellular targets for sodium (or lithium) toxicity are still poorly defined, although several likely candidates have been proposed (63, 161, 182). In any case, to survive under the high external sodium salt conditions, cells must react to the loss of water caused by the osmotic shock (as reviewed many times elsewhere; see, e.g., reference 108), as well as to the unfavorably low intracellular K+/Na+ ratio. Besides involvement of plasma membrane transporters, intracellular transporters, mainly Vnx1 and Nhx1 antiporters, play an important role in decreasing the cytosolic concentration of Na+, as they help to sequester toxic sodium cations in the vacuole, where the intracellular supply of potassium is normally kept. Transport and storage of alkali metal cations in the vacuole serve not only to ensure optimal cytosolic potassium concentrations or to diminish the amount of toxic sodium in the cytosol but also to compensate for the negative charges of the vacuolar stock of polyphosphates (126).
CATION TRANSPORT AT THE PLASMA MEMBRANE LEVEL
Although for the sake of clarity we will classify plasma membrane cation transporters according to the most relevant cation transported (or at least the one originally identified), it is important to stress that in many cases they are not fully specific for a given cation (e.g., Trk transporters import both potassium and sodium).
Potassium Uptake
A historical introduction.
The pioneering biochemical work by Conway and O'Malley (50) and Rothstein and Enns (240) during more than 2 decades established an important framework for the study of potassium transport in S. cerevisiae. Those authors showed that in yeast, K+ was transported in exchange with H+, and they proposed for the first time the existence of a plasma membrane carrier with higher affinity for K+ than for other cations (49), the effect of external pH on the ability of yeast cells to discriminate between K+ and Na+ (10), and the competitive inhibition of potassium uptake by other alkali metal cations in yeast (11). Moreover, in that work, a model accounting for the inhibition kinetics was presented. That model was based on two cation-binding sites for which cations would compete: a carrier or transporting site and a second, nontransporting (modifier) site with a different array of affinities for cations. In the following years, several laboratories contributed to the characterization of potassium transport in Saccharomyces and in other yeasts (130, 132, 196, 204). A useful review, published by Borst-Pauwels, compiled the status of the field of energetics and kinetics of ion transport in yeast as of the late 1970s (32).
The state of the art significantly changed during the second half of the 1980s, and several reports brought our understanding to the molecular level. While by that time it was already known that bacteria or plants could transport potassium through high-affinity processes (67, 68), no evidence for something similar in fungi was available. A first study, reported in 1981 (42), defining potassium requirements in S. cerevisiae provided the basis to propose, 3 years later, the existence of a dual mode for potassium transport (238). The functioning of high-affinity or low-affinity transport processes was shown to be dependent on the growth history of the cells. For instance, when cells were inoculated in a medium in which arginine was the only N source and neither ammonium nor sodium was present, they could grow at micromolar concentrations of external potassium and developed a transport system with high affinity for the cation (Km in the micromolar range) (238). In addition, the isolation and characterization of the first yeast potassium transport mutant (229) laid the groundwork for the identification of the genes involved in K+ transport (82, 128).
Trk1 and Trk2 high-affinity transporters.
In a series of related papers, Gaber and coworkers reported the identification and characterization of the genes encoding the two main transporters involved in potassium uptake in S. cerevisiae: TRK1 and TRK2 (82, 127, 128).
TRK1 (YJL129c) was the first gene isolated and studied which encodes a K+ transporter in eukaryotic nonanimal cells and it was cloned on the basis of its ability to suppress the potassium transport defect in S. cerevisiae trk1 mutant cells. TRK1 encodes a 180-kilodalton plasma membrane protein, 1,235 amino acids (aa) long, for which hydrophobicity analysis predicts 12 potential membrane-spanning domains (82). Significant amino acid sequence identity between Trk1 and the nucleotide-binding domains of a number of prokaryotic and eukaryotic proteins was observed, and later TRK1 orthologs were identified in other yeasts, fungi, and higher plants (235). Like the H+-ATPase Pma1, Trk1 is an integral plasma membrane protein localized to the “raft” domains (293), which are glycolipid-enriched microdomains of the plasma membrane postulated to form a platform for lipid and protein sorting and trafficking. The Trk1 protein contains four repeated motifs and resembles the structure of the KcsA channel of Streptomyces lividans, which is made up of four identical subunits. Therefore, it appears as a tetra-M1PM2 structure (where M corresponds to a hydrophobic segment and P to an α-helix that enters the membrane and connects the M segments) instead of standard 12-transmembrane-domain (TMS) structure. Haro and Rodríguez-Navarro have proposed a schematic model of the transporter and have identified residues which are important in the transport process (103).
TRK1 is nonessential in S. cerevisiae. Haploid cells that contain a null allele of TRK1 (trk1Δ) rely on a remaining, less efficient transport activity for potassium uptake (82). While the wild-type S. cerevisiae strain grows at low-micromolar potassium concentrations and exhibits a transport process with high velocity and high affinity for K+ and Rb+ (Vmax of 30 nmol/mg cells/min and Kms of 0.024 and 0.08 mM, respectively) (238), trk1 mutants show increased potassium requirements and defective K+ (Rb+) transport (82). These cells do not grow on 0.1 mM KCl, and the Vmax of the high-affinity component of rubidium transport decreases from 30 nmol/mg/min in the wild type to 5 nmol/mg/min in the mutant. Whereas the role of Trk1 as the major high-affinity K+ transporter is well documented, more recently an electrophysiological approach has revealed an additional function for Trk proteins, the efflux of chloride ions (133). A hypothetical structural model for Trk1 has been proposed, suggesting that chloride ions flow through a central pore formed by symmetric aggregation of four Trk1 monomers.
TRK2 (YKR050w) is the second TRK gene in S. cerevisiae. It encodes a shorter protein (889 amino acids) and is 55% identical to Trk1 in its overall sequence. It also contains 12 putative transmembrane domains (127, 128) arranged in four MPM segments, whose identity to those of Trk1 rises to 70 to 90%. The principal difference between Trk1 and Trk2 lies between the first and second MPM motifs; in Trk1 this loop is 642 aa long, while in Trk2 this intracellular segment is much shorter (326 aa). Interestingly, most TRK genes identified in other yeasts are more closely related to TRK2 than to TRK1. It is worth noting that although the existence of a Trk1,2 system is often mentioned in the literature, this has to be taken just as a semantic notion, since there are no results supporting the possible existence of a hybrid multimeric complex or a physical interaction between the two Trk proteins.
The viability and the increased K+ requirements of trk1 mutants suggested the existence of an additional, functionally independent potassium transporter, whose function could be negligible in a wild-type strain. Therefore, the group of R. Gaber used trk1Δ cells to screen for mutants (named Kla−) that required higher concentrations of K+ in the medium to support growth. They isolated 38 independent mutants that contained a mutation in the same gene, TRK2. Double trk1Δ trk2 mutants require up to 10-fold-higher concentrations of K+ than trk1Δ TRK2 cells for normal growth. K+ and Rb+ transport assays demonstrated that the mutant phenotype was due to defective K+ uptake. Therefore, the primary function of TRK2, at least in trk1 cells, was proposed to be K+ influx (127). This has been subsequently confirmed by other laboratories (168, 228). It has been reported that strains deficient for both K+ transporters (trk1Δ trk2) exhibit hypersensitivity to low extracellular pH that can be suppressed by high concentrations of K+ but not Na+. This additional phenotype is most probably a side effect derived from the fact that at acidic pH, the cells become depolarized and K+ requirements are higher. This is probably the reason why either TRK1 or TRK2 can completely suppress both the K+ transport defect and low-pH hypersensitivity of trk1 trk2 cells (127, 168).
It is worth mentioning that the secondary function of S. cerevisiae Trk1, chloride efflux, can also be performed by Trk2, since patch clamp experiments using several mutant strains identified chloride currents via both Trk proteins (29, 133). It must be noted that trk1 trk2 cells, while severely limited in their ability to take up K+, are viable when the growth medium is supplemented with sufficient amounts of potassium, which reveals the existence of one or more additional, functionally independent systems able to transport potassium (see below).
High-affinity versus low-affinity potassium transport.
The relationship between genes encoding K+ transporters and transport modes has been somewhat controversial. We have mentioned that even before the isolation of the TRK genes, the existence of a dual mode for K+ uptake showing low or high affinities for the cation was reported (238). Since the lack of Trk1 produced increased potassium requirements and impaired transport, this protein was proposed to be a high-affinity K+ transporter, and, for the same reason, the orthologous TRK2 gene was considered to encode the putative low-affinity system. This idea was reinforced by the modest contribution of Trk2 to total K+ uptake in wild-type cells and by the low Trk2-mediated potassium uptake activity measured in trk1 TRK2 strains (127, 278). However, this scenario was too simplistic, since it was shown that Trk2 may be a high/moderate-affinity K+ transporter (168, 228) and, on the other hand, a residual potassium transport process with a Km in the mM range was present in mutants lacking both the TRK1 and TRK2 genes (148).
The following summarizes our view about the relationship between TRK genes and the existence of different modes of potassium transport. In a wild-type strain, most if not all potassium influx is mediated by Trk1, which can work as a high- or low-affinity transporter according to the growth history and the K+ status of the cell. Thus, wild-type cells grown without K+ limitations would show the so-called low-affinity transport mode, but after starvation, they would display the high-affinity system. Trk2 would also transport potassium with high/moderate affinity, but the gene is very poorly expressed under standard conditions. Its activity, if any, would be masked in the presence of TRK1. Finally, in strains lacking the two transporters, an ectopic potassium transport process is still evident. This Trk-independent process shows very low affinity (Km in the mM range), and the system(s) mediating transport has not been definitely established, although some clues about the nature of the transporters involved can be found when the activities of other transporters, such as the NSC1 channel or the Qdr2 drug exporter, are analyzed.
Low-affinity transport system.
Using electrophysiological techniques, potassium currents were detected in trk1 trk2 mutants, and the putative channel involved in that process was named NSC1 (nonspecific cation “channel”). Although the gene responsible has not been identified yet, this channel appears to be a good potential candidate to explain the “very-low-affinity” potassium uptake process in trk1 trk2 mutants. Its activity is blocked by mM concentrations of calcium and other divalent metal ions. It is unblocked by decreasing the amount of extracellular free divalent metals to below approximately 10 μM and is independent of the other identified potassium transporters, Tok1, Trk1, and Trk2 (30). Analysis of this nonselective cation channel by whole-cell patch clamping (92, 234) suggested that this inwardly rectifying pathway could relieve the growth inhibition normally imposed on yeast by disruption of its potassium transporters, Trk1 and Trk2. Later on, it was proposed as the primary low-affinity uptake route for potassium ions in yeast. Factors which suppress NSC1-mediated inward currents and inhibit growth of trk1 trk2 cells include high calcium, low extracellular pH, hygromycin B, and, to a lesser extent, tetraethylammonium. Growth of trk1 trk2 cells is also inhibited by lithium and ammonium; however, these ions do not inhibit NSC1 but instead enter yeast cells via this channel. Growth inhibition by lithium ions is probably a toxic effect, whereas growth inhibition by ammonium ions probably results from competitive inhibition, i.e., replacement of intracellular potassium by entering ammonium (31).
The identification of the gene(s) encoding NSC1 is an urgent task that would clarify the final contribution of this nonselective channel to the low-affinity ectopic transport process present in trk1 trk2 S. cerevisiae mutants.
The QDR2 gene of S. cerevisiae encodes a putative plasma membrane drug/H+ antiporter that confers resistance against quinidine, barban, bleomycin, and cisplatin. The presence of quinidine in the medium produces increased potassium requirements and defective Rb+ (K+) transport, and these effects are more dramatic in a qdr2 strain than in the wild type. The growth differences registered between the parental strain and qdr2 cells under K+-limiting conditions, even in the absence of quinidine, suggested that the capacity to transport K+ may be altered in this mutant. The lack of QDR2 slightly reduces the K+ content of cells compared with that in the parental strain. However, in quinidine-stressed cells, the values of internal K+ measured in the qdr2 mutant were about one-half of those determined in the wild-type strain. Results from Rb+ transport assays indicated a defective uptake in the absence of QDR2. The direct involvement of Qdr2 in K+ uptake is reinforced by the fact that increased Rb+ uptake due to QDR2 expression is independent of the Trk1 and Trk2 systems (272).
Gaber and coworkers have shown that mutations in sugar or amino acid transporters can partially suppress the increased K+ requirements in trk1 trk2 cells by increasing Rb+ uptake (129, 290). Their hypothesis was that under certain conditions, sugar or amino acid carriers can accept and transport amounts of alkali metal cations that would improve growth at limiting external K+ concentrations. Similarly, Navarre and Goffeau (188) reported the existence of Pmp3, a 55-amino-acid hydrophobic plasma membrane polypeptide that is conserved in yeast and plants. Yeast cells deleted for the PMP3 gene showed increased plasma membrane potential and sensitivity to toxic cations, such as Na+ and hygromycin B. This effect appeared to be unrelated to changes in Pma1 activity. Lack of Pmp3 also exacerbated the sensitivity of a mutant strain lacking the Ena1 and Nha1 proteins to Na+ and suppressed the potassium dependency of a trk1 trk2 strain. Interestingly, these effects were reversed by addition of 10 mM CaCl2. The authors proposed that the absence of Pmp3 facilitates a Trk1,2-independent, nonspecific monovalent cation uptake through an increase in the membrane potential, although no further progress has been made in this direction.
In summary, the remaining ectopic potassium uptake process observed in trk1 trk2 double mutants can be explained by the activity of one or, most probably, several processes that mediate potassium influx in a nonspecific manner. It is conceivable that the contribution of these transporters could vary depending on the growth conditions.
Mechanism of potassium transport.
The mechanisms mediating potassium uptake in S. cerevisiae are still unresolved. Potassium is accumulated by yeast cells against a very wide range of concentration gradients. While intracellular potassium reaches concentrations of around 200 to 300 mM, extracellular potassium can be in the low mM or micromolar range. Therefore, yeast cells have to adapt to changing environmental conditions, and the mechanism of transport may not be the same under different growth conditions.
We have mentioned above that the plasma membrane ATPase extrudes H+ and creates a membrane potential that draws potassium into the cell. Despite several decades of investigation, a reliable experimental determination of the membrane potential in S. cerevisiae has remained elusive. The quantitative results obtained using diverse probes (−100/−125 mV) seem to underestimate the real membrane potential. According to the Nernst's equation and assuming membrane potentials of close to −300 mV (reported for other organisms, such as Neurospora crassa [236]), the existence of a K+ uniporter can explain the K+i/K+e gradients usually observed. However, under certain conditions, such as low potassium in combination with acidic external pH, where the cells are less hyperpolarized, uniport could not fulfill the cell's requirements, and an active process is likely to be required in S. cerevisiae. In this case, a K+-ATPase or a symport mechanism could explain active K+ uptake. The possible existence of a K+-ATPase in S. cerevisiae has received little support during the last 2 decades, and furthermore, systems similar to the ACU (alkali cation uptake) ATPases working in Ustilago maydis or Pichia sorbitophila are not present in S. cerevisiae (21). On the other hand, the functioning of a K+-H+ symporter has been supported by the proposal that two binding sites are present in the yeast K+ transporter and by the existence of a K+-H+ symport in Neurospora crassa (241). However, the possibility that Trk systems work as a K+-Na+ symporter similar to the one described in plants (241) cannot be ruled out, and the fact that the HKT/TRK family of transporters is very specific for alkali metal cations in plants may be taken as an indication that a putative K+-Na+ transport mechanism may operate in S. cerevisiae (101).
Efflux of Potassium
The existence of potassium efflux processes in S. cerevisiae has been known for a long time. Nowadays, strong experimental evidence exists that at least three different transporters can contribute to the potassium efflux, depending on the physiological conditions. Two of them have been first identified as Na+ efflux systems, but they can also mediate K+ efflux (i.e., Ena1 and Nha1 [see below]). The third system is the only K+-specific efflux system (Tok1) existing in S. cerevisiae.
Tok1 channel.
Tok1 is the only outward-rectifying potassium channel present in the plasma membrane of yeast cells. Tok1 is 691 residues long (169), and its sequence conceptually corresponds to a channel protein with eight transmembrane domains and two P loops. The TOK1 (YJL093c) open reading frame (ORF) (116) is also known as YKC1 (296), DUK1 (231), YORK (140), and YPK1 (26).
The two Tok1 pore-forming P domains are structural entities common to potassium-selective ion channels (1). Each of the two pores forms a functional channel permeable to potassium, and the carboxyl tail would function to prevent inner gate closures. As deduced from its structure and demonstrated by electrophysiological experiments, the primary function of Tok1 is K+ efflux. Gating of the channel is promoted by membrane depolarization; the channel is open at positive and very low negative membrane voltages (26, 72). Additionally, the activity of Tok1p is modulated by external K+ (143, 275), both in yeast and when expressed in Xenopus oocytes (116, 231, 296).
Tok1 currents show a clear outward rectification above the equilibrium potential of K+ (EK+); i.e., when the membrane potential is more positive than the EK+, the outward K+ current increases disproportionately, indicating increased channel-open probability. This was observed in the K+ current native to yeast (24, 26, 98, 231, 296) as well as in oocytes heterologously expressing TOK1 (116, 140, 143). Deletion of TOK1 abolishes the native yeast K+ current observed in patch clamp experiments, whereas overexpression of TOK1 enhances it (231, 296). Despite extensive efforts to find conditions that specifically affect the growth of tok1 mutants, no clear growth-related phenotypes have been reported (231, 296). In addition, tok1 mutants did not display changes in K+ content, Rb+ uptake, or K+ efflux when the cations were measured by atomic absorption spectrophotometry, and the effect of the mutation was observed only by following an electrophysiological approach (25). Thus, although the biophysical properties of the Tok1 channel are well described, the physiological role of Tok1 is presently unclear (25). What we know is that Tok1 is activated by plasma membrane depolarization (26) and that K+ accumulated in the cells is probably released in order to regenerate the membrane potential (24). In fact, the relationship between membrane potential regulation and Tok1 has been more recently confirmed by the observation that tok1 mutants are depolarized and cells overexpressing TOK1 are hyperpolarized (154).
Tok1 has been reported to be a molecular target of the yeast viral killer toxin K1 (1), which is thought to act on Tok1 from both sides of the plasma membrane (257). However, the physiological significance of the binding of K1 to Tok1 has been questioned (34). A secondary function has also been assigned to Tok1. It has been proposed that under certain conditions Tok1 could mediate some K+ influx, but this possible passive influx would ordinarily be overshadowed by the strong uptake by Trk1 or even by Trk2. To maximize the chances of detecting this passive influx, a trk1 trk2 strain overexpressing TOK1 was examined; growth patterns and internal K+ and membrane K+ currents were studied, and evidence consistent with K+ influx through Tok1 was found (72).
Sodium Uptake
There is no single, specific sodium transporter in the budding yeast S. cerevisiae, and this cation enters the cells significantly only if it is present at high external concentrations. This idea has been generally accepted since Conway and Duggan (49) and Armstrong and Rothstein (10) proposed the existence of a cation carrier transporting different alkali metal cations and was reinforced when it was proposed that Na+ binds the activation site of the K+ carrier, thus affecting Rb+ and K+ transport (32). Sodium competitively inhibits potassium uptake in wild-type cells, and therefore Trk1 must accept and transport Na+ in addition to K+, although with much lower affinity (229). trk1 trk2 cells accumulate more Na+ and less K+ than wild-type cells, suggesting that the low-affinity and nonspecific transporters acting in these cells do not discriminate between K+ and Na+ (96). In this respect, it is important to point out that in the absence of calcium, the NSC1 channel mentioned above also transports sodium or lithium (30).
On the other hand, it was proposed in 1977 that phosphate uptake in S. cerevisiae was mediated by two mechanisms, with one of them being dependent on the presence of sodium (239). The same investigators reported that the mechanism involved two sites with affinity for sodium and that lithium, but not rubidium, also stimulated phosphate uptake. Subsequently, the PHO84 and PHO89 genes were found to encode two derepressible high-affinity phosphate cotransporters localized to the plasma membrane. Whereas Pho84 catalyzes a proton-coupled phosphate transport at acidic pH, Pho89 catalyzes a sodium-dependent phosphate uptake at alkaline pH (207). Northern analysis showed that PHO89 is transcribed under conditions of Pi limitation, although it was shown later that it is also strongly induced by alkaline pH even when the cells are grown in medium with normal phosphate concentrations (256). Remarkably, induction of PHO89 at high pH seems to be largely dependent on calcineurin, and the gene also transcriptionally responds to increased extracellular calcium (246, 280). Kinetic characterization of Pho89 showed that it has an apparent Km for phosphate of 0.5 μM, has an optimum pH of 9.5, and is highly specific for Na+; activation of Pi uptake is maximal at an Na+ concentration of 25 mM (156). Pho89 represents the only Na+-coupled secondary anion transport system so far identified in S. cerevisiae. As far as we know, no studies on sodium entry have been performed with the triple mutant lacking both TRK genes and PHO89. The results provided by those studies may be of interest to evaluate the actual relevance of this transporter in Na+ uptake.
Sodium Efflux
To maintain a low cytosolic concentration of toxic sodium (or lithium) cations, S. cerevisiae cells use two types of efflux systems at their plasma membrane. As discussed below, while these systems complement each other in detoxification function, they differ in both transport mechanism and regulation of their expression and activity.
Ena ATPases.
P-type ATPases couple the hydrolysis of ATP to the transport of cations against electrochemical gradients by forming a typical phosphoenzyme intermediate (44). The IID subtype, exemplified by the ENA system, corresponds to a subfamily of ATPases able to extrude Na+, Li+, or K+ with different capacities (20). As discussed below, S. cerevisiae contains various copies of genes encoding identical or very similar Ena proteins (86, 102, 157, 288). On the basis of its sequence, ENA1 was initially proposed to encode a putative calcium P-type ATPase (242), and the locus was named PMR2 (for plasma membrane ATPase related), but in 1991 Rodriguez-Navarro and coworkers reported its capacity to extrude Na+, Li+, and probably K+ (102), thus establishing its primary role in the cell. Therefore, ENA1 (exitus natru) is currently the accepted gene name, whereas PMR2 and HOR6 are listed as aliases. Remarkably, in S. cerevisiae these genes are usually found in tandem repeats on chromosome IV. The number of copies varies from strain to strain. The FY1679 strain contains 3 ENA genes, ENA1 (YDR040c), ENA2 (YDR039c), and ENA5 (YDR038c), encoding proteins of identical length (1,091 amino acids) and displaying very similar sequences (>97%), although ENA1 is slightly more distant from ENA2 and -5. Under standard growth conditions Ena1, -2, and -5 are present in the cell at low levels (around 600 molecules/cell) (91), although, as will be discussed below in detail, ENA1 expression markedly increases in response to saline or alkaline pH stresses. Other strains, such as DBY746 and W303-1A, contain four copies of ENA (86, 237), while strains S288C, A364A, and D273-10B contain five repeats and Σ1278b only one (288). A recent report has confirmed the existence of a single ENA gene in the CEN.PK113-7D strain (named ENA6) and found that the encoded protein shows a higher-than-normal degree of sequence divergence compared to those encoded in other genetic backgrounds. This difference may be responsible for the unusually high sensitivity of CEN.PK derivatives to Na+ and Li+ cations (54).
The ENA cluster (particularly ENA1), is a major determinant of sodium tolerance in S. cerevisiae. Deletion of the entire cluster results in strong sensitivity to sodium and lithium cations (86, 102, 213, 237, 288) and a significant growth defect at alkaline pH (102, 209), whereas expression of ENA1 restores tolerance to these cations. Furthermore, expression of S. cerevisiae ENA1 in other fungi, such as Schizosaccharomyces pombe (13), or in plant cells (184) increases tolerance to Na+ and Li+ and decreases the intracellular content of these cations. Although the molecular basis for cation transport has not been solved, it has been proposed that the slight divergence in structure between Ena1 and Ena2 may explain the observation of the former being more effective in extruding Na+ cations and the latter more specific for Li+ (288).
The amino acid sequence of Ena proteins leads to the prediction of 9 or 10 transmembrane domains, and their presence at the plasma membrane has been demonstrated for S. cerevisiae Ena1 and Ena2 (288) and for Debaryomyces (formerly Schwanniomyces) occidentalis (15). However, overexpressed ENA1 is also present in intracellular membrane structures (22). The SRO7/SOP gene (homolog of the Drosophila Lgl tumor suppressor gene), which encodes a protein involved in exocytic docking and fusion of Golgi-derived vesicles with the plasma membrane, was initially identified as being required for normal salt tolerance (137). This requirement could be explained by its involvement in the possibly specific delivery of Ena1 to the plasma membrane (283). In contrast, whereas alteration of Ena1 function has been proposed as an explanation for the salt-sensitive phenotype of diverse vps mutants (141), Ena1 seems to be properly localized to the plasma membrane in all of them. Therefore, the precise mechanisms for this effect remain unclear.
Nha1 antiporter.
Although plasma membrane Na+/H+ antiporters represent conserved transport systems existing in all types of organisms (so far identified in, e.g., archaea, bacteria, fungi, parasites, insects, plants, and mammals), their structures, substrate specificities, and probable cell functions have diverged during their evolution. While most microorganisms and plants use the inward gradient of protons created by the plasma membrane H+-ATPase as a driving force to pump sodium cations out, animal cells usually consume the Na+ gradient resulting from Na+/K+-ATPase activity in order to force the surplus protons out and regulate intracellular pH.
In S. cerevisiae, the Na+/H+ antiporter encoded by the NHA1 gene was selected from a multicopy genomic library on the basis of its ability to improve the growth of a salt-sensitive strain (a derivative of Σ1278b) on a medium supplemented with a high concentration of NaCl (219). Sequencing revealed that the cloned DNA fragment was about 300 nucleotides (nt) shorter than the complete ORF (which is 2,958 nt long), and later studies showed that this truncation improved the transporter's capacity to mediate the Na+ and Li+ efflux (120). Further studies on Nha1 activity revealed potassium (and its analog rubidium) as additional substrates (17). These data represented the first evidence showing that a typical member of the Na+/H+ antiporter family displays specificity for multiple substrates and is directly involved in potassium efflux. The two yeast antiporters previously characterized, S. pombe sod2 and Zygosaccharomyces rouxii Sod2, were shown to be specific for sodium and lithium (see below). Construction of a mutant strain with deletions of genes encoding both the Na+-ATPase and the Na+/H+ antiporter (ena1-4Δ nha1Δ) confirmed that both transport systems are necessary for tolerance to high external concentrations of salts and that they both mediate efflux of all four alkali metal cations. The plasmid-based overexpression of both ENA1 and NHA1 gene in the double-deleted strain showed complementary action in the maintenance of a high intracellular K+/Na+ ratio. The Nha1 antiporter is responsible for cell growth with high concentrations of KCl and NaCl at acidic external pH values, and the Ena1 ATPase is necessary at higher pH values (17).
In contrast to their complementary function, the expression of NHA1 differs from the tightly regulated expression of ENA1 (described below). NHA1 transcription is constitutive and very low, and it does not seem to be induced by salts, pH changes, or osmotic shock, suggesting a housekeeping role for Nha1 in potassium homeostasis and intracellular pH (17). The involvement of Nha1 in the maintenance of intracellular pH was first shown by measurements of intracellular pH in cells lacking or overexpressing Nha1 (269) and was later confirmed by the observation of an internal pH increase in nha1Δ cells (36).
Besides its function in detoxification of Na+ and Li+ cations and its involvement in the maintenance of stable intracellular K+ and pH levels, the participation of Nha1 in several other cell processes, e.g., regulation of cell cycle, cell volume, or membrane potential, was clearly demonstrated. Overexpression of NHA1 results in a rescue of the G1/S cell cycle blockage in cells with conditional sit4 hal3 mutations, implying that Nha1 plays a role in some aspect of cell cycle regulation (262). Nha1 participates, together with other K+ transporters (Tok1, Trk1, Trk2, and Ena1), in the maintenance of stable plasma membrane potential (122, 154), and it is involved in the rapid-rescue mechanism during the immediate cell response to osmotic or saline shock (119, 120) (see below). Nha1 also acts as a safety valve upon sudden alkalinization of the cytosol, when the outward gradient of potassium cations can serve as a driving force for proton influx (17, 120, 198). Nha1 is very stable, with a half-life longer than 6 h (H. Flegelová-Kučerová and H. Sychrová, unpublished results). Detailed analysis of the antiporter's activity in isolated secretory vesicles revealed the electrogenicity of its transport mechanism, i.e., the observed requirement for downhill transport of more than one proton for the extrusion of one sodium ion (195). Biochemical studies of Nha1 revealed that (i) it is most probably present in membranes as a dimer and the interaction of monomers is important for the activity (175), and (ii) it localizes predominantly to detergent-insoluble and ergosterol-rich membrane fractions. The interaction with lipid rafts probably occurs only once Nha1 reaches the plasma membrane, and for its stabilization, the presence of sphingolipids is necessary (172).
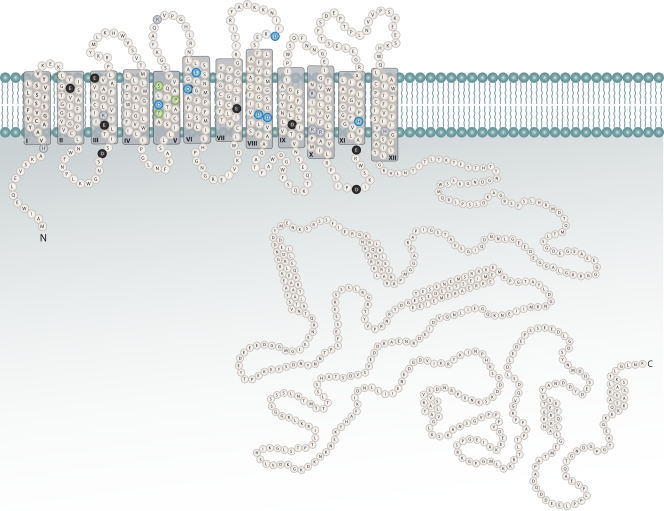
The Nha1 antiporter is a typical membrane protein and is 985 amino acid residues long (Fig. 2). It has a very short hydrophilic N terminus (only 12 amino acids) with a predicted cytosolic orientation, followed by probably 12 transmembrane hydrophobic segments and rather short connecting loops and terminated with a long terminal repeat hydrophilic C terminus. The membrane-spanning part of the protein, approximately 440 residues long, is highly conserved among yeast plasma membrane Na+/H+ antiporters (218), and it contains several, mainly charged, amino acid residues that have been shown to be important for alkali metal cation and/or proton translocation (Fig. 2). At least five highly conserved negatively charged amino acid residues and H367 have been identified as being crucial for the plasma membrane Na+/H+ antiporter function, first in S. pombe (62, 77, 287) and later in other yeast species. A tandem of aspartic residues (D266 and D267 in Fig. 2) in the eighth transmembrane segment was shown to be vital for the function of the Z. rouxii Sod2 antiporter as well (285), and mutations of these conserved residues to asparagine also abolished the sodium transport activity of Candida albicans Cnh1 and S. cerevisiae Nha1 (ScNha1) (262, 264). Interestingly, in the case of the ScNha1(D266N) mutant, the potassium efflux activity was not changed, and only the sodium export activity was abolished (195), whereas mutation of another aspartic residue (D241) affected K+ extrusion (261). In addition, one proline (P146) and two polar residues (T142 and S151) in the fifth transmembrane domain were shown to be involved in the yeast plasma membrane Na+/H+ antiporter activity and/or substrate specificity (124, 125, 189).
FIG. 2.
Structure of the Nha1 Na+, K+/H+ antiporter. Highly conserved charged (blue) and polar (green) residues important for function are highlighted in color; other highly conserved charged residues are shown in black (negatively charged) or gray (positively charged).
Most of the known functions of Nha1 in cell physiology mentioned above are related to its very long hydrophilic C terminus, which represents approximately 56% of the total protein (Fig. 2). Though the overall level of identity of the C termini of the yeast plasma membrane Na+/H+ antiporters is much lower than that of the transmembrane domains (218), six conserved C-terminal regions (C1 to C6) have been identified (175). While the truncation of most of the C terminus (approximately last 510 amino acid residues) does not influence plasma membrane localization of S. cerevisiae Nha1 (120), the first conserved region, which is 16 residues long (aa 434 to 449) and overlaps the end of the 12th transmembrane segment and the beginning of the C terminus, is crucial both for proper targeting and activity of the protein (173, 174). Some of the C-terminal regions (e.g., amino acid residues 920 to 930) have been shown to be important for the full transport capacity for Li+ cations (120), and some (located mainly in two clusters, residues 869 to 876 and 918 to 927) were shown to be involved in the ability of Nha1 to suppress the G1/S transition block observed in cells lacking the Sit4 and Hal3 proteins (261). Based on the analogy with mammalian NHE Na+/H+ exchangers of the same family (197), the long C terminus might be the target of the regulation of Nha1 activity and connect the Nha1 transport capacity with its physiological functions (see further).
Altogether, the main physiological function of Nha1 is probably housekeeping in S. cerevisiae and contributing to the essential continuous recycling of potassium cations across the plasma membrane. Its contribution to the detoxification of Na+ and Li+ cations is significant but not crucial. Nha1 seems to be more involved, via its potassium-transporting capacity, in intracellular potassium and pH homeostases and, consequently, in the rapid response to changes of external osmolarity, to cell volume adjustment, and to maintenance of plasma membrane potential. Probably in all these physiological functions, the Nha1 antiporter is linked to the activity of the Trk1 (and/or Trk2) potassium uptake system, as it has been shown that the deletion of NHA1 affects the kinetics of high-affinity potassium uptake via Trk systems (16) and, vice versa, that deletion of TRK1,2 results in a diminished potassium efflux upon potassium starvation (J. Zahrádka and H. Sychrová, unpublished results).
INTRACELLULAR CATION TRANSPORT
The concentration of alkali metal cations inside yeast cells is probably not homogeneous, and there is a presumed difference in the sodium and/or potassium amounts in the cytosol and in individual organelles. The physiological necessity of alkali metal cation transport across the organellar membranes is connected to the maintenance of potassium homeostasis and sodium detoxification in the cytosol as well as to the regulation of organellar pH and volume. So far, the antiport mechanism exchanging protons for potassium and/or sodium cations has been described for four S. cerevisiae organelles. Two of the antiporters involved, endosomal Nhx1 and Kha1 (Golgi apparatus) belong to the family of typical Na+/H+ exchangers, which also includes the plasma membrane-localized Nha1 (35, 218). In contrast, the vacuolar Vnx1 antiporter and the mitochondrial Mdm38 have different structures. The activities of Nhx1, Kha1, and Vnx1 antiporters are closely interconnected with the function of the vacuolar V-type H+-ATPase, which creates the gradient of protons across membranes of these organelles (see above and reference 250).
Transport across the Inner Mitochondrial Membrane
Though the exchange of potassium and protons across the mitochondrial membranes had been predicted already by Peter Mitchell (171) and validated by direct measurements of proton and potassium fluxes in isolated mitochondria from many organisms, including yeast, the number, nature, and structure of the corresponding transporter(s) in yeast remain obscure. The MDM38/MKH1/YOL027c gene, whose product is localized in the inner mitochondrial membrane, has been shown to be indispensable for K+/H+ exchange. However, this gene is not likely to encode the transporter itself, as its product contains only one transmembrane hydrophobic segment. MDM38, which is well conserved among all eukaryotes, was isolated in a screen for genes involved in mitochondrial cation homeostasis (192). Mitochondria isolated from mdm38 deletion mutants show highly reduced K+/H+ exchange, low membrane potential, high potassium content in the matrix and increased volume (80, 192). Detailed analysis of events following the downregulation of MDM38 expression revealed that upon loss of K+/H+ antiport, mitochondrial swelling and reduction in membrane potential ensued, followed by fragmentation of mitochondrial reticulum, close association of mitochondria with vacuoles, and finally, uptake of mitochondrial material into the vacuoles (193). All these data confirm the important role of the active exchange of K+ and H+ in mitochondrial physiology, but nevertheless, the identification of the transporter molecule(s) remains open. Recently, two other proteins, Mrs7 and Ydl183, with a putative role in the mitochondrial K+/H+ exchange have been identified, and the existence of a multicomponent complex responsible for transport has been suggested (L. Zotova, M. Aleschko, G. Sponder, R. Baumgartner, S. Reipert, M. Prinz, R. J. Schweyen, and K. Nowikovsky, submitted for publication).
Vacuolar Transport
The vacuole is the place where cells sequester toxic sodium and store a substantial part of the intracellular potassium. A recent report (18) has proposed the existence of two types of fungi in regard to Na+ tolerance: those tolerant to high Na+ contents in the cytoplasm, which include U. maydis, and those intolerant to high Na+ contents, which include the model yeast S. cerevisiae. However, in both types, vacuolar transport may play an important role in cation homeostasis. Exchange of sodium and potassium cations for protons across the vacuolar membranes was predicted a long time ago as the transport mechanism for accumulation of these cations in vacuoles. The existence of such a transport mechanism has been confirmed in experiments measuring the fluxes of cations and protons with the use of permeabilized cells, selective electrodes, and/or fluorescent probes (see, e.g., references 38, 107, and 159).
Vnx1 antiporter.
For about 10 years, Nhx1 was believed to be the main antiporter involved in the transport of sodium and lithium cations to the vacuole, though its localization in the endosomal membranes and not in the membranes of vacuoles had been shown (186). This hypothesis was based mainly on the fact that the S. cerevisiae genome harbors only three genes (NHA1, KHA1, and NHX1) encoding typical members of the Na+/H+ antiporter family, with none of them being localized in the vacuolar membrane. Only recently, a gene encoding an alkali metal cation/H+ antiporter has been identified and its product characterized (37). The VNX1 gene (YNL321w) was identified using a reverse genetics approach (screen of the Euroscarf mutant collection), and, surprisingly, its sequence corresponds to a member of the Ca2+/H+ antiporter family, although with some atypical characteristics. Nevertheless, Vnx1 has been shown to reside in the vacuolar membrane and not to mediate calcium uptake but to be indispensable for the vacuolar exchange of both sodium and potassium cations for protons (37). Besides the sequestration of alkali metal cations into the vacuolar lumen, Vnx1 also is likely to play a role in cytosolic ion homeostasis and pHin regulation, similar to the case for the Nha1 and Nhx1 antiporters. Due to the unexpected nature of the transport mediated by Vnx1, additional studies to further validate its proposed cellular function seem to be necessary. Interestingly, a “residual” monovalent cation exchange activity could be detected in vacuolar fractions of vnx1Δ cells by using a slightly modified in vitro transport method. Disruption of potential candidate genes in vnx1Δ background strains permitted assignment of this “residual” activity to Vcx1 (O. Cagnac, personal communication), originally identified as a highly specific Ca2+/H+ vacuolar antiporter (53, 216) with possible affinities only for other divalent cations (208).
Endosomes and Golgi Apparatus
Kha1.
Among the three yeast Na+/H+ antiporters, Kha1 (encoded by YJL094c) has the highest level of similarity to bacterial sodium or potassium/proton antiporters (218, 227). Although for some time it was believed to be a plasma membrane transporter (227), later it was shown to reside in the membrane of the Golgi apparatus (78, 153). Deletion of KHA1 results in a growth defect in media with high pH or containing hygromycin B. There is no direct evidence regarding the substrate specificity of Kha1, but potassium is most probably its substrate, as the inability of the kha1Δ mutant to grow at higher pH can be suppressed by the addition of moderate concentrations of KCl (78, 153). Similar to the case for the other yeast intracellular alkali metal cation/H+ antiporters, Kha1 is believed to be involved in the regulation of intraorganellar potassium and pH homeostasis and most probably acts in collaboration with the Gef1 anion channel, as high cellular levels of Kha1 are required for the functional expression of a mammalian ClC-2 channel in S. cerevisiae and for complementation of the gef1Δ phenotype (78).
Nhx1 antiporter.
Nhx1, encoded by YDR456w, was the first discovered yeast intracellular antiporter (185), and it is the best characterized. It was identified upon characterization of Na+-tolerant phenotypes of the H+-ATPase pma1-α4 mutant (185), and though there were some reports indicating its localization in mitochondria (194), it has been repeatedly shown and confirmed that it is localized in the membranes of prevacuolar compartments, i.e., late endosomes (33, 186). Deletion of the NHX1 gene results in an increased salt and low-pHout sensitivity of cells, together with a drop in the cytosolic pH (36). Deletion of the NHX1 gene in a background lacking the plasma membrane Nha1 and Ena1-4 transporters brings about an extreme sensitivity not only to Na+ but also to K+, Li+, and Rb+ cations (121, 225), which suggests the broad substrate specificity of this antiporter. Its ability to transport Rb+ was confirmed in a transport assay (35). The Nhx1 antiporter, like the plasma membrane Nha1, has multiple functions in yeast cells. It contributes to the sequestration of toxic sodium and lithium cations, as well as surplus potassium, in the endosomes and vacuoles (225) and is involved in the early stage of cell adaptation to hyperosmotic shock (187). In addition, its activity contributes significantly to the maintenance of a stable intracellular pH, and its presence is required for proper protein trafficking among endosomes and other organelles (33, 36). Mutational analysis of Nhx1 revealed a direct connection between its function in the regulation of pH homeostasis and vesicle trafficking (178). Nhx1 homologs are conserved in both plant and mammalian cells (35), and its primary structure has a higher level of similarity with the NHE and NHA/SOS families of plasma membrane and intracellular Na+/H+ exchangers of mammalian and plant cells, respectively (see below). In fact, plasma membrane mammalian and plant antiporters are more closely related to S. cerevisiae Nhx1 than to Nha1 or Kha1 antiporters (218). Although the hydrophilic C-terminal part of Nhx1 is much shorter than that of Nha1 (175 versus 546 amino acid residues), it has important significance for proper functioning as well. Most probably it is N glycosylated (286), and it interacts with the Gyp6 GTPase-activating protein, which is proposed to be one of the coordinators of the vesicle trafficking between the Golgi apparatus and late endosomes (4), in accord with Nhx1's presumed role in intracellular vesicle trafficking. The NHX1 gene is conserved in yeast species, but, as is the case for Kha1, only the Nhx1 homolog from Debaryomyces hansenii has been studied (see below).
The endosomal and Golgi apparatus antiporters Nhx1 and Kha1 are likely to have a similar function, but in different organelles. It is worth noting that although the deletion of both genes results in a decreased tolerance to hygromycin B, the overexpression of Kha1 does not suppress the hygromycin B sensitivity of nhx1Δ cells (153). On the other hand, deletion of the two genes affects the cell's ability to grow at limiting values of external pH: it leads to sensitivity to low (in the case of Nhx1) or high (for Kha1 absence) pHout. Altogether, the available data suggest that Nhx1 and Kha1 antiporters, together with the vacuolar H+-ATPase, are involved in intraorganellar pH and alkali metal cation balance, which in turn influences vesicle trafficking among the organelles of the secretory and endosomal pathways.
REGULATORY AND SIGNALING NETWORKS
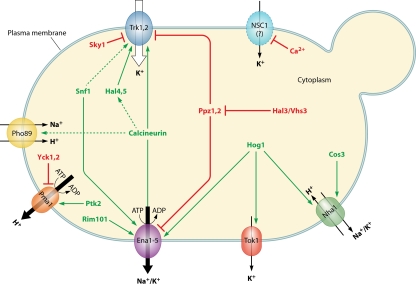
Multiple signaling pathways are involved in the maintenance of cation homeostasis. A remarkable feature is that whereas in some cases (HOG [(high-osmolarity glycerol] and calcineurin) a single pathway has, either simultaneously or sequentially, various relevant targets (e.g., HOG signaling on Nha1, Tok1, and ENA1), a given target can also integrate multiple signals that define its specific behavior (e.g., ENA1 integrating HOG, calcineurin, and other signals). This network of functional interactions is depicted in Fig. 3.
FIG. 3.
The regulatory network for the plasma membrane alkali metal cation transporters. Discontinuous lines indicate interactions not fully documented.
Regulation of Pma1
Because of its role in modulating the osmotic and ionic balance as well as driving uptake of nutrients, the activity of the electrogenic Pma1 proton pump must be finely regulated. As described by Serrano in a seminal paper (254), glucose is an important signal for Pma1 regulation. This regulation occurs at two different levels: an initial rapid 5- to 10-fold increase in ATPase catalytic activity (254), affecting both Km (which decreases) and Vmax (which increases), is then followed by a second phase involving a slow and moderate increase in the expression of the gene in a process that involves the Rap1 and Gcr1 transcription factors (see reference 210 and references therein for early reports). Expression levels of PMA1 (and also of PMA2) can be modulated in response to changing growth conditions, such as carbon sources, diauxic shift, or entry into stationary phase, and stress conditions (73, 230).
Early evidence suggested that short-term regulation of Pma1 activity by activators may involve protein phosphorylation at both Ser and Thr residues (46) targeting its inhibitory C-terminal tail, since mutations in this domain affecting Ser899 or Thr912 abolished the glucose-dependent Km or Vmax change, respectively (211). A role for Ptk2, a protein kinase belonging to the NPR/HAL5 family, has been proposed to activate Pma1 by phosphorylation of Ser899 (97). Ptk2 is localized to the plasma membrane, and in vitro phosphorylation of Pma1 Ser899 upon incubation with yeast membranes has been determined to be Ptk2 dependent. However, mass spectrometry analysis of Pma1 purified from plasma membranes of cells cultured in the presence or absence of glucose detected tandem phosphorylation of Ser911 and Thr912 in response to the sugar (138). In contrast, phosphorylation of Pma1 by the CK1 protein kinase, encoded by the YCK1 and YCK2 genes, at Ser507 results in decreased H+ pumping activity (71). Therefore, it appears that site-specific phosphorylation of Pma1 may result in both down- and upregulation of its activity. Pma1 may also be posttranscriptionally regulated by additional inputs that are still poorly defined. For instance, Hrk1, a protein kinase related to the Npr1/Hal5 family, may also activate Pma1 (97). It has been recently proposed that the glucose-mediated increase in calcium concentrations may serve as a signal for Pma1 activation, in a Pkc1-dependent manner (205). An inhibitory interaction of Pma1 with acetylated tubulin has been reported. Addition of glucose to the cells disrupts the complex, leading to activation of the H+-ATPase (43). In any case, the precise mechanisms controlling this essential plasma membrane transporter are still obscure.
Regulation of Trk1 and Trk2
Although TRK1 seems to be less expressed at stationary phase (88), there is no experimental evidence for transcriptional regulation of TRK1 under cation-related stresses. Several reports indicate that TRK2 undergoes a rather complex transcriptional regulation; in this respect, it has been shown that TRK2 expression is negatively regulated by the Rpd3/Sin3 histone deacetylase complex and by a URS1 element located within the promoter region (278, 279). Peña's group has also reported that Northern blot analysis showed no TRK2 transcripts in a wild-type strain or in a trk1Δ mutant, strongly suggesting that in these strains TRK2 was not expressed and that deletion of SIN3 resulted in derepression of TRK2 transcription (168). However, as for TRK1 expression, TRK2 expression is not altered by perturbations in cation homeostasis.
Despite the work of several laboratories, our understanding of the posttranscriptional regulation of Trk1 or Trk2 is still quite limited. Work by Serrano's laboratory identified the HAL4/SAT4 and HAL5 protein kinases as genes able to confer tolerance to toxic cations in a wild-type strain. The characterization of these genes revealed that they were partially redundant activators of potassium transport that acted by positively modulating Trk1 and Trk2 (180). This gave rise to the possibility that Trk systems may be directly controlled by phosphorylation events. Recent work has demonstrated that Hal4 and Hal5 may not be directly required for Trk1 activity, but they are required to stabilize the transporter at the plasma membrane under low-K+ conditions, preventing its endocytosis and vacuolar degradation (206). The role of these kinases is not restricted to Trk1, and several nutrient transporters, such as Can1, Fur4, and Hxt1, were found to be destabilized in the hal4 hal5 mutant under low-K+ conditions. The characterization of the molecular mechanism responsible for this mode of regulation will require the identification of the substrates of the Hal4 and Hal5 kinases.
The SR protein kinase Sky1 was isolated in a genetic screen for clones capable of restoring spermine sensitivity of spermine-tolerant mutants. Strains lacking SKY1 were tolerant to toxic levels of spermine, while overexpression of SKY1 in wild-type cells increased their sensitivity to this polyamine (69). In addition to spermine tolerance, sky1Δ cells were tolerant to lithium and sodium ions. The role of Sky1 in cation tolerance and potassium homeostasis is not clear. Some reports, on the basis of genetic analysis and Rb+ uptake measurements, postulate that this kinase modulates the Trk1 and Trk2 transporters (79). In contrast, other authors support a Trk-independent role and favor the involvement of the Nha1 and Kha1 antiporters and of the Tok1 channel (70). Interestingly, it has been reported that the ability of overexpressed Sky1 to increase sensitivity to LiCl depends on the integrity of PPZ1 but not on that of ENA1 (69). Since Ppz1 has been reported to regulate ENA1 expression and Trk activity (see below), this observation may support a regulatory role of Sky1 on the Trk uptake systems.
Further involvement of phosphorylation-dephosphorylation mechanisms in regulation of potassium uptake was derived from the identification of Hal3 as a modulator of both sodium and lithium efflux and potassium influx (74). Hal3 was identified as a negative regulator of Ppz1 (59), a Ser/Thr protein phosphatase known to influence salt tolerance (213) (see below). Yenush and coworkers demonstrated that the Hal3/Ppz1 pair is involved in the proper regulation of intracellular potassium concentrations and pH in a Trk-dependent manner (294). More recently, it has been shown that Trk1 is present in plasma membrane “rafts,” where it physically interacts with Ppz1. Trk1 is phosphorylated in vivo, and its level of phosphorylation increases in ppz1 ppz2 mutants. Protein stability and/or subcellular localization is not altered in the ppz1 ppz2 mutant strain (293). The authors observed that both the interaction with and inhibition of Ppz1 by Hal3 are pH dependent, which led to the proposal that the Ppz1-Hal3 interaction could be a sensor of intracellular pH that modulates H+ and K+ homeostases through the regulation of Trk1 activity. It must be noted, however, that direct dephosphorylation of Trk1 by Ppz1 remains to be demonstrated. Similarly, an early report identified the calcium-activated protein phosphatase calcineurin as a necessary element for the transition of the Trk transporter system to the high-affinity potassium state induced by Na+ stress, which helps to discriminate K+ from Na+ (167). The basis for this effect is still unknown, although calcineurin seems to be necessary for Trk1 activity even under basal conditions (C. Casado et al., submitted for publication).
Strains lacking ARL1, a gene encoding a conserved guanine nucleotide-binding protein, were found to be sensitive to several toxic cations, and these phenotypes were suppressed by K+ (181). The arl1 strain also displayed impaired Rb+ uptake and exhibited normal K+ and H+ efflux. In spite of the recognized role of Arl1 in intracellular trafficking, loss of ARL1 had no effect on the steady-state level or the localization of a tagged Trk1 version (181), as confirmed later by Perez-Valle and coworkers (206). Although from the reported data, it is likely that Arl1 controls Trk1 activity, its precise role is still poorly defined. The observation that localization of Trk1 seems unaffected upon arl1 mutation makes it unlikely that Arl1 acts as a regulator of the Hal4/5 kinases, as was initially proposed (181).
The Snf1 stress-activated protein kinase has been proposed as an additional regulator of potassium influx via Trk1 and/or Trk2 (212). snf1Δ cells were found to be unable to fully activate K+ import, and genetic analysis suggested that the weak kinase activity of the nonphosphorylated form of Snf1 activates high-affinity K+ uptake in glucose-metabolizing yeast cells. However, this is most probably an indirect effect, perhaps mediated by the Sip4 transcriptional activator (212). A striking link between carbohydrate metabolism and potassium transport is highlighted by the observation that TPS1, a gene encoding trehalose-6-phosphate synthase and known to modulate glucose metabolism, activates Trk and reduces the sensitivity of yeast cells to many toxic cations independently of diverse Trk1 regulators (Hal4, Hal5, or calcineurin). Mutants defective in PGM2 and HXK2 exhibited phenotypes (reduced Trk1 activity and increased sensitivity to toxic cations) similar to those of tps1 mutants. The authors observed a positive correlation between levels of glucose phosphates (Glc-1-P and Glc-6-P) caused by these mutations and Trk activity, leading to the proposal that these metabolites, directly or indirectly, activate potassium uptake via Trk systems (179). These findings would support the early observation that potassium uptake in yeast is activated by glucose and other fermentable sugars and that phosphorylation of the sugar was sufficient to trigger the activating pathway (5).
Regulation of Nha1
Expression of the NHA1 gene seems to be rather stable and not affected by any form of stress. However, Nha1 is an important component of the early cellular response to sodium stress and is necessary for additional adaptive transcriptional responses. For instance, osmotic shock, as produced by high external NaCl concentrations, causes almost immediate dissociation of most proteins (transcription factors, TATA-binding protein [TBP], etc.) from chromatin, thus preventing the necessary adaptive transcriptional response (i.e., expression of ENA1). Upon osmotic shock, the Hog1 kinase is activated via the HOG signaling pathway and very rapidly phosphorylates Nha1 at its C-terminal cytosolic tail (presumably at T765 and T876). This phosphorylation event changes the activity of Nha1, by an unknown mechanism, and is proposed to be necessary for the reassociation of the transcriptional machinery (224). The same work also shows a participation (although less relevant) of the Tok1 channel in this early response. Therefore, Hog1 would behave as a key regulator for both pretranscriptional and transcriptional responses relevant for adaptation to cation stress. Later, it was shown that in response to osmotic shock mediated by sorbitol, the phosphorylation of Nha1 by Hog1 results in a transient inhibition of potassium efflux activity (123). This decrease in potassium loss via Nha1 helps cells to cope with the osmotic shock, and in the case of NaCl stress, fewer sodium cations enter the cells if less potassium is liberated, thus resulting in a detectable phenotype of increased sodium tolerance.
Overexpression of Sit4, a Ser/Thr phosphatase whose expression is induced by exposure to Li+, Na+, and K+, causes increased K+ and Rb+ efflux upon salt stress (160). Further work identified SAP155 as a high-copy-number suppressor of the hygromycin B-sensitive phenotype of the arl1Δ mutant (150). Sap155, together with Sap4, Sap185, and Sap190, have been previously identified as Sit4-interacting proteins (144). Overexpression of SAP155 or deletion of SAP185 decreased K+ efflux, whereas overexpression of SAP155 or deletion of SAP155 had the opposite effect. In contrast, manipulation of Sap4 and Sap190 levels did not alter K+ homeostasis (150). Therefore, SAP155 and SAP185 encode negative and positive modulators of K+ efflux, respectively. These effects are mediated by Sit4 and require the presence of Nha1. Therefore, Sap155 and Sap185 could modulate the effect of Sit4 on Nha1 K+ transport activity. Since Sit4 was initially identified as a regulator of the G1-S transition (268), this functional link is consistent with the previous identification of Nha1 as a high-copy suppressor of the cell cycle blockage of a sit4 hal3 mutant (262). However, no direct dephosphorylation of Nha1 by Sit4 has been demonstrated so far.
Though our knowledge on the posttranslational modification and activity regulation of Nha1p is still rather limited, it seems that regulatory events are concentrated in the C-terminal cytosolic tail of the protein (123, 261, 262) (Fig. 2). This notion would fit with the identification of Cos3 as a binding partner of Nha1p. Cos3 interacts with the first two conserved regions of the C terminus (C1 and C2), and its absence or overexpression results in decreased or increased salt tolerance, respectively, of S. cerevisiae cells, most probably as a consequence of Nha1 activity changes (174). The Nha1 C terminus also contains putative nuclear localization sequences (NLS) that can be functional, as the expression of the Nha1 C terminus as an independent protein targets it to the nucleus (123). Nevertheless, splitting off of the C terminus from the membrane segment and its subsequent trafficking to the nucleus has never been observed in vivo.
Regulation of Ena1
Whereas the expression of most structural components of the influx or efflux systems is essentially constitutive, expression of ENA1 (but not that of other members of the cluster) is dramatically increased by exposure to high Na+ or Li+ concentrations or alkaline pH (86, 167, 213, 288). As first reported by Marquez and Serrano (155), exposure to salt or high pH causes activation of diverse signaling pathways that are integrated by the ENA1 promoter. Remarkably, some of these pathways are also relevant for the control of other key components of cation homeostasis, as will be emphasized when appropriate.
One of the signaling pathways involved in the regulation of ENA1 expression (calcium/calmodulin [see below]) was proposed in an early report to be also involved in posttranscriptional regulation of Ena1 activity (288), although no further progress has been made since then.
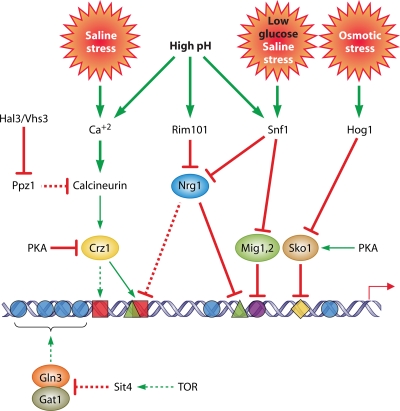
HOG osmoresponsive pathway.
In addition to saline toxicity, exposure of yeast cells to relatively low extracellular concentrations of NaCl (∼0.4 M) also provokes a situation of osmotic stress. Activation of the HOG (high-osmolarity glycerol) pathway in response to high external osmolarity results in phosphorylation and activation of the Hog1 mitogen-activated protein (MAP) kinase (57). Hog1 promotes at least two important events for saline tolerance: control of Nha1 activity, as a fast response that decreases the intracellular amount of toxic cations (see above), and subsequent cooperation in the induction of ENA1 expression. Lack of Hog1 largely attenuates the transcriptional response of ENA1 to NaCl stress (155). Regulation of ENA1 by Hog1 is accomplished through a downstream target of the kinase, the bZip transcription factor Sko1, which acts as a repressor by recruiting the general corepressor complex Ssn6-Tup1 to a cyclic AMP (cAMP) response element (CRE) site in the ENA1 promoter (223). The Sko1-Ssn6-Tup1 repressor complex is disrupted upon phosphorylation by Hog1 of diverse residues at the Sko1 N-terminal region (222). In contrast, phosphorylation of Sko1 by cAMP-dependent protein kinase (PKA) increases its repressor activity (222). In addition to the Sko1-mediated mechanism, activated Hog1 recruits the specific Rpd3-Sin3 histone deacetylase complex to the promoter of ENA1, leading to histone deacetylation, entry of RNA polymerase II, and induction of the expression of the gene upon saline stress (60). In contrast to the relatively important role of Hog1-mediated signaling in the response to toxic concentrations of NaCl, exposure of yeast cells to relatively low concentrations of LiCl (50 to 100 mM) causes a growth defect that is not aggravated by deletion of HOG1 (243) and triggers a significant increase in ENA1 expression that is only slightly decreased by the deletion of HOG1 (A. Ruiz, A. González and J. Ariño, unpublished results). Several lines of evidence (209, 256) suggest that the transcriptional response of ENA1 to alkaline pH does not involve activation of the Hog1 kinase.
Calcineurin pathway.
In S. cerevisiae, calcineurin is a heterodimer composed of one of two redundant catalytic subunit-encoding genes (CNA1 and CNA2) plus a single regulatory subunit gene (CNB1). The relevance of calcineurin in ionic homeostasis is demonstrated by the sensitivity to Na+, Li+, or high pH of cnb1 mutants or wild-type cells treated with the specific inhibitor FK506 (167, 183). Both the vacuole, through the Yvc1 channel (61), and the extracellular milieu, through the Mid1-Cch1 channel (163), have been proposed as the source for the burst of cytosolic calcium upon saline stress. Activation of calcineurin affects expression of a substantial number of genes (295). Gene activation occurs mainly through the dephosphorylation of the transcription factor Crz1/Tcn1/Hal8 (162, 166, 265), which then enters the nucleus and binds to specific sequences, known as CDRE, present in calcineurin-responsible promoters. ENA1 has been known for a long time to be a transcriptional target of the calcineurin/Crz1 tandem, and the accumulation of sodium or lithium cations in calcineurin-deficient cells could be attributed, at least in part, to a decrease in the efflux capacity as a result of impaired induction of ENA1 expression (106, 155, 167). Two Crz1-binding regions have been identified in the ENA1 promoter at positions −813/−821 and −727/−719, with the downstream element being the most relevant for induction of the gene (165). The proposed antagonistic regulation of ENA1 by the calcineurin and PKA pathways (106), supported by the observation that NaCl stress decreases intracellular levels of cAMP (155), can probably be explained by the fact that PKA can phosphorylate Crz1, thus opposing the action of calcineurin (112). Approximately 40% of the total transcriptional response to high pH seems to be mediated by activation of calcineurin (209, 256), in this case as a result of almost immediate entry of external calcium into the cytosol through the Cch1-Mid1 calcium channel (280).
Rim101 pathway.
Yeast cells lacking Rim101, a C2H2 zinc finger transcription factor that positively regulates the expression of genes involved in meiosis and sporulation (266), are sensitive to both high salt concentrations and high pH (135). rim101 cells display decreased expression of ENA1 upon alkaline (135, 256) or NaCl and LiCl (M. Platara and J. Ariño, unpublished results) stresses. Remarkably, the transcriptional role of Rim101 appears to be mediated by repression of other regulatory genes (134), thus acting itself as a repressor. In the case of ENA1, Rim101 would repress the expression of Nrg1, a repressor protein that negatively controls the expression of several glucose-repressed genes and invasive growth. It is worth noting that Nrg1 is also regulated by the Snf1 protein kinase (282), thus integrating two salt-activated signaling pathways (see below). Accordingly, the nrg1 mutation confers tolerance to Na+ and Li+, suppresses the rim101 growth defect at high pH (134), and leads to higher-than-normal ENA1 levels even in the absence of stress (134, 281). Two putative binding sites for Nrg1 in the ENA1 promoter were suggested (CCCTC and CCCCT), located at positions −725/−729 and −651/−647, respectively (134). While the upstream element is probably functional, the downstream one appears to be less important or ineffective (256). A third binding site at positions −561/−573 (AGACCCT) has been identified more recently as relevant for alkaline stress response (209). Taken together, these data suggest that Rim101 may control ENA1 expression in response to alkaline stress through the Nrg1 repressor protein. In fact, the role of this pathway in the regulation of ENA1 expression may be more relevant under alkaline stress than under saline stress, since lack of Rim101 significantly reduces ENA1 expression at high pH but has little effect on the ENA1 response to 0.4 M NaCl (209) or to 0.9 M NaCl or 0.2 M LiCl (M. Platara, A. Ruiz, and J. Ariño, unpublished results).
Regulation by nutrients.
ENA1 expression is higher on derepressing carbon sources, such as galactose or raffinose, than on glucose (2, 233). On the other hand, a strain with a mutation in the SNF1 gene, encoding a protein kinase central to adaptation to glucose depletion, displays enhanced sensitivity to sodium or lithium, probably as a result of impaired induction of ENA1 by high salt concentrations. The Snf1-mediated effect on ENA1 expression was found to be independent of the HOG and calcineurin pathways (2). Glucose-mediated ENA1 repression is based in the URSMIG-ENA1 element at positions −533/−544, which serves as a recognition site for the Mig1/2-Ssn6-Tup1 complex (223). Snf1 activation by low glucose concentrations would then prevent Mig1/2-Ssn6-Tup1-mediated repression of ENA1. However, whereas Snf1 is phosphorylated and activated by saline stress (109, 164), this does not result in phosphorylation of Mig1 (164, 291), and Snf1 remains excluded from the nucleus (109). Instead, it has been proposed that Snf1 may mediate upregulation of ENA1 through the Nrg1 repressor (291). A functional link between Gis4 (whose absence decreases saline tolerance, sodium and lithium efflux, and ENA1 expression) and Snf1 has been proposed (292), although its nature is still unclear. Induction of ENA1 by alkaline pH is markedly reduced in cells lacking SNF1 (2, 209). In this case, Snf1 becomes enriched in the nucleus, similarly to what happens during carbon stress (109). Recent work (209) has demonstrated that activation of Snf1 by high pH increases expression of ENA1 by releasing the repression mediated through the MIG element and by deactivating the Nrg1 repressor. Thus, the Nrg1 repressor would be under the control of two regulators, Snf1 and Rim101 (see above) (Fig. 4).
FIG. 4.
Regulation of ENA1 expression by saline and alkaline stress. Symbols denote known or predicted regulatory sites (○, GATA; ▪, CDRE; ▵, NRG; •, MIG; ⧫, CRE). The promoter is not drawn to scale, but the relative positions of the regulatory sites are maintained. Discontinuous lines denote regulatory interactions still to be fully documented. See the text for details. (Adapted from reference 247a.)
The TOR (target of rapamycin) pathway plays an important role in regulating nutrient-responsive transcription (52). Tor kinases are inhibited by poor nitrogen sources or rapamycin treatment. Crespo and coworkers (52) demonstrated that treatment of yeast cells with rapamycin transiently increased ENA1 expression and that this response required the presence of Gln3 and Gat1 (two Tor-regulated GATA factors) for the full effect. Similarly, induction triggered by exposure to 0.4 M NaCl was markedly reduced in these mutants. Moreover, gln3 or gat1 cells are sensitive to sodium, and the absence of the transcription factors eliminates the salt tolerance of ure2 cells (51). These observations were in agreement with the early finding that mutations in URE2 suppressed the sensitivity of calcineurin mutants to Na+ or Li+ cations and that this effect required the presence of the ENA1 gene (289). Although at least six putative GATA motifs (GATAA and GATAAG) can be detected in the ENA1 promoter (mostly in its upstream region), their functional role has not been tested so far. In fact, the regulation of ENA1 by the TOR pathway is still controversial. For instance, whereas the Sit4 phosphatase (see above) plays a prominent role in Gln3 localization upon Tor signaling, it has been reported that neither deletion nor overexpression of SIT4 (160) affects basal or salt-induced ENA1 mRNA levels (although these results were obtained using cells grown on galactose, which strongly affects the ENA1 basal expression level [2, 233]). In addition, the observation that stressing cells with 1 M NaCl under conditions in which Gln3 is normally nuclear (i.e., nitrogen starvation) results in rapid relocation of Gln3 to the cytoplasm (270) is difficult to reconcile with a direct role of Gln3 in ENA1 expression.
Hal3/Ppz system.
Ppz1 and Ppz2 are atypical Ser/Thr protein phosphatases related to the type 1 enzyme encoded in yeast by GLC7 (48, 139, 214, 215; reviewed in reference 9. Mutants lacking Ppz1 display a marked tolerance to Na+ and Li+ (213). ppz2 mutants display no phenotype, but deletion of PPZ2 further enhances ppz1 phenotypes. Single ppz1 and double ppz1 ppz2 mutants show increased ENA1 mRNA levels even in the absence of stress and an enhanced response in salt-stressed cells. Therefore, Ppz1,2 control ENA1 expression. HAL3/SIS2 was identified almost simultaneously as a gene conferring halotolerance when overexpressed (74) and as high-copy-number suppressor of the growth defect of a Sit4 phosphatase mutant (64). Hal3 is necessary for full induction of ENA1 upon saline stress. The role of Hal3 in salt tolerance and the cell cycle was clarified when de Nadal and coworkers discovered that Hal3 was a negative regulatory subunit of Ppz1 that acts by binding to its C-terminal catalytic moiety (47, 59). Hal3 is also able to bind and inhibit Ppz2 (247). Vhs3, a second regulatory subunit of Ppz1, was identified by means of a multicopy suppressor screen of the hal3 sit4 synthetically lethal phenotype (245). Vhs3 also binds the C-terminal half of Ppz1 and inhibits its phosphatase activity in vitro with a potency similar to that of Hal3. However, Vhs3 has a less important role in vivo than Hal3, probably due to lower expression levels (245).
The precise mechanism for regulation of ENA1 expression by the Ppz phosphatases is not completely solved. The higher-than-normal basal levels of ENA1 mRNA in the ppz1 single mutant seem to be fully caused by constitutive activation of the calcineurin/Crz1 pathway (247). In ppz1 ppz2 mutants, however, additional regulatory mechanisms are activated, leading to a higher expression of ENA1 even in the absence of stress. This may be explained on the basis of the negative control exerted by Ppz1,2 in regulating the Trk transporters, which has a substantial effect on saline tolerance and intracellular pH (294). It was shown that deregulated activity of Trk transporters in the ppz1 ppz2 mutant causes intracellular alkalinization, which would further activate the ENA1 promoter in a calcineurin-independent fashion (247).
A striking finding was the observation that the hal3 and vhs3 mutations resulted in a synthetically lethal phenotype that was independent of their role as Ppz1,2 inhibitors (245), suggesting an additional essential function for these proteins. This paradox has been solved very recently with the discovery that Hal3 and/or Vhs3 cooperate with the still-uncharacterized protein Ykl088w to constitute a heterotrimer responsible for the essential phosphopantothenoylcysteine decarboxylase activity in S. cerevisiae (244), a key step in coenzyme A (CoA) biosynthesis. Therefore, Hal3 and Vhs3 are moonlighting proteins. It is tempting to speculate on the evolutionary advantage gained by functionally linking the inhibition of an energy-consuming, stress-related ATPase such as the ENA1 product and the generation of CoA.
Other possible regulatory inputs.
Mutation or overexpression of a number of other genes has been shown to affect salt tolerance and, in some cases, ENA1 expression. For instance, a link between ENA1 expression and inositol metabolism has been established through the observation that isc1 mutants, lacking inositol phosphosphingolipid phospholipase C activity, are sensitive to sodium or lithium and display decreased ENA1 expression (27). Furthermore, overexpression of the genes encoding the inositol monophosphatases Imp1 and Imp2 conferred tolerance to both cations in an ENA1-dependent fashion (142), although without effect on the expression levels of the ATPase gene.
Mutation of genes encoding the regulatory subunits (CKB1 or CKB2) of the conserved Ser/Thr protein kinase CK2 yields cells sensitive to high sodium or lithium concentrations (28, 58, 271). Tenney and Glover (271) proposed that lack of Ckb1 or Ckb2 decreased ENA1 expression in cells stressed with 0.4 M NaCl. In contrast, de Nadal and coworkers (58) did not observe changes in ENA1 promoter activity in ckb1 cells challenged with higher concentrations of NaCl (0.75 M) for 60 min. The relevance of phosphorylation events in the regulation of ENA1 expression is further stressed by the observation that ptc1 cells (lacking one of the seven type 2C Ser/Thr protein phosphatases in S. cerevisiae) were sensitive to Li+ (but not to Na+) and that this phenotype was not additive to that of the ena1-ena4 deletion (243). Both basal and lithium-induced expression levels of ENA1 were decreased in the ptc1 strain, and this effect was not attributable to the role of Ptc1 in Hog1 regulation (249). Several lines of evidence suggest that Ptc1 may be regulating the Hal3/Ppz system (and hence ENA1 expression [see above]) through a still-unknown mechanism.
Other gene products, such as Psr1 and Psr2 (two similar membrane proteins with phosphatase activity [263]), Hal9 (a putative transcription factor containing a zinc finger that, when overexpressed, was able to confer increased tolerance to sodium or lithium ions [166]), or the nuclear phosphoprotein Lic4/Atc1 (related to cation homeostasis several years ago [105]) have been proposed to modulate saline tolerance by means of the control of ENA1 expression, although no precise mechanism is known.
The HAL1 gene plays an important role in maintaining a normal intracellular K+/Na+ ratio and confers salt tolerance when overexpressed in yeast cells. Mutants lacking HAL1 are salt sensitive and display decreased ENA1 expression, whereas overexpression of HAL1 confers salt tolerance and increases expression of the ATPase gene (89, 233). Overexpression of HAL1 also increased intracellular K+ in a TRK1- and TOK1-independent fashion. This is probably caused by a reduced K+ loss from the cells upon salt stress. The expression of HAL1 itself is upregulated by osmotic stress via the Sko1 repressor and the Gcn4 activator, both competing for the CRE-like sequence present in the HAL1 promoter (203). A dose-dependent effect of the STD1 and MTH1 genes on both HAL1 and ENA1 expression levels has been described (83).
Very recently, a role for Ref2 (a regulatory subunit of the Glc7 type 1 protein phosphatase) in cation homeostasis has been proposed (75). The ref2 deletion mutant displays a marked decrease in lithium efflux, which can be explained by the inability of these cells to fully induce the ENA1 gene. The effect of lack of Ref2 was shown to be additive to that of blockage of the calcineurin pathway and might disrupt multiple mechanisms controlling ENA1 expression. Remarkably, this role seems to be unrelated to the previously identified function of Ref2 as a component of the APT complex (75).
ALKALI METAL CATION TRANSPORTERS IN OTHER YEASTS: SIMILARITIES AND DIFFERENCES
Genes encoding putative alkali metal cation transporters (orthologous to those of S. cerevisiae) have been identified in the genomes of other yeast species. However, characterization of corresponding proteins has revealed extremely interesting exceptions and peculiarities that we summarize here, focusing mainly on some of the best known nonconventional yeasts.
K+ Uptake Systems and Na+ Efflux ATPases
Although the S. pombe genome contains two TRK genes (Sptrk1+ and Sptrk2+), similar to the case for S. cerevisiae, the situation concerning potassium uptake is far from identical in the two yeasts. Sptrk1+ and Sptrk2+ encode proteins of 833 and 880 amino acids, respectively, that are 34% identical to each other. Unlike in budding yeast, they seem to be equally important for the cell when it is growing under standard conditions. The presence of either of these genes is sufficient to allow growth at low K+ and to transport the cation similarly to the manner in the wild-type strain. Northern analysis revealed that the two genes are expressed under different external conditions. Consequently, removal of both transporter genes is necessary to provoke increased potassium requirements for growth and impaired transport characteristics (39, 41). Only in the presence of some specific stress conditions, such as very low pH or salt concentration, does SpTrk1 seem to contribute more efficiently than SpTrk2 to the fitness of the fission yeast (40).
In the case of D. hansenii, most of the potassium and sodium transporters identified have been partially characterized by heterologous expression in S. cerevisiae mutants lacking the equivalent transporters, since molecular tools to manipulate and delete genes are not extensively developed for Debaryomyces. However, an interesting approach was followed by Prista and coworkers (221), who showed that transformation with genes from D. hansenii can improve the salt tolerance of a wild-type S. cerevisiae strain. Concerning K+ transporters, the most important novelty compared to the situation in S. cerevisiae or S. pombe is the existence of a single TRK gene and an additional and completely different transporter encoded by D. hansenii HAK1 (DhHAK1) (220).
Hak1 (high-affinity K+ transporter) was first identified in D. occidentalis, and it was shown to have significant amino acid homology with the Kup gene product of Escherichia coli (12, 253). Subsequently, HAK1-orthologous genes have been found in many other organisms, such as the nonconventional yeasts D. hansenii (220) and Hansenula polymorpha (J. M. Siverio, personal communication), the micelial fungus N. crassa (104), the moss Physcomitrella patens (84), and higher plants (259, 276). When expressed heterologously in an S. cerevisiae mutant lacking its own K+ transporters, DhHak1 improved growth under low-K+ conditions, K+ accumulation, and Rb+ transport (220). Research with N. crassa and D. occidentalis has shown that Hak transporters work as K+-H+ symporters with a high concentrative capacity. When D. occidentalis HAK1 was expressed in an S. cerevisiae trk1 trk2 mutant, it was observed that it could deplete K+ from the external medium to almost the same extent as seen with the wild-type strain of D. occidentalis (12).
For other yeasts, such as C. albicans or Z. rouxii, potassium transporters have not been characterized in detail yet. Database searches indicate that while HAK- and TRK-orthologous genes are present in C. albicans, Z. rouxii possesses only the Trk-type K+ transporter. A recent paper reports that the primary function of Trk proteins, transport of K+ ions, is quantitatively conserved between C. albicans and S. cerevisiae. However, the secondary function, chloride efflux channeling, is present but poorly conserved between the two species, being highly variant with respect to activation velocity, amplitude, flickering (channel-like) behavior, pH dependence, and inhibitor sensitivity (170).
D. hansenii is also endowed with Ena-type ATPases. While a variable number of ENA copies are present in S. cerevisiae genomes, only two ENA genes have been identified so far in D. hansenii (DhENA1 and DhENA2). Heterologous expression of DhENA genes in an S. cerevisiae mutant indicated their function in sodium detoxification and extrusion (6). In addition, ENA orthologs are present in the genomes of C. albicans, Candida dubliniensis (66), and Z. rouxii.
With regard to cation efflux ATPases, additional differences between S. cerevisiae and S. pombe are also worth mentioning. Sequence comparisons indicate that S. pombe Cta3 belongs to the group of the ENA ATPases. However, when the function was studied, it was observed that Cta3 does not pump sodium. The cta3+ gene product was initially proposed to work as an ATP-dependent calcium pump and not as an Na+-ATPase (100). This notion is supported by the fact that Cta3 conserves several amino acids typical of Ca2+-ATPases and is consistent with the functional absence of an Na+-ATPase in S. pombe, where Na+ tolerance and Na+ efflux are dominated by the Na+/H+ antiporter Sod2 (99, 111). However, with the increasing number of P-ATPases isolated and studied, the phylogenetic isolation of Cta3 from other Ca2+-ATPases became more evident, and doubts about its real function arose. Consistent with its capacity to increase the K+ tolerance of the S. cerevisiae ena1-4 nha1 mutant, Cta3 also induced a rapid K+ efflux. Taken together, these results demonstrate that Cta3 is a K+-ATPase (20).
Finally it is worth pointing out that a novel type of P-type ATPase, the ACU (alkali cation uptake)-ATPases, mediating high-affinity K+ or Na+ uptake, has been identified in nonconventional yeasts such as U. maydis or P. sorbitophila (21).
Na+/H+ Antiporters
So far, the existence of plasma membrane Na+/H+ antiporters has been confirmed by functional characterization in 11 yeast species. According to the number of ScNHA1 homologs in the plasma membrane and to the specialization of their function, these yeast species can be divided in two subgroups; one contains three members in which the original NHA1 gene has probably been duplicated and gained new functions, whereas in the larger subgroup, only one antiporter with broad substrate specificity and multiple physiological functions exists.
The identification of the very first gene encoding a yeast Na+/H+ antiporter, sod2, was based on the selection for increased tolerance to Li+ in salt-sensitive S. pombe cells (111). The second member of the yeast Na+/H+ antiporter family characterized was Sod2 from the osmotolerant yeast Z. rouxii (284). In the Z. rouxii strain used (ATCC 42981), another highly similar gene, designated SOD22, was identified (110). Both Z. rouxii Sod2 (ZrSod2) and ZrSod22 were shown to enhance the NaCl tolerance of a salt-sensitive S. cerevisiae strain, but only ZrSOD2 was confirmed to be transcribed in Z. rouxii cells (110, 284). In another, also highly salt-tolerant, Z. rouxii strain (CBS732), only one SOD2-like gene was identified. As it was highly similar to ZrSOD2, but contained an additional stretch of sequence unique for ZrSOD22, it was named ZrSOD2-22 (119) Heterologous expression in an S. cerevisiae strain lacking its own alkali metal cation exporters revealed that all three Z. rouxii antiporters transport only sodium and lithium (110, 118, 119), similar to what was observed for the S. pombe Sod2 antiporter. This Li+ and Na+ specificity was in contrast to the case for the S. cerevisiae Nha1, which also transports potassium and its analog rubidium (see above), and thus presents the question of how these cells eliminate surplus potassium. The question was answered later by the characterization of two members of the Na+/H+ antiporter family in Yarrowia lipolytica (199). The Y. lipolytica genome encodes two putative plasma membrane Na+/H+ antiporters, and their heterologous expression in S. cerevisiae cells revealed that while the Y. lipolytica Nha1 (YlNha1) protein mainly increased cell tolerance to potassium, YlNha2 displayed a remarkable transport capacity for sodium. Thus, Y. lipolytica was the first example of a yeast species with two plasma membrane alkali metal cation/H+ antiporters differing in their function in cell physiology, i.e., cation detoxification versus maintenance of stable intracellular potassium content. Findings with Y. lipolytica led to the reexamination of the S. pombe and Z. rouxii genomes and to the identification of second members of the NHA family of yeast antiporters in both species (201, 217). Their characterization revealed that SpSod22 and ZrNha1 contribute significantly to potassium homeostasis. In the case of Z. rouxii, the difference between both Sod2-22 and Nha1 transporters in substrate preferences and physiological functions has been demonstrated directly by the characterization of Z. rouxii cells lacking or overexpressing the two antiporters (201, 217). In general, the sodium-specific antiporters in the three yeast species are shorter in the C-terminal hydrophilic parts than their potassium-transporting paralogs, and ZrSod2-22 and YlNha2 proteins have extremely high capacities to export sodium cations (119, 200), much higher than those of ScNha1 or other yeast antiporters with broad substrate specificity.
A single plasma membrane antiporter with broad substrate specificity has been characterized in eight yeast species. Besides S. cerevisiae, it was identified in the osmotolerant D. hansenii (273), in P. sorbitophila (14), and in five members of the Candida family, i.e., C. albicans, C. dubliniensis, C. parapsilosis, C. glabrata, and C. tropicalis (113, 117, 131). All of these transporters have been characterized by heterologous expression in S. cerevisiae cells lacking the NHA1 and ENA1-4 genes, i.e., in cells unable to export alkali metal cations efficiently. Phenotypes of increased salt tolerance as well as direct measurements of cation efflux showed that the individual transporters, though having the same wide substrate specificity, differ in their capacity to transport cations; e.g., C. parapsilosis and C. albicans antiporters are the most efficient, whereas those of C. dubliniensis and C. glabrata are less effective.
ScKHA1 and ScNHX1 homologs have been identified in the genomes of most yeast species (218), but only the Kha1 and Nhx1 antiporters of the osmotolerant yeast D. hansenii have been functionally characterized. Expression of DhKHA1 in S. cerevisiae complemented kha1Δ phenotypes and, surprisingly, improved tolerance to Na+ (87). Similarly, heterologous expression of the DhNHX1 gene partially suppressed the alkali metal cation sensitivity of an S. cerevisiae ena1-4 nha1 nhx1 mutant, and it led to the enhanced accumulation of Na+ and K+ cations in the vacuole (176).
CONSERVATION OF “YEAST” ALKALI METAL CATION TRANSPORTERS IN PLANTS
Most yeast alkali metal cation transporters have been conserved throughout evolution, and proteins similar to those reviewed in this work usually appear in higher plants. The only relevant exception is the ENA genes, which encode sodium pumps extremely important for salt tolerance in S. cerevisiae and other fungi but definitely absent in higher plants (85). Additionally, plant cells have developed other transporters that frequently carry out redundant functions, which makes the situation quite complex, especially if we consider that our knowledge on cation homeostasis in higher plants is not as detailed as it is for yeast (see references 235, 259, 276, and 277 for reviews). Below we briefly summarize the current knowledge concerning plasma membrane and intracellular alkali metal cation transporters in plants in comparison with what is known for S. cerevisiae.
K+ Transporters and Na+ Efflux ATPases
Regarding plasma membrane potassium transporters, the two different families identified in yeasts (Trk and Hak) are also present in plants (277). The HKT/TRK family, equivalent to the yeast Trk, contains a variety of members that can work in different ways, and, for example, it has been proposed that Hkt1 mediates a sodium uniport in roots (101). Also, many different genes belong to the KUP/HAK family, equivalent to the yeast Hak transporters. Different HAK genes may have different functions, depending, for example, on their location in the plant. The precise role of these genes in potassium transport is being determined, and it has been proposed that HAK5 in Arabidopsis thaliana (93) or in Solanum lycopersicum (191) contributes to the high-affinity K+ uptake by plant roots and work in a manner similar to that of yeast Hak proteins.
Concerning K+ efflux, we noted above that the only outward-rectifying K+ channel in the plasma membrane of S. cerevisiae is encoded by TOK1. From a sequence homology point of view, the TPK (KCO) plant K+ channels belong to the same family. KCO channels display a hydrophobic core composed of either four TMS and two P domains (KCO-2P family) or two TMS domains and one P domain (KCO-1P family). In Arabidopsis, the KCO-2P family has five members and the KCO-1P family has a single member. Their specific function is still unknown, although it has been recently shown that the A. thaliana Tpk1 (AtTpk1) mediates K+-selective currents between cytoplasmic and vacuolar compartments and plays a role in K+ homeostasis (94). Plant K+ channels of the so-called Shaker family are good examples of additional channels that are not represented in yeast. Both inward K+ channels (AKT1 and KAT1) and outward rectifiers (SKOR/GORK) have been described in plants (259, 277).
The Ena transporters, the main determinants of halotolerance in S. cerevisiae and probably present in most fungi (15, 18, 19), do not exist in flowering plants (85). Remarkably, two Na+-ATPases in the moss Physcomitrella patens have been characterized (23) and at least one of them (PpEna1), appears to be functional as a sodium pump both in S. cerevisiae (23) and in planta (145). This observation has led to the proposal that Ena-like sodium ATPases were present in early land plants but that they have been lost during the evolution of bryophytes to flowering plants (23).
Alkali Metal Cation/Proton Antiporters
Our knowledge on the number and physiological functions of Na+/H+ antiporters in higher eukaryotes has dramatically increased during recent years, often boosted by the efficient expression of these proteins in S. cerevisiae mutant strains lacking their own transport systems. This phenotype complementation approach has been successfully used to characterize the localization and function of mammalian and (mainly) plant plasma membrane antiporters (76, 226) as well as the intracellular homologs of ScNhx1 (81, 90, 121, 225) and ScKha1 (152). However, much more research is needed to fully understand the role of alkali metal cation/H+ antiporters in multicellular organisms because of the gene redundancy (e.g., 9 NHE isoforms in mammalian cells [202] or 40 genes encoding putative Na+/H+ antiporters in A. thaliana [161]) and the different expression levels and various functions according to tissue, organ, and phase of development (see, e.g., references 202 and 302).
Mineral nutrition and ion homeostasis are crucial to plant growth and development (146, 255). Moreover, maintenance of a high intracellular K+/Na+ ratio has been shown to be very important for plant growth in soils with increased salinity (147, 151, 202). Most of the plasma membrane and vacuolar antiporters characterized so far in different plants (263) belong to the ScNhx1 subfamily (151), although ScKha1 homologs (forming the CHX subfamily) have also been identified in plant chloroplast and endosomal membranes (45). Several members of the Nhx1 family have been shown to be crucial for plant salt tolerance. Sos1, the plasma membrane-localized plant homolog of ScNhx1, is a very important sodium extrusion system, and its overexpression can improve the plant's capacity to grow upon salt stress (8, 260). Sos1 is tightly regulated by the Sos2 protein kinase and the Sos3 Ca2+ sensor (149, 226). The general hypothesis is that the Ca2+ sensor, Sos3, interacts with and activates the Sos2 kinase. This Sos3-Sos2 complex activates the Na+/H+ antiporter activity of Sos1 and thereby reestablishes cellular ion homeostasis. However, the very interesting question regarding the molecular mechanism of how this phosphorylation event regulates transporter activity remains unsolved. Similarly, overexpression of the AtNHX1 gene, encoding the vacuolar antiporter (8, 260), improves plant salt tolerance. Besides its role in Na+ detoxification, Nhx1 from Arabidopsis vacuoles has been shown to transport K+ with affinity similar to that of Na+, and thus its role in K+ homeostasis and consequently osmoregulation and internal pH regulation has been suggested (274).
PERSPECTIVES AND FUTURE CHALLENGES
Although our knowledge of alkali metal cation transport and homeostasis in yeast organelles has increased significantly during the last few years and certainly the major transport systems have been identified since the discovery of the Trk1 and Ena systems in the early 1990s, many questions still remain open. For instance, at the plasma membrane level, the residual low-affinity potassium transport system and the molecular nature of the NSC1 channel await identification. Similarly, the nature of the protein(s) involved, together with Mdm38, in the exchange of protons for potassium cations across the mitochondrial inner membrane remains to be solved, and our knowledge about how the endoplasmic reticulum and peroxisomes regulate their potassium content and pH homeostasis is still very poor. Therefore, although a detailed in silico analysis of the yeast “transportome” has identified and classified virtually all putative transporters of alkali metal cations (56, 190), the example of Vnx1 (classified by sequence comparison as a calcium/proton antiporter) and the presence of several ORFs with unknown function and weak homology to mammalian transporters whose substrates are probably alkali metal cations, along with the open questions mentioned above, suggest the existence of so-far-unidentified transporters for potassium and/or sodium in S. cerevisiae cells.
Our knowledge regarding the regulation of alkali metal cation transport is still quite fragmentary. Whereas we have a relatively good idea about how ENA1 transcriptional regulation is controlled by saline and alkaline stress, no real advance has been made in understanding its possible posttranslational control. Similarly, the picture of the control of high-affinity K+ uptake by posttranslational regulation of Trk1 and/or Trk2, which seems to involve diverse protein kinases and phosphatases, lacks molecular detail. The regulation of organelle transporters is currently a fully open field that deserves further investigation. However, it is of utmost importance to obtain a complete and integrated picture of how cells regulate alkali metal cation transport and content and how these parameters influence yeast biology. Therefore, in addition to advances in specific fields, a systems biology approach seems most desirable. Of course, this will probably require the development or improvement of current technologies, such as those that will permit the quantitative determination of actual membrane potential in S. cerevisiae or fast time-resolved measurements of cation fluxes across plasma and organellar membranes.
Acknowledgments
We thank Lynne Yenush for excellent critical review of the manuscript and Alonso Rodríguez-Navarro for fruitful discussions. We appreciate the help of the colleagues who shared with us data prior to publication. We collaborate within the context of Translucent, a SysMo ERA-NET-funded research consortium, and express our gratitude to all members of the consortium for many hours of fruitful and exciting scientific interaction.
Work in J.A.'s laboratory was supported by grants GEN2006-27748-C2-1-E/SYS and BFU2008-04188-C03-01 (MICINN, Spain). J.A. is the recipient of an “Ajut de Suport a les Activitats dels Grups de Recerca” (grant 2009SGR-1091, Generalitat de Catalunya). Work in J.R.'s laboratory was supported by grants GEN2006-27748-C2-2-E/SYS and BFU2008-04188-C03-03 (MICINN, Spain). Work in H.S.'s laboratory was supported by grants MSMT LC531, GA AS CR IAA500110801, GA CR 204/08/0354, and AV0Z 50110509.
Biography
 Joaquín Ariño was born in Barcelona (Spain) in 1957. He was awarded a Ph.D. degree in pharmacy at the Department of Biochemistry (School of Pharmacy, University of Barcelona) in 1986 and was a Fulbright postdoctoral fellow at the Department of Biochemistry, University of Massachusetts (1986 to 1987). In the early 1990s he was involved in the European S. cerevisiae sequencing project, and in 1993 became a professor of biochemistry at the Universitat Autònoma de Barcelona. His laboratory is interested in the control of cellular processes in yeast by phosphorylation-dephosphorylation mechanisms and is focused primarily on Ser/Thr protein phosphatases and stress responses (saline, alkaline pH, nutrients, etc.). He has published around 100 research papers or reviews in the fields of biochemistry, genetics, and microbiology. Together with the other authors, he participates in an international consortium (Translucent) that investigates cation homeostasis in yeast from a systems biology approach.
Joaquín Ariño was born in Barcelona (Spain) in 1957. He was awarded a Ph.D. degree in pharmacy at the Department of Biochemistry (School of Pharmacy, University of Barcelona) in 1986 and was a Fulbright postdoctoral fellow at the Department of Biochemistry, University of Massachusetts (1986 to 1987). In the early 1990s he was involved in the European S. cerevisiae sequencing project, and in 1993 became a professor of biochemistry at the Universitat Autònoma de Barcelona. His laboratory is interested in the control of cellular processes in yeast by phosphorylation-dephosphorylation mechanisms and is focused primarily on Ser/Thr protein phosphatases and stress responses (saline, alkaline pH, nutrients, etc.). He has published around 100 research papers or reviews in the fields of biochemistry, genetics, and microbiology. Together with the other authors, he participates in an international consortium (Translucent) that investigates cation homeostasis in yeast from a systems biology approach.
 José Ramos was born in Córdoba (Spain) in 1957. He was awarded a Ph.D. degree in microbiology at the Department of Microbiology (University of Córdoba) in 1984. He was a visiting professor at the Department of Biochemistry, State University of New York (1987 to 1988) and at the Molecular and Cellular Biology Department, Catholic University of Leuven (1992). He had been a full professor in microbiology since 2006 (University of Córdoba). His research is focused on the study of ion homeostasis and abiotic stress responses in cell-walled eukaryotic organisms. He has published around 100 research papers in the fields of microbiology and biochemistry. They deal mostly with model yeasts such as Saccharomyces cerevisiae or nonconventional yeasts such as Debaryomyces hansenii. Together with the other authors, he participates in an international consortium (Translucent) that investigates cation homeostasis in yeast from a systems biology approach.
José Ramos was born in Córdoba (Spain) in 1957. He was awarded a Ph.D. degree in microbiology at the Department of Microbiology (University of Córdoba) in 1984. He was a visiting professor at the Department of Biochemistry, State University of New York (1987 to 1988) and at the Molecular and Cellular Biology Department, Catholic University of Leuven (1992). He had been a full professor in microbiology since 2006 (University of Córdoba). His research is focused on the study of ion homeostasis and abiotic stress responses in cell-walled eukaryotic organisms. He has published around 100 research papers in the fields of microbiology and biochemistry. They deal mostly with model yeasts such as Saccharomyces cerevisiae or nonconventional yeasts such as Debaryomyces hansenii. Together with the other authors, he participates in an international consortium (Translucent) that investigates cation homeostasis in yeast from a systems biology approach.
 Hana Sychrová was born in Prague (Czechoslovakia) in 1959. She was awarded a Ph.D. degree in biochemistry at the Academy of Sciences in Prague in 1988 and a D.Sc. degree in molecular biology at the same institution in 2001. She spent almost 3 years as a postdoctoral fellow at the Luis Pasteur University in Strasbourg, France, and she became the head of the Department of Membrane Transport, Institute of Physiology AS CR, Prague, in 2006. Her laboratory is interested in characterization of membrane transport systems, in the use of yeast as a model organism to study transport processes in animal and plant cells, and in the study of specific properties of the nonconventional yeasts. She has published around 70 research papers in the fields of biochemistry, genetics, and microbiology. Together with the other authors, she participates in an international consortium (Translucent) that investigates cation homeostasis in yeast from a systems biology approach.
Hana Sychrová was born in Prague (Czechoslovakia) in 1959. She was awarded a Ph.D. degree in biochemistry at the Academy of Sciences in Prague in 1988 and a D.Sc. degree in molecular biology at the same institution in 2001. She spent almost 3 years as a postdoctoral fellow at the Luis Pasteur University in Strasbourg, France, and she became the head of the Department of Membrane Transport, Institute of Physiology AS CR, Prague, in 2006. Her laboratory is interested in characterization of membrane transport systems, in the use of yeast as a model organism to study transport processes in animal and plant cells, and in the study of specific properties of the nonconventional yeasts. She has published around 70 research papers in the fields of biochemistry, genetics, and microbiology. Together with the other authors, she participates in an international consortium (Translucent) that investigates cation homeostasis in yeast from a systems biology approach.
REFERENCES
- 1.Ahmed, A., F. Sesti, N. Ilan, T. M. Shih, S. L. Sturley, and S. A. Goldstein. 1999. A molecular target for viral killer toxin: TOK1 potassium channels. Cell 99:283-291. [DOI] [PubMed] [Google Scholar]
- 2.Alepuz, P. M., K. W. Cunningham, and F. Estruch. 1997. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol. Microbiol. 26:91-98. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Ali, R., C. L. Brett, S. Mukherjee, and R. Rao. 2004. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279:4498-4506. [DOI] [PubMed] [Google Scholar]
- 5.Alijo, R., and J. Ramos. 1993. Several routes of activation of the potassium uptake system of yeast. Biochim. Biophys. Acta 1179:224-228. [DOI] [PubMed] [Google Scholar]
- 6.Almagro, A., C. Prista, B. Benito, M. C. Loureiro-Dias, and J. Ramos. 2001. Cloning and expression of two genes coding for sodium pumps in the salt-tolerant yeast Debaryomyces hansenii. J. Bacteriol. 183:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambesi, A., M. Miranda, V. V. Petrov, and C. W. Slayman. 2000. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J. Exp. Biol. 203:155-160. [DOI] [PubMed] [Google Scholar]
- 8.Apse, M. P., G. S. Aharon, W. A. Snedden, and E. Blumwald. 1999. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256-1258. [DOI] [PubMed] [Google Scholar]
- 9.Arino, J. 2002. Novel protein phosphatases in yeast. Eur. J. Biochem. 269:1072-1077. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong, W. M., and A. Rothstein. 1964. Discrimination between alkali metal cations by yeast. I. Effect of pH on uptake. J. Gen. Physiol. 48:61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong, W. M., and A. Rothstein. 1967. Discrimination between alkali metal cations by yeast. II. Cation interactions in transport. J. Gen. Physiol. 50:967-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banuelos, M. A., R. D. Klein, S. J. Alexander-Bowman, and A. Rodriguez-Navarro. 1995. A potassium transporter of the yeast Schwanniomyces occidentalis homologous to the Kup system of Escherichia coli has a high concentrative capacity. EMBO J. 14:3021-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banuelos, M. A., F. J. Quintero, and A. Rodriguez-Navarro. 1995. Functional expression of the ENA1(PMR2)-ATPase of Saccharomyces cerevisiae in Schizosaccharomyces pombe. Biochim. Biophys. Acta 1229:233-238. [DOI] [PubMed] [Google Scholar]
- 14.Banuelos, M. A., J. Ramos, F. Calero, V. Braun, and S. Potier. 2002. Cation/H+ antiporters mediate potassium and sodium fluxes in Pichia sorbitophila. Cloning of the PsNHA1 and PsNHA2 genes and expression in Saccharomyces cerevisiae. Yeast 19:1365-1372. [DOI] [PubMed] [Google Scholar]
- 15.Banuelos, M. A., and A. Rodriguez-Navarro. 1998. P-type ATPases mediate sodium and potassium effluxes in Schwanniomyces occidentalis. J. Biol. Chem. 273:1640-1646. [DOI] [PubMed] [Google Scholar]
- 16.Banuelos, M. A., M. C. Ruiz, A. Jimenez, J. L. Souciet, S. Potier, and J. Ramos. 2002. Role of the Nha1 antiporter in regulating K(+) influx in Saccharomyces cerevisiae. Yeast 19:9-15. [DOI] [PubMed] [Google Scholar]
- 17.Banuelos, M. A., H. Sychrova, C. Bleykasten-Grosshans, J. L. Souciet, and S. Potier. 1998. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 144:2749-2758. [DOI] [PubMed] [Google Scholar]
- 18.Benito, B., B. Garciadeblas, J. Perez-Martin, and A. Rodriguez-Navarro. 2009. Growth at high pH and sodium and potassium tolerance in media above the cytoplasmic pH depend on ENA ATPases in Ustilago maydis. Eukaryot. Cell 8:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benito, B., B. Garciadeblas, and A. Rodriguez-Navarro. 2000. Molecular cloning of the calcium and sodium ATPases in Neurospora crassa. Mol. Microbiol. 35:1079-1088. [DOI] [PubMed] [Google Scholar]
- 20.Benito, B., B. Garciadeblas, and A. Rodriguez-Navarro. 2002. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology 148:933-941. [DOI] [PubMed] [Google Scholar]
- 21.Benito, B., B. Garciadeblas, P. Schreier, and A. Rodriguez-Navarro. 2004. Novel p-type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eukaryot. Cell 3:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benito, B., F. J. Quintero, and A. Rodriguez-Navarro. 1997. Overexpression of the sodium ATPase of Saccharomyces cerevisiae: conditions for phosphorylation from ATP and Pi. Biochim. Biophys. Acta 1328:214-226. [DOI] [PubMed] [Google Scholar]
- 23.Benito, B., and A. Rodriguez-Navarro. 2003. Molecular cloning and characterization of a sodium-pump ATPase of the moss Physcomitrella patens. Plant J. 36:382-389. [DOI] [PubMed] [Google Scholar]
- 24.Bertl, A., H. Bihler, J. D. Reid, C. Kettner, and C. L. Slayman. 1998. Physiological characterization of the yeast plasma membrane outward rectifying K+ channel, DUK1 (TOK1), in situ. J. Membr. Biol. 162:67-80. [DOI] [PubMed] [Google Scholar]
- 25.Bertl, A., J. Ramos, J. Ludwig, H. Lichtenberg-Frate, J. Reid, H. Bihler, F. Calero, P. Martinez, and P. O. Ljungdahl. 2003. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2 and tok1 null mutations. Mol. Microbiol. 47:767-780. [DOI] [PubMed] [Google Scholar]
- 26.Bertl, A., C. L. Slayman, and D. Gradmann. 1993. Gating and conductance in an outward-rectifying K+ channel from the plasma membrane of Saccharomyces cerevisiae. J. Membr. Biol. 132:183-199. [DOI] [PubMed] [Google Scholar]
- 27.Betz, C., D. Zajonc, M. Moll, and E. Schweizer. 2002. ISC1-encoded inositol phosphosphingolipid phospholipase C is involved in Na+/Li+ halotolerance of Saccharomyces cerevisiae. Eur. J. Biochem. 269:4033-4039. [DOI] [PubMed] [Google Scholar]
- 28.Bidwai, A. P., J. C. Reed, and C. V. Glover. 1995. Cloning and disruption of CKB1, the gene encoding the 38-kDa beta subunit of Saccharomyces cerevisiae casein kinase II (CKII). Deletion of CKII regulatory subunits elicits a salt-sensitive phenotype. J. Biol. Chem. 270:10395-10404. [DOI] [PubMed] [Google Scholar]
- 29.Bihler, H., R. F. Gaber, C. L. Slayman, and A. Bertl. 1999. The presumed potassium carrier Trk2p in Saccharomyces cerevisiae determines an H+-dependent, K+-independent current. FEBS Lett. 447:115-120. [DOI] [PubMed] [Google Scholar]
- 30.Bihler, H., C. L. Slayman, and A. Bertl. 1998. NSC1: a novel high-current inward rectifier for cations in the plasma membrane of Saccharomyces cerevisiae. FEBS Lett. 432:59-64. [DOI] [PubMed] [Google Scholar]
- 31.Bihler, H., C. L. Slayman, and A. Bertl. 2002. Low-affinity potassium uptake by Saccharomyces cerevisiae is mediated by NSC1, a calcium-blocked non-specific cation channel. Biochim. Biophys. Acta 1558:109-118. [DOI] [PubMed] [Google Scholar]
- 32.Borst-Pauwels, G. W. 1981. Ion transport in yeast. Biochim. Biophys. Acta 650:88-127. [DOI] [PubMed] [Google Scholar]
- 33.Bowers, K., B. P. Levi, F. I. Patel, and T. H. Stevens. 2000. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 11:4277-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breinig, F., D. J. Tipper, and M. J. Schmitt. 2002. Kre1p, the plasma membrane receptor for the yeast K1 viral toxin. Cell 108:395-405. [DOI] [PubMed] [Google Scholar]
- 35.Brett, C. L., M. Donowitz, and R. Rao. 2005. Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell Physiol. 288:C223-C239. [DOI] [PubMed] [Google Scholar]
- 36.Brett, C. L., D. N. Tukaye, S. Mukherjee, and R. Rao. 2005. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 16:1396-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cagnac, O., M. Leterrier, M. Yeager, and E. Blumwald. 2007. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J. Biol. Chem. 282:24284-24293. [DOI] [PubMed] [Google Scholar]
- 38.Calahorra, M., G. A. Martinez, A. Hernandez-Cruz, and A. Pena. 1998. Influence of monovalent cations on yeast cytoplasmic and vacuolar pH. Yeast 14:501-515. [DOI] [PubMed] [Google Scholar]
- 39.Calero, F., N. Gomez, J. Arino, and J. Ramos. 2000. Trk1 and Trk2 define the major K(+) transport system in fission yeast. J. Bacteriol. 182:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calero, F., V. Montiel, Z. Caracuel, F. Cabello-Hurtado, and J. Ramos. 2004. On the role of Trk1 and Trk2 in Schizosaccharomyces pombe under different ion stress conditions. FEMS Yeast Res. 4:619-624. [DOI] [PubMed] [Google Scholar]
- 41.Calero, F., and J. Ramos. 2003. K+ fluxes in Schizosaccharomyces pombe. FEMS Yeast Res. 4:1-6. [DOI] [PubMed] [Google Scholar]
- 42.Camacho, M., J. Ramos, and A. Rodriguez-Navarro. 1981. Potassium requirements of Saccharomyces cerevisiae. Curr. Microbiol. 6:295-299. [Google Scholar]
- 43.Campetelli, A. N., G. Previtali, C. A. Arce, H. S. Barra, and C. H. Casale. 2005. Activation of the plasma membrane H-ATPase of Saccharomyces cerevisiae by glucose is mediated by dissociation of the H(+)-ATPase-acetylated tubulin complex. FEBS J. 272:5742-5752. [DOI] [PubMed] [Google Scholar]
- 44.Catty, P., A. de Kerchove d'Exaerde, and A. Goffeau. 1997. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 409:325-332. [DOI] [PubMed] [Google Scholar]
- 45.Cellier, F., G. Conejero, L. Ricaud, D. T. Luu, M. Lepetit, F. Gosti, and F. Casse. 2004. Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J. 39:834-846. [DOI] [PubMed] [Google Scholar]
- 46.Chang, A., and C. W. Slayman. 1991. Maturation of the yeast plasma membrane [H+]ATPase involves phosphorylation during intracellular transport. J. Cell Biol. 115:289-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clotet, J., E. Gari, M. Aldea, and J. Arino. 1999. The yeast ser/thr phosphatases sit4 and ppz1 play opposite roles in regulation of the cell cycle. Mol. Cell. Biol. 19:2408-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clotet, J., F. Posas, A. Casamayor, I. Schaaff-Gerstenschlager, and J. Arino. 1991. The gene DIS2S1 is essential in Saccharomyces cerevisiae and is involved in glycogen phosphorylase activation. Curr. Genet. 19:339-342. [DOI] [PubMed] [Google Scholar]
- 49.Conway, E. J., and F. Duggan. 1958. A cation carrier in the yeast cell wall. Biochem. J. 69:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conway, E. J., and E. O'Malley. 1946. The nature of the cation exchanges during yeast fermentation, with formation of 0.02n-H ion. Biochem. J. 40:59-67. [PMC free article] [PubMed] [Google Scholar]
- 51.Crespo, J. L., K. Daicho, T. Ushimaru, and M. N. Hall. 2001. The GATA transcription factors GLN3 and GAT1 link TOR to salt stress in Saccharomyces cerevisiae. J. Biol. Chem. 276:34441-34444. [DOI] [PubMed] [Google Scholar]
- 52.Crespo, J. L., and M. N. Hall. 2002. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 66:579-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunningham, K. W., and G. R. Fink. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daran-Lapujade, P., J. M. Daran, M. A. Luttik, M. J. Almering, J. T. Pronk, and P. Kotter. 2009. An atypical PMR2 locus is responsible for hypersensitivity to sodium and lithium cations in the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D. FEMS Yeast Res. 9:789-792. [DOI] [PubMed] [Google Scholar]
- 55.De Hertogh, B., E. Carvajal, E. Talla, B. Dujon, P. Baret, and A. Goffeau. 2002. Phylogenetic classification of transporters and other membrane proteins from Saccharomyces cerevisiae. Funct. Integr. Genomics 2:154-170. [DOI] [PubMed] [Google Scholar]
- 56.De Hertogh, B., F. Hancy, A. Goffeau, and P. V. Baret. 2006. Emergence of species-specific transporters during evolution of the hemiascomycete phylum. Genetics 172:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Nadal, E., P. M. Alepuz, and F. Posas. 2002. Dealing with osmostress through MAP kinase activation. EMBO Rep. 3:735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Nadal, E., F. Calero, J. Ramos, and J. Arino. 1999. Biochemical and genetic analyses of the role of yeast casein kinase 2 in salt tolerance. J. Bacteriol. 181:6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Nadal, E., J. Clotet, F. Posas, R. Serrano, N. Gomez, and J. Arino. 1998. The yeast halotolerance determinant Hal3p is an inhibitory subunit of the Ppz1p Ser/Thr protein phosphatase. Proc. Natl. Acad. Sci. U. S. A. 95:7357-7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Nadal, E., M. Zapater, P. M. Alepuz, L. Sumoy, G. Mas, and F. Posas. 2004. The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427:370-374. [DOI] [PubMed] [Google Scholar]
- 61.Denis, V., and M. S. Cyert. 2002. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 156:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dibrov, P., and L. Fliegel. 1998. Comparative molecular analysis of Na+/H+ exchangers: a unified model for Na+/H+ antiport? FEBS Lett. 424:1-5. [DOI] [PubMed] [Google Scholar]
- 63.Dichtl, B., A. Stevens, and D. Tollervey. 1997. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J. 16:7184-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Como, C. J., R. Bose, and K. T. Arndt. 1995. Overexpression of SIS2, which contains an extremely acidic region, increases the expression of SWI4, CLN1 and CLN2 in sit4 mutants. Genetics 139:95-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eddy, A. A., and J. A. Barnett. 2007. A history of research on yeasts 11. The study of solute transport: the first 90 years, simple and facilitated diffusion. Yeast 24:1023-1059. [DOI] [PubMed] [Google Scholar]
- 66.Enjalbert, B., G. P. Moran, C. Vaughan, T. Yeomans, D. M. Maccallum, J. Quinn, D. C. Coleman, A. J. Brown, and D. J. Sullivan. 2009. Genome-wide gene expression profiling and a forward genetic screen show that differential expression of the sodium ion transporter Ena21 contributes to the differential tolerance of Candida albicans and Candida dubliniensis to osmotic stress. Mol. Microbiol. 72:216-228. [DOI] [PubMed] [Google Scholar]
- 67.Epstein, E. 1966. Dual pattern of ion absorption by plant cells and by plants. Nature 212:1324-1327. [Google Scholar]
- 68.Epstein, W., and L. Laimins. 1980. Potassium transport in Escherichia coli: diverse systems with common control by osmotic forces. Trends Biochem. Sci. 5:21-23. [Google Scholar]
- 69.Erez, O., and C. Kahana. 2001. Screening for modulators of spermine tolerance identifies Sky1, the SR protein kinase of Saccharomyces cerevisiae, as a regulator of polyamine transport and ion homeostasis. Mol. Cell. Biol. 21:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erez, O., and C. Kahana. 2002. Deletions of SKY1 or PTK2 in the Saccharomyces cerevisiae trk1Deltatrk2Delta mutant cells exert dual effect on ion homeostasis. Biochem. Biophys. Res. Commun. 295:1142-1149. [DOI] [PubMed] [Google Scholar]
- 71.Estrada, E., P. Agostinis, J. R. Vandenheede, J. Goris, W. Merlevede, J. Francois, A. Goffeau, and M. Ghislain. 1996. Phosphorylation of yeast plasma membrane H+-ATPase by casein kinase I. J. Biol. Chem. 271:32064-32072. [DOI] [PubMed] [Google Scholar]
- 72.Fairman, C., X. Zhou, and C. Kung. 1999. Potassium uptake through the TOK1 K+ channel in the budding yeast. J. Membr. Biol. 168:149-157. [DOI] [PubMed] [Google Scholar]
- 73.Fernandes, A. R., and I. Sa-Correia. 2003. Transcription patterns of PMA1 and PMA2 genes and activity of plasma membrane H+-ATPase in Saccharomyces cerevisiae during diauxic growth and stationary phase. Yeast 20:207-219. [DOI] [PubMed] [Google Scholar]
- 74.Ferrando, A., S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1995. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell. Biol. 15:5470-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrer-Dalmau, J., A. Gonzalez, M. Platara, C. Navarrete, J. L. Martinez, L. Barreto, J. Ramos, J. Arino, and A. Casamayor. 2009. Ref2, a regulatory subunit of the yeast protein phosphatase 1, is a novel component of cation homeostasis. Biochem. J. [Epub ahead of print.] doi: 10.1042/BJ20091909. [DOI] [PubMed]
- 76.Flegelova, H., R. Haguenauer-Tsapis, and H. Sychrova. 2006. Heterologous expression of mammalian Na/H antiporters in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1760:504-516. [DOI] [PubMed] [Google Scholar]
- 77.Fliegel, L. 2005. Identification of conserved polar residues important for salt tolerance by the Na+/H+ exchanger of Schizosaccharomyces pombe. Mol. Cell. Biochem. 268:83-92. [DOI] [PubMed] [Google Scholar]
- 78.Flis, K., A. Hinzpeter, A. Edelman, and A. Kurlandzka. 2005. The functioning of mammalian ClC-2 chloride channel in Saccharomyces cerevisiae cells requires an increased level of Kha1p. Biochem. J. 390:655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forment, J., J. M. Mulet, O. Vicente, and R. Serrano. 2002. The yeast SR protein kinase Sky1p modulates salt tolerance, membrane potential and the Trk1,2 potassium transporter. Biochim. Biophys. Acta 1565:36-40. [DOI] [PubMed] [Google Scholar]
- 80.Froschauer, E., K. Nowikovsky, and R. J. Schweyen. 2005. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim. Biophys. Acta 1711:41-48. [DOI] [PubMed] [Google Scholar]
- 81.Fukuda, A., A. Nakamura, A. Tagiri, H. Tanaka, A. Miyao, H. Hirochika, and Y. Tanaka. 2004. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na(+)/H(+) antiporter from rice. Plant Cell Physiol. 45:146-159. [DOI] [PubMed] [Google Scholar]
- 82.Gaber, R. F., C. A. Styles, and G. R. Fink. 1988. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:2848-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganster, R. W., R. R. McCartney, and M. C. Schmidt. 1998. Identification of a calcineurin-independent pathway required for sodium ion stress response in Saccharomyces cerevisiae. Genetics 150:31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garciadeblas, B., J. Barrero-Gil, B. Benito, and A. Rodriguez-Navarro. 2007. Potassium transport systems in the moss Physcomitrella patens: pphak1 plants reveal the complexity of potassium uptake. Plant J. 52:1080-1093. [DOI] [PubMed] [Google Scholar]
- 85.Garciadeblas, B., B. Benito, and A. Rodríguez-Navarro. 2001. Plant cells express several stress calcium ATPases but apparently no sodium ATPase. Plant Soil. 235:181-192. [Google Scholar]
- 86.Garciadeblas, B., F. Rubio, F. J. Quintero, M. A. Banuelos, R. Haro, and A. Rodriguez-Navarro. 1993. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol. Gen. Genet. 236:363-368. [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Salcedo, R., V. Montiel, F. Calero, and J. Ramos. 2007. Characterization of DhKHA1, a gene coding for a putative Na(+) transporter from Debaryomyces hansenii. FEMS Yeast Res. 7:905-911. [DOI] [PubMed] [Google Scholar]
- 88.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaxiola, R., I. F. de Larrinoa, J. M. Villalba, and R. Serrano. 1992. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 11:3157-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaxiola, R. A., R. Rao, A. Sherman, P. Grisafi, S. L. Alper, and G. R. Fink. 1999. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. U. S. A. 96:1480-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghaemmaghami, S., W. K. Huh, K. Bower, R. W. Howson, A. Belle, N. Dephoure, E. K. O'Shea, and J. S. Weissman. 2003. Global analysis of protein expression in yeast. Nature 425:737-741. [DOI] [PubMed] [Google Scholar]
- 92.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 93.Gierth, M., P. Maser, and J. I. Schroeder. 2005. The potassium transporter AtHAK5 functions in K(+) deprivation-induced high-affinity K(+) uptake and AKT1 K(+) channel contribution to K(+) uptake kinetics in Arabidopsis roots. Plant Physiol. 137:1105-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gobert, A., S. Isayenkov, C. Voelker, K. Czempinski, and F. J. Maathuis. 2007. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. U. S. A. 104:10726-10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goffeau, A., B. G. Barrell, H. Bussey, R. W. Davis, B. Dujon, H. Feldmann, F. Galibert, J. D. Hoheisel, C. Jacq, M. Johnston, E. J. Louis, H. W. Mewes, Y. Murakami, P. Philippsen, H. Tettelin, and S. G. Oliver. 1996. Life with 6000 genes. Science 274:546, 563-567. [DOI] [PubMed] [Google Scholar]
- 96.Gomez, M. J., K. Luyten, and J. Ramos. 1996. The capacity to transport potassium influences sodium tolerance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 135:157-160. [DOI] [PubMed] [Google Scholar]
- 97.Goossens, A., N. de La Fuente, J. Forment, R. Serrano, and F. Portillo. 2000. Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 20:7654-7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gustin, M. C., B. Martinac, Y. Saimi, M. R. Culbertson, and C. Kung. 1986. Ion channels in yeast. Science 233:1195-1197. [DOI] [PubMed] [Google Scholar]
- 99.Hahnenberger, K. M., Z. Jia, and P. G. Young. 1996. Functional expression of the Schizosaccharomyces pombe Na+/H+ antiporter gene, sod2, in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 93:5031-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Halachmi, D., M. Ghislain, and Y. Eilam. 1992. An intracellular ATP-dependent calcium pump within the yeast Schizosaccharomyces pombe, encoded by the gene cta3. Eur. J. Biochem. 207:1003-1008. [DOI] [PubMed] [Google Scholar]
- 101.Haro, R., M. A. Banuelos, M. E. Senn, J. Barrero-Gil, and A. Rodriguez-Navarro. 2005. HKT1 mediates sodium uniport in roots. Pitfalls in the expression of HKT1 in yeast. Plant Physiol. 139:1495-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haro, R., B. Garciadeblas, and A. Rodriguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 103.Haro, R., and A. Rodriguez-Navarro. 2002. Molecular analysis of the mechanism of potassium uptake through the TRK1 transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1564:114-122. [DOI] [PubMed] [Google Scholar]
- 104.Haro, R., L. Sainz, F. Rubio, and A. Rodriguez-Navarro. 1999. Cloning of two genes encoding potassium transporters in Neurospora crassa and expression of the corresponding cDNAs in Saccharomyces cerevisiae. Mol. Microbiol. 31:511-520. [DOI] [PubMed] [Google Scholar]
- 105.Hemenway, C. S., and J. Heitman. 1999. Lic4, a nuclear phosphoprotein that cooperates with calcineurin to regulate cation homeostasis in Saccharomyces cerevisiae. Mol. Gen. Genet. 261:388-401. [DOI] [PubMed] [Google Scholar]
- 106.Hirata, D., S. Harada, H. Namba, and T. Miyakawa. 1995. Adaptation to high-salt stress in Saccharomyces cerevisiae is regulated by Ca2+/calmodulin-dependent phosphoprotein phosphatase (calcineurin) and cAMP-dependent protein kinase. Mol. Gen. Genet. 249:257-264. [DOI] [PubMed] [Google Scholar]
- 107.Hirata, T., Y. Wada, and M. Futai. 2002. Sodium and sulfate ion transport in yeast vacuoles. J. Biochem. 131:261-265. [DOI] [PubMed] [Google Scholar]
- 108.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong, S. P., and M. Carlson. 2007. Regulation of snf1 protein kinase in response to environmental stress. J. Biol. Chem. 282:16838-16845. [DOI] [PubMed] [Google Scholar]
- 110.Iwaki, T., Y. Higashida, H. Tsuji, Y. Tamai, and Y. Watanabe. 1998. Characterization of a second gene (ZSOD22) of Na+/H+ antiporter from salt-tolerant yeast Zygosaccharomyces rouxii and functional expression of ZSOD2 and ZSOD22 in Saccharomyces cerevisiae. Yeast 14:1167-1174. [DOI] [PubMed] [Google Scholar]
- 111.Jia, Z. P., N. McCullough, R. Martel, S. Hemmingsen, and P. G. Young. 1992. Gene amplification at a locus encoding a putative Na+/H+ antiporter confers sodium and lithium tolerance in fission yeast. EMBO J. 11:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kafadar, K. A., and M. S. Cyert. 2004. Integration of stress responses: modulation of calcineurin signaling in Saccharomyces cerevisiae by protein kinase A. Eukaryot. Cell 3:1147-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamauchi, S., K. Mitsui, S. Ujike, M. Haga, N. Nakamura, H. Inoue, S. Sakajo, M. Ueda, A. Tanaka, and H. Kanazawa. 2002. Structurally and functionally conserved domains in the diverse hydrophilic carboxy-terminal halves of various yeast and fungal Na+/H+ antiporters (Nha1p). J. Biochem. 131:821-831. [DOI] [PubMed] [Google Scholar]
- 114.Kane, P. M. 2005. Close-up and genomic views of the yeast vacuolar H+-ATPase. J. Bioenerg. Biomembr. 37:399-403. [DOI] [PubMed] [Google Scholar]
- 115.Kane, P. M. 2006. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol. Mol. Biol. Rev. 70:177-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ketchum, K. A., W. J. Joiner, A. J. Sellers, L. K. Kaczmarek, and S. A. Goldstein. 1995. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 376:690-695. [DOI] [PubMed] [Google Scholar]
- 117.Kinclova, O., S. Potier, and H. Sychrova. 2001. The Candida albicans Na(+)/H(+) antiporter exports potassium and rubidium. FEBS Lett. 504:11-15. [DOI] [PubMed] [Google Scholar]
- 118.Kinclova, O., S. Potier, and H. Sychrova. 2002. Difference in substrate specificity divides the yeast alkali-metal-cation/H(+) antiporters into two subfamilies. Microbiology 148:1225-1232. [DOI] [PubMed] [Google Scholar]
- 119.Kinclova, O., S. Potier, and H. Sychrova. 2001. The Zygosaccharomyces rouxii strain CBS732 contains only one copy of the HOG1 and the SOD2 genes. J. Biotechnol. 88:151-158. [DOI] [PubMed] [Google Scholar]
- 120.Kinclova, O., J. Ramos, S. Potier, and H. Sychrova. 2001. Functional study of the Saccharomyces cerevisiae Nha1p C-terminus. Mol. Microbiol. 40:656-668. [DOI] [PubMed] [Google Scholar]
- 121.Kinclova-Zimmermannova, O., H. Flegelova, and H. Sychrova. 2004. Rice Na+/H+-antiporter Nhx1 partially complements the alkali-metal-cation sensitivity of yeast strains lacking three sodium transporters. Folia Microbiol. (Praha) 49:519-525. [DOI] [PubMed] [Google Scholar]
- 122.Kinclova-Zimmermannova, O., D. Gaskova, and H. Sychrova. 2006. The Na+,K+/H+-antiporter Nha1 influences the plasma membrane potential of Saccharomyces cerevisiae. FEMS Yeast Res. 6:792-800. [DOI] [PubMed] [Google Scholar]
- 123.Kinclova-Zimmermannova, O., and H. Sychrova. 2006. Functional study of the Nha1p C-terminus: involvement in cell response to changes in external osmolarity. Curr. Genet. 49:229-236. [DOI] [PubMed] [Google Scholar]
- 124.Kinclova-Zimmermannova, O., M. Zavrel, and H. Sychrova. 2005. Identification of conserved prolyl residue important for transport activity and the substrate specificity range of yeast plasma membrane Na+/H+ antiporters. J. Biol. Chem. 280:30638-30647. [DOI] [PubMed] [Google Scholar]
- 125.Kinclova-Zimmermannova, O., M. Zavrel, and H. Sychrova. 2006. Importance of the seryl and threonyl residues of the fifth transmembrane domain to the substrate specificity of yeast plasma membrane Na+/H+ antiporters. Mol. Membr. Biol. 23:349-361. [DOI] [PubMed] [Google Scholar]
- 126.Klionsky, D. J., P. K. Herman, and S. D. Emr. 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54:266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ko, C. H., A. M. Buckley, and R. F. Gaber. 1990. TRK2 is required for low affinity K+ transport in Saccharomyces cerevisiae. Genetics 125:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ko, C. H., and R. F. Gaber. 1991. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ko, C. H., H. Liang, and R. F. Gaber. 1993. Roles of multiple glucose transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:638-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kotyk, A., L. Rihova, and M. Ponec. 1971. Uptake of amino acids by actidione-treated yeast cells. 3. Effect of sodium and potassium ions. Folia Microbiol. (Praha) 16:451-453. [DOI] [PubMed] [Google Scholar]
- 131.Krauke, Y., and H. Sychrova. 2008. Functional comparison of plasma-membrane Na+/H+ antiporters from two pathogenic Candida species. BMC Microbiol. 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kunemund, A., and M. Hofer. 1983. Passive fluxes of K+ and H+ in wild strain and nystatin-resistant mutant of Rhodotorula gracilis (ATCC 26194). Biochim. Biophys. Acta 735:203-210. [DOI] [PubMed] [Google Scholar]
- 133.Kuroda, T., H. Bihler, E. Bashi, C. L. Slayman, and A. Rivetta. 2004. Chloride channel function in the yeast TRK-potassium transporters. J. Membr. Biol. 198:177-192. [DOI] [PubMed] [Google Scholar]
- 134.Lamb, T. M., and A. P. Mitchell. 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 136.Lapathitis, G., and A. Kotyk. 1998. Univalent cation fluxes in yeast. Biochem. Mol. Biol. Int. 44:371-380. [DOI] [PubMed] [Google Scholar]
- 137.Larsson, K., F. Bohl, I. Sjostrom, N. Akhtar, D. Strand, B. M. Mechler, R. Grabowski, and L. Adler. 1998. The Saccharomyces cerevisiae SOP1 and SOP2 genes, which act in cation homeostasis, can be functionally substituted by the Drosophila lethal(2) giant larvae tumor suppressor gene. J. Biol. Chem. 273:33610-33618. [DOI] [PubMed] [Google Scholar]
- 138.Lecchi, S., C. J. Nelson, K. E. Allen, D. L. Swaney, K. L. Thompson, J. J. Coon, M. R. Sussman, and C. W. Slayman. 2007. Tandem phosphorylation of Ser-911 and Thr-912 at the C terminus of yeast plasma membrane H+-ATPase leads to glucose-dependent activation. J. Biol. Chem. 282:35471-35481. [DOI] [PubMed] [Google Scholar]
- 139.Lee, K. S., L. K. Hines, and D. E. Levin. 1993. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol. Cell. Biol. 13:5843-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lesage, F., E. Guillemare, M. Fink, F. Duprat, M. Lazdunski, G. Romey, and J. Barhanin. 1996. A pH-sensitive yeast outward rectifier K+ channel with two pore domains and novel gating properties. J. Biol. Chem. 271:4183-4187. [DOI] [PubMed] [Google Scholar]
- 141.Logg, K., J. Warringer, S. H. Hashemi, M. Kall, and A. Blomberg. 2008. The sodium pump Ena1p provides mechanistic insight into the salt sensitivity of vacuolar protein sorting mutants. Biochim. Biophys. Acta 1783:974-984. [DOI] [PubMed] [Google Scholar]
- 142.Lopez, F., M. Leube, R. Gil-Mascarell, J. P. Navarro-Avino, and R. Serrano. 1999. The yeast inositol monophosphatase is a lithium- and sodium-sensitive enzyme encoded by a non-essential gene pair. Mol. Microbiol. 31:1255-1264. [DOI] [PubMed] [Google Scholar]
- 143.Loukin, S. H., B. Vaillant, X. L. Zhou, E. P. Spalding, C. Kung, and Y. Saimi. 1997. Random mutagenesis reveals a region important for gating of the yeast K+ channel Ykc1. EMBO J. 16:4817-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luke, M. M., S. F. Della, C. J. Di Como, H. Sugimoto, R. Kobayashi, and K. T. Arndt. 1996. The SAP, a new family of proteins, associate and function positively with the SIT4 phosphatase. Mol. Cell. Biol. 16:2744-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lunde, C., D. P. Drew, A. K. Jacobs, and M. Tester. 2007. Exclusion of Na+ via sodium ATPase (PpENA1) ensures normal growth of Physcomitrella patens under moderate salt stress. Plant Physiol. 144:1786-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maathuis, F. J. 2006. The role of monovalent cation transporters in plant responses to salinity. J. Exp. Bot. 57:1137-1147. [DOI] [PubMed] [Google Scholar]
- 147.Maathuis, F. J., and D. Sanders. 1999. Plasma membrane transport in context—making sense out of complexity. Curr. Opin. Plant Biol. 2:236-243. [DOI] [PubMed] [Google Scholar]
- 148.Madrid, R., M. J. Gomez, J. Ramos, and A. Rodriguez-Navarro. 1998. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem. 273:14838-14844. [DOI] [PubMed] [Google Scholar]
- 149.Mahajan, S., G. K. Pandey, and N. Tuteja. 2008. Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Arch. Biochem. Biophys. 471:146-158. [DOI] [PubMed] [Google Scholar]
- 150.Manlandro, C. M., D. H. Haydon, and A. G. Rosenwald. 2005. Ability of Sit4p to promote K+ efflux via Nha1p is modulated by Sap155p and Sap185p. Eukaryot. Cell 4:1041-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mansour, M. M. F., K. H. A. Salama, and M. M. Al-Mutawa. 2003. Transport proteins and salt tolerance in plants. Plant Sci. 164:891-900. [Google Scholar]
- 152.Maresova, L., and H. Sychrova. 2006. Arabidopsis thaliana CHX17 gene complements the kha1 deletion phenotypes in Saccharomyces cerevisiae. Yeast 23:1167-1171. [DOI] [PubMed] [Google Scholar]
- 153.Maresova, L., and H. Sychrova. 2005. Physiological characterization of Saccharomyces cerevisiae kha1 deletion mutants. Mol. Microbiol. 55:588-600. [DOI] [PubMed] [Google Scholar]
- 154.Maresova, L., E. Urbankova, D. Gaskova, and H. Sychrova. 2006. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 6:1039-1046. [DOI] [PubMed] [Google Scholar]
- 155.Marquez, J. A., and R. Serrano. 1996. Multiple transduction pathways regulate the sodium-extrusion gene PMR2/ENA1 during salt stress in yeast. FEBS Lett. 382:89-92. [DOI] [PubMed] [Google Scholar]
- 156.Martinez, P., and B. L. Persson. 1998. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 258:628-638. [DOI] [PubMed] [Google Scholar]
- 157.Martinez, R., M. T. Latreille, and M. Mirande. 1991. A PMR2 tandem repeat with a modified C-terminus is located downstream from the KRS1 gene encoding lysyl-tRNA synthetase in Saccharomyces cerevisiae. Mol. Gen. Genet. 227:149-154. [DOI] [PubMed] [Google Scholar]
- 158.Martinez-Munoz, G. A., and P. Kane. 2008. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J. Biol. Chem. 283:20309-20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martinez-Munoz, G. A., and A. Pena. 2005. In situ study of K+ transport into the vacuole of Saccharomyces cerevisiae. Yeast 22:689-704. [DOI] [PubMed] [Google Scholar]
- 160.Masuda, C. A., J. Ramirez, A. Pena, and M. Montero-Lomeli. 2000. Regulation of monovalent ion homeostasis and pH by the Ser-Thr protein phosphatase SIT4 in Saccharomyces cerevisiae. J. Biol. Chem. 275:30957-30961. [DOI] [PubMed] [Google Scholar]
- 161.Masuda, C. A., M. A. Xavier, K. A. Mattos, A. Galina, and M. Montero-Lomeli. 2001. Phosphoglucomutase is an in vivo lithium target in yeast. J. Biol. Chem. 276:37794-37801. [DOI] [PubMed] [Google Scholar]
- 162.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matsumoto, T. K., A. J. Ellsmore, S. G. Cessna, P. S. Low, J. M. Pardo, R. A. Bressan, and P. M. Hasegawa. 2002. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 277:33075-33080. [DOI] [PubMed] [Google Scholar]
- 164.McCartney, R. R., and M. C. Schmidt. 2001. Regulation of Snf1 kinase. Activation requires phosphorylation of threonine 210 by an upstream kinase as well as a distinct step mediated by the Snf4 subunit. J. Biol. Chem. 276:36460-36466. [DOI] [PubMed] [Google Scholar]
- 165.Mendizabal, I., A. Pascual-Ahuir, R. Serrano, and I. F. de Larrinoa. 2001. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genomics 265:801-811. [DOI] [PubMed] [Google Scholar]
- 166.Mendizabal, I., G. Rios, J. M. Mulet, R. Serrano, and I. F. de Larrinoa. 1998. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 425:323-328. [DOI] [PubMed] [Google Scholar]
- 167.Mendoza, I., F. Rubio, A. Rodriguez-Navarro, and J. M. Pardo. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792-8796. [PubMed] [Google Scholar]
- 168.Michel, B., C. Lozano, M. Rodriguez, R. Coria, J. Ramirez, and A. Pena. 2006. The yeast potassium transporter TRK2 is able to substitute for TRK1 in its biological function under low K and low pH conditions. Yeast 23:581-589. [DOI] [PubMed] [Google Scholar]
- 169.Miosga, T., A. Witzel, and F. K. Zimmermann. 1994. Sequence and function analysis of a 9.46 kb fragment of Saccharomyces cerevisiae chromosome X. Yeast 10:965-973. [DOI] [PubMed] [Google Scholar]
- 170.Miranda, M., E. Bashi, S. Vylkova, M. Edgerton, C. Slayman, and A. Rivetta. 2009. Conservation and dispersion of sequence and function in fungal TRK potassium transporters: focus on Candida albicans. FEMS Yeast Res. 9:278-292. [DOI] [PubMed] [Google Scholar]
- 171.Mitchell, P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144-148. [DOI] [PubMed] [Google Scholar]
- 172.Mitsui, K., K. Hatakeyama, M. Matsushita, and H. Kanazawa. 2009. Saccharomyces cerevisiae Na+/H+ antiporter Nha1p associates with lipid rafts and requires sphingolipid for stable localization to the plasma membrane. J. Biochem. 145:709-720. [DOI] [PubMed] [Google Scholar]
- 173.Mitsui, K., S. Kamauchi, N. Nakamura, H. Inoue, and H. Kanazawa. 2004. A conserved domain in the tail region of the Saccharomyces cerevisiae Na+/H+ antiporter (Nha1p) plays important roles in localization and salinity-resistant cell-growth. J. Biochem. 135:139-148. [DOI] [PubMed] [Google Scholar]
- 174.Mitsui, K., F. Ochi, N. Nakamura, Y. Doi, H. Inoue, and H. Kanazawa. 2004. A novel membrane protein capable of binding the Na+/H+ antiporter (Nha1p) enhances the salinity-resistant cell growth of Saccharomyces cerevisiae. J. Biol. Chem. 279:12438-12447. [DOI] [PubMed] [Google Scholar]
- 175.Mitsui, K., H. Yasui, N. Nakamura, and H. Kanazawa. 2005. Oligomerization of the Saccharomyces cerevisiae Na+/H+ antiporter Nha1p: implications for its antiporter activity. Biochim. Biophys. Acta 1720:125-136. [DOI] [PubMed] [Google Scholar]
- 176.Montiel, V., and J. Ramos. 2007. Intracellular Na and K distribution in Debaryomyces hansenii. Cloning and expression in Saccharomyces cerevisiae of DhNHX1. FEMS Yeast Res. 7:102-109. [DOI] [PubMed] [Google Scholar]
- 177.Morsomme, P., C. W. Slayman, and A. Goffeau. 2000. Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H(+)-ATPase. Biochim. Biophys. Acta 1469:133-157. [DOI] [PubMed] [Google Scholar]
- 178.Mukherjee, S., L. Kallay, C. L. Brett, and R. Rao. 2006. Mutational analysis of the intramembranous H10 loop of yeast Nhx1 reveals a critical role in ion homoeostasis and vesicle trafficking. Biochem. J. 398:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mulet, J. M., S. Alejandro, C. Romero, and R. Serrano. 2004. The trehalose pathway and intracellular glucose phosphates as modulators of potassium transport and general cation homeostasis in yeast. Yeast 21:569-582. [DOI] [PubMed] [Google Scholar]
- 180.Mulet, J. M., M. P. Leube, S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19:3328-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Munson, A. M., D. H. Haydon, S. L. Love, G. L. Fell, V. R. Palanivel, and A. G. Rosenwald. 2004. Yeast ARL1 encodes a regulator of K+ influx. J. Cell Sci. 117:2309-2320. [DOI] [PubMed] [Google Scholar]
- 182.Murguia, J. R., J. M. Belles, and R. Serrano. 1996. The yeast HAL2 nucleotidase is an in vivo target of salt toxicity. J. Biol. Chem. 271:29029-29033. [DOI] [PubMed] [Google Scholar]
- 183.Nakamura, T., Y. Liu, D. Hirata, H. Namba, S. Harada, T. Hirokawa, and T. Miyakawa. 1993. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 12:4063-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nakayama, H., K. Yoshida, and A. Shinmyo. 2004. Yeast plasma membrane Ena1p ATPase alters alkali-cation homeostasis and confers increased salt tolerance in tobacco cultured cells. Biotechnol. Bioeng. 85:776-789. [DOI] [PubMed] [Google Scholar]
- 185.Nass, R., K. W. Cunningham, and R. Rao. 1997. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J. Biol. Chem. 272:26145-26152. [DOI] [PubMed] [Google Scholar]
- 186.Nass, R., and R. Rao. 1998. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 273:21054-21060. [DOI] [PubMed] [Google Scholar]
- 187.Nass, R., and R. Rao. 1999. The yeast endosomal Na+/H+ exchanger, Nhx1, confers osmotolerance following acute hypertonic shock. Microbiology 145:3221-3228. [DOI] [PubMed] [Google Scholar]
- 188.Navarre, C., and A. Goffeau. 2000. Membrane hyperpolarization and salt sensitivity induced by deletion of PMP3, a highly conserved small protein of yeast plasma membrane. EMBO J. 19:2515-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ndayizeye, M., N. Touret, and L. Fliegel. 2009. Proline 146 is critical to the structure, function and targeting of sod2, the Na+/H+ exchanger of Schizosaccharomyces pombe. Biochim. Biophys. Acta 1788:983-992. [DOI] [PubMed] [Google Scholar]
- 190.Nelissen, B., W. R. De, and A. Goffeau. 1997. Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:113-134. [DOI] [PubMed] [Google Scholar]
- 191.Nieves-Cordones, M., A. J. Miller, F. Aleman, V. Martinez, and F. Rubio. 2008. A putative role for the plasma membrane potential in the control of the expression of the gene encoding the tomato high-affinity potassium transporter HAK5. Plant Mol. Biol. 68:521-532. [DOI] [PubMed] [Google Scholar]
- 192.Nowikovsky, K., E. M. Froschauer, G. Zsurka, J. Samaj, S. Reipert, M. Kolisek, G. Wiesenberger, and R. J. Schweyen. 2004. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 279:30307-30315. [DOI] [PubMed] [Google Scholar]
- 193.Nowikovsky, K., S. Reipert, R. J. Devenish, and R. J. Schweyen. 2007. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ. 14:1647-1656. [DOI] [PubMed] [Google Scholar]
- 194.Numata, M., K. Petrecca, N. Lake, and J. Orlowski. 1998. Identification of a mitochondrial Na+/H+ exchanger. J. Biol. Chem. 273:6951-6959. [DOI] [PubMed] [Google Scholar]
- 195.Ohgaki, R., N. Nakamura, K. Mitsui, and H. Kanazawa. 2005. Characterization of the ion transport activity of the budding yeast Na+/H+ antiporter, Nha1p, using isolated secretory vesicles. Biochim. Biophys. Acta 1712:185-196. [DOI] [PubMed] [Google Scholar]
- 196.Opekarova, M., and K. Sigler. 1985. CO2-dependent K+ efflux in yeast utilizing endogenous substrates. Cell Mol. Biol. 31:195-200. [PubMed] [Google Scholar]
- 197.Orlowski, J., and S. Grinstein. 1997. Na+/H+ exchangers of mammalian cells. J. Biol. Chem. 272:22373-22376. [DOI] [PubMed] [Google Scholar]
- 198.Ortega, M. D., and A. Rodríguez-Navarro. 1985. Potassium and rubidium effluxes in Saccharomyces cerevisiae. Z. Naturforsch. 40c:721-725. [Google Scholar]
- 199.Papouskova, K., and H. Sychrova. 2006. Yarrowia lipolytica possesses two plasma membrane alkali metal cation/H+ antiporters with different functions in cell physiology. FEBS Lett. 580:1971-1976. [DOI] [PubMed] [Google Scholar]
- 200.Papouskova, K., and H. Sychrova. 2007. Production of Yarrowia lipolytica Nha2 Na+/H+ antiporter improves the salt tolerance of Saccharomyces cerevisiae. Folia Microbiol. (Praha) 52:600-602. [DOI] [PubMed] [Google Scholar]
- 201.Papouskova, K., and H. Sychrova. 2007. Schizosaccharomyces pombe possesses two plasma membrane alkali metal cation/H antiporters differing in their substrate specificity. FEMS Yeast Res. 7:188-195. [DOI] [PubMed] [Google Scholar]
- 202.Pardo, J. M., B. Cubero, E. O. Leidi, and F. J. Quintero. 2006. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 57:1181-1199. [DOI] [PubMed] [Google Scholar]
- 203.Pascual-Ahuir, A., R. Serrano, and M. Proft. 2001. The Sko1p repressor and Gcn4p activator antagonistically modulate stress-regulated transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pena, A. 1975. Studies on the mechanism of K+ transport in yeast. Arch. Biochem. Biophys. 167:397-409. [DOI] [PubMed] [Google Scholar]
- 205.Pereira, M. B., R. Tisi, L. G. Fietto, A. S. Cardoso, M. M. Franca, F. M. Carvalho, M. J. Tropia, E. Martegani, I. M. Castro, and R. L. Brandao. 2008. Carbonyl cyanide m-chlorophenylhydrazone induced calcium signaling and activation of plasma membrane H(+)-ATPase in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 8:622-630. [DOI] [PubMed] [Google Scholar]
- 206.Perez-Valle, J., H. Jenkins, S. Merchan, V. Montiel, J. Ramos, S. Sharma, R. Serrano, and L. Yenush. 2007. Key role for intracellular K+ and protein kinases Sat4/Hal4 and Hal5 in the plasma membrane stabilization of yeast nutrient transporters. Mol. Cell. Biol. 27:5725-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Persson, B. L., A. Berhe, U. Fristedt, P. Martinez, J. Pattison, J. Petersson, and R. Weinander. 1998. Phosphate permeases of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1365:23-30. [DOI] [PubMed] [Google Scholar]
- 208.Pittman, J. K., N. H. Cheng, T. Shigaki, M. Kunta, and K. D. Hirschi. 2004. Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol. Microbiol. 54:1104-1116. [DOI] [PubMed] [Google Scholar]
- 209.Platara, M., A. Ruiz, R. Serrano, A. Palomino, F. Moreno, and J. Arino. 2006. The transcriptional response of the yeast Na+-ATPase ENA1 gene to alkaline stress involves three main signaling pathways. J. Biol. Chem. 281:36632-36642. [DOI] [PubMed] [Google Scholar]
- 210.Portillo, F. 2000. Regulation of plasma membrane H(+)-ATPase in fungi and plants. Biochim. Biophys. Acta 1469:31-42. [DOI] [PubMed] [Google Scholar]
- 211.Portillo, F., P. Eraso, and R. Serrano. 1991. Analysis of the regulatory domain of yeast plasma membrane H+-ATPase by directed mutagenesis and intragenic suppression. FEBS Lett. 287:71-74. [DOI] [PubMed] [Google Scholar]
- 212.Portillo, F., J. M. Mulet, and R. Serrano. 2005. A role for the non-phosphorylated form of yeast Snf1: tolerance to toxic cations and activation of potassium transport. FEBS Lett. 579:512-516. [DOI] [PubMed] [Google Scholar]
- 213.Posas, F., M. Camps, and J. Arino. 1995. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J. Biol. Chem. 270:13036-13041. [DOI] [PubMed] [Google Scholar]
- 214.Posas, F., A. Casamayor, and J. Arino. 1993. The PPZ protein phosphatases are involved in the maintenance of osmotic stability of yeast cells. FEBS Lett. 318:282-286. [DOI] [PubMed] [Google Scholar]
- 215.Posas, F., A. Casamayor, N. Morral, and J. Arino. 1992. Molecular cloning and analysis of a yeast protein phosphatase with an unusual amino-terminal region. J. Biol. Chem. 267:11734-11740. [PubMed] [Google Scholar]
- 216.Pozos, T. C., I. Sekler, and M. S. Cyert. 1996. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16:3730-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pribylova, L., K. Papouskova, and H. Sychrova. 2008. The salt tolerant yeast Zygosaccharomyces rouxii possesses two plasma-membrane Na+/H+-antiporters (ZrNha1p and ZrSod2-22p) playing different roles in cation homeostasis and cell physiology. Fungal Genet. Biol. 45:1439-1447. [DOI] [PubMed] [Google Scholar]
- 218.Pribylova, L., K. Papouskova, M. Zavrel, J. L. Souciet, and H. Sychrova. 2006. Exploration of yeast alkali metal cation/H+ antiporters: sequence and structure comparison. Folia Microbiol. (Praha) 51:413-424. [DOI] [PubMed] [Google Scholar]
- 219.Prior, C., S. Potier, J. L. Souciet, and H. Sychrova. 1996. Characterization of the NHA1 gene encoding a Na+/H+-antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett. 387:89-93. [DOI] [PubMed] [Google Scholar]
- 220.Prista, C., J. C. Gonzalez-Hernandez, J. Ramos, and M. C. Loureiro-Dias. 2007. Cloning and characterization of two K+ transporters of Debaryomyces hansenii. Microbiology 153:3034-3043. [DOI] [PubMed] [Google Scholar]
- 221.Prista, C., A. Soeiro, P. Vesely, A. Almagro, J. Ramos, and M. C. Loureiro-Dias. 2002. Genes from Debaryomyces hansenii increase salt tolerance in Saccharomyces cerevisiae W303. FEMS Yeast Res. 2:151-157. [DOI] [PubMed] [Google Scholar]
- 222.Proft, M., A. Pascual-Ahuir, E. de Nadal, J. Arino, R. Serrano, and F. Posas. 2001. Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J. 20:1123-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Proft, M., and R. Serrano. 1999. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol. Cell. Biol. 19:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Proft, M., and K. Struhl. 2004. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell 118:351-361. [DOI] [PubMed] [Google Scholar]
- 225.Quintero, F. J., M. R. Blatt, and J. M. Pardo. 2000. Functional conservation between yeast and plant endosomal Na(+)/H(+) antiporters. FEBS Lett. 471:224-228. [DOI] [PubMed] [Google Scholar]
- 226.Quintero, F. J., M. Ohta, H. Shi, J. K. Zhu, and J. M. Pardo. 2002. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. U. S. A. 99:9061-9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ramirez, J., O. Ramirez, C. Saldana, R. Coria, and A. Pena. 1998. A Saccharomyces cerevisiae mutant lacking a K+/H+ exchanger. J. Bacteriol. 180:5860-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ramos, J., R. Alijo, R. Haro, and A. Rodriguez-Navarro. 1994. TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae. J. Bacteriol. 176:249-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ramos, J., P. Contreras, and A. Rodriguez-Navarro. 1985. A potassium transport mutant of Saccharomyces cerevisiae. 143:88-93. [Google Scholar]
- 230.Rao, R., D. Drummond-Barbosa, and C. W. Slayman. 1993. Transcriptional regulation by glucose of the yeast PMA1 gene encoding the plasma membrane H(+)-ATPase. Yeast 9:1075-1084. [DOI] [PubMed] [Google Scholar]
- 231.Reid, J. D., W. Lukas, R. Shafaatian, A. Bertl, C. Scheurmann-Kettner, H. R. Guy, and R. A. North. 1996. The S. cerevisiae outwardly-rectifying potassium channel (DUK1) identifies a new family of channels with duplicated pore domains. Receptors Channels 4:51-62. [PubMed] [Google Scholar]
- 232.Reference deleted.
- 233.Rios, G., A. Ferrando, and R. Serrano. 1997. Mechanisms of salt tolerance conferred by overexpression of the HAL1 gene in Saccharomyces cerevisiae. Yeast 13:515-528. [DOI] [PubMed] [Google Scholar]
- 234.Roberts, S. K., M. Fischer, G. K. Dixon, and D. Sanders. 1999. Divalent cation block of inward currents and low-affinity K+ uptake in Saccharomyces cerevisiae. J. Bacteriol. 181:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rodriguez-Navarro, A. 2000. Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469:1-30. [DOI] [PubMed] [Google Scholar]
- 236.Rodriguez-Navarro, A., M. R. Blatt, and C. L. Slayman. 1986. A potassium-proton symport in Neurospora crassa. J. Gen. Physiol. 87:649-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rodriguez-Navarro, A., F. J. Quintero, and B. Garciadeblas. 1994. Na(+)-ATPases and Na+/H+ antiporters in fungi. Biochim. Biophys. Acta 1187:203-205. [DOI] [PubMed] [Google Scholar]
- 238.Rodriguez-Navarro, A., and J. Ramos. 1984. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Roomans, G. M., F. Blasco, and G. W. Borst-Pauwels. 1977. Cotransport of phosphate and sodium by yeast. Biochim. Biophys. Acta 467:65-71. [DOI] [PubMed] [Google Scholar]
- 240.Rothstein, A., and L. H. Enns. 1946. The relationship of potassium to carbohydrate metabolism in baker's yeast. J. Cell Comp. Physiol. 28:231-252. [DOI] [PubMed] [Google Scholar]
- 241.Rubio, F., W. Gassmann, and J. I. Schroeder. 1995. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270:1660-1663. [DOI] [PubMed] [Google Scholar]
- 242.Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman, J. LeVitre, L. S. Davidow, J. I. Mao, and D. T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133-145. [DOI] [PubMed] [Google Scholar]
- 243.Ruiz, A., A. González, R. García-Salcedo, J. Ramos, and J. Arino. 2006. Role of protein phosphatases 2C on tolerance to lithium toxicity in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 62:263-277. [DOI] [PubMed] [Google Scholar]
- 244.Ruiz, A., A. Gonzalez, I. Munoz, R. Serrano, J. A. Abrie, E. Strauss, and J. Arino. 2009. Moonlighting proteins Hal3 and Vhs3 form a heteromeric PPCDC with Ykl088w in yeast CoA biosynthesis. Nat. Chem. Biol. 5:920-928. [DOI] [PubMed] [Google Scholar]
- 245.Ruiz, A., I. Munoz, R. Serrano, A. Gonzalez, E. Simon, and J. Arino. 2004. Functional characterization of the Saccharomyces cerevisiae VHS3 gene: a regulatory subunit of the Ppz1 protein phosphatase with novel, phosphatase-unrelated functions. J. Biol. Chem. 279:34421-34430. [DOI] [PubMed] [Google Scholar]
- 246.Ruiz, A., R. Serrano, and J. Arino. 2008. Direct regulation of genes involved in glucose utilization by the calcium/calcineurin pathway. J. Biol. Chem. 283:13923-13933. [DOI] [PubMed] [Google Scholar]
- 247.Ruiz, A., L. Yenush, and J. Arino. 2003. Regulation of ENA1 Na(+)-ATPase gene expression by the Ppz1 protein phosphatase is mediated by the calcineurin pathway. Eukaryot. Cell 2:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247a.Ruiz, A., and J. Ariño. 2007. Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot. Cell 6:2175-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Saier, M. H., Jr. 2000. Families of transmembrane sugar transport proteins. Mol. Microbiol. 35:699-710. [DOI] [PubMed] [Google Scholar]
- 249.Saito, H., and K. Tatebayashi. 2004. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136:267-272. [DOI] [PubMed] [Google Scholar]
- 250.Samarao, S. S., C. E. Teodoro, F. E. Silva, C. C. Ribeiro, T. M. Granato, N. R. Bernardes, C. A. Retamal, A. R. Facanha, A. L. Okorokova-Facanha, and L. A. Okorokov. 2009. V H+-ATPase along the yeast secretory pathway: energization of the ER and Golgi membranes. Biochim. Biophys. Acta 1788:303-313. [DOI] [PubMed] [Google Scholar]
- 251.Saroussi, S., and N. Nelson. 2009. Vacuolar H(+)-ATPase-an enzyme for all seasons. Pflugers Arch. 457:581-587. [DOI] [PubMed] [Google Scholar]
- 252.Schlesser, A., S. Ulaszewski, M. Ghislain, and A. Goffeau. 1988. A second transport ATPase gene in Saccharomyces cerevisiae. J. Biol. Chem. 263:19480-19487. [PubMed] [Google Scholar]
- 253.Schleyer, M., and E. P. Bakker. 1993. Nucleotide sequence and 3′-end deletion studies indicate that the K(+)-uptake protein kup from Escherichia coli is composed of a hydrophobic core linked to a large and partially essential hydrophilic C terminus. J. Bacteriol. 175:6925-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Serrano, R. 1983. In vivo glucose activation of the yeast plasma membrane ATPase. FEBS Lett. 156:11-14. [DOI] [PubMed] [Google Scholar]
- 255.Serrano, R., and A. Rodriguez-Navarro. 2001. Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 13:399-404. [DOI] [PubMed] [Google Scholar]
- 256.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Arino. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319-1333. [DOI] [PubMed] [Google Scholar]
- 257.Sesti, F., T. M. Shih, N. Nikolaeva, and S. A. Goldstein. 2001. Immunity to K1 killer toxin: internal TOK1 blockade. Cell 105:637-644. [DOI] [PubMed] [Google Scholar]
- 258.Seto-Young, D., and D. S. Perlin. 1991. Effect of membrane voltage on the plasma membrane H(+)-ATPase of Saccharomyces cerevisiae. J. Biol. Chem. 266:1383-1389. [PubMed] [Google Scholar]
- 259.Shabala, S., and T. A. Cuin. 2008. Potassium transport and plant salt tolerance. Physiol. Plant. 133:651-669. [DOI] [PubMed] [Google Scholar]
- 260.Shi, H., B. H. Lee, S. J. Wu, and J. K. Zhu. 2003. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat. Biotechnol. 21:81-85. [DOI] [PubMed] [Google Scholar]
- 261.Simon, E., A. Barcelo, and J. Arino. 2003. Mutagenesis analysis of the yeast Nha1 Na+/H+ antiporter carboxy-terminal tail reveals residues required for function in cell cycle. FEBS Lett. 545:239-245. [DOI] [PubMed] [Google Scholar]
- 262.Simon, E., J. Clotet, F. Calero, J. Ramos, and J. Arino. 2001. A screening for high copy suppressors of the sit4 hal3 synthetically lethal phenotype reveals a role for the yeast Nha1 antiporter in cell cycle regulation. J. Biol. Chem. 276:29740-29747. [DOI] [PubMed] [Google Scholar]
- 263.Siniossoglou, S., E. C. Hurt, and H. R. Pelham. 2000. Psr1p/Psr2p, two plasma membrane phosphatases with an essential DXDX(T/V) motif required for sodium stress response in yeast. J. Biol. Chem. 275:19352-19360. [DOI] [PubMed] [Google Scholar]
- 264.Soong, T. W., T. F. Yong, N. Ramanan, and Y. Wang. 2000. The Candida albicans antiporter gene CNH1 has a role in Na+ and H+ transport, salt tolerance, and morphogenesis. Microbiology 146:1035-1044. [DOI] [PubMed] [Google Scholar]
- 265.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Su, S. S. Y., and A. P. Mitchell. 1993. Identification of functionally related genes that stimulate early meiotic gene-expression in yeast. Genetics 133:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Supply, P., A. Wach, and A. Goffeau. 1993. Enzymatic properties of the PMA2 plasma membrane-bound H(+)-ATPase of Saccharomyces cerevisiae. J. Biol. Chem. 268:19753-19759. [PubMed] [Google Scholar]
- 268.Sutton, A., D. Immanuel, and K. T. Arndt. 1991. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol. 11:2133-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sychrova, H., J. Ramirez, and A. Pena. 1999. Involvement of Nha1 antiporter in regulation of intracellular pH in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 171:167-172. [DOI] [PubMed] [Google Scholar]
- 270.Tate, J. J., and T. G. Cooper. 2007. Stress-responsive Gln3 localization in Saccharomyces cerevisiae is separable from and can overwhelm nitrogen source regulation. J. Biol. Chem. 282:18467-18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tenney, K. A., and C. V. Glover. 1999. Transcriptional regulation of the S. cerevisiae ENA1 gene by casein kinase II. Mol. Cell Biochem. 191:161-167. [PubMed] [Google Scholar]
- 272.Vargas, R. C., R. Garcia-Salcedo, S. Tenreiro, M. C. Teixeira, A. R. Fernandes, J. Ramos, and I. Sa-Correia. 2007. Saccharomyces cerevisiae multidrug resistance transporter Qdr2 is implicated in potassium uptake, providing a physiological advantage to quinidine-stressed cells. Eukaryot. Cell 6:134-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Velkova, K., and H. Sychrova. 2006. The Debaryomyces hansenii NHA1 gene encodes a plasma membrane alkali-metal-cation antiporter with broad substrate specificity. Gene 369:27-34. [DOI] [PubMed] [Google Scholar]
- 274.Venema, K., F. J. Quintero, J. M. Pardo, and J. P. Donaire. 2002. The arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J. Biol. Chem. 277:2413-2418. [DOI] [PubMed] [Google Scholar]
- 275.Vergani, P., T. Miosga, S. M. Jarvis, and M. R. Blatt. 1997. Extracellular K+ and Ba2+ mediate voltage-dependent inactivation of the outward-rectifying K+ channel encoded by the yeast gene TOK1. FEBS Lett. 405:337-344. [DOI] [PubMed] [Google Scholar]
- 276.Very, A. A., and H. Sentenac. 2002. Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 7:168-175. [DOI] [PubMed] [Google Scholar]
- 277.Very, A. A., and H. Sentenac. 2003. Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54:575-603. [DOI] [PubMed] [Google Scholar]
- 278.Vidal, M., A. M. Buckley, C. Yohn, D. J. Hoeppner, and R. F. Gaber. 1995. Identification of essential nucleotides in an upstream repressing sequence of Saccharomyces cerevisiae by selection for increased expression of TRK2. Proc. Natl. Acad. Sci. U. S. A. 92:2370-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Vidal, M., and R. F. Gaber. 1991. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:6317-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Viladevall, L., R. Serrano, A. Ruiz, G. Domenech, J. Giraldo, A. Barcelo, and J. Arino. 2004. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 279:43614-43624. [DOI] [PubMed] [Google Scholar]
- 281.Vyas, V. K., C. D. Berkey, T. Miyao, and M. Carlson. 2005. Repressors Nrg1 and Nrg2 regulate a set of stress-responsive genes in Saccharomyces cerevisiae. Eukaryot. Cell 4:1882-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Vyas, V. K., S. Kuchin, and M. Carlson. 2001. Interaction of the repressors Nrg1 and Nrg2 with the Snf1 protein kinase in Saccharomyces cerevisiae. Genetics 158:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wadskog, I., A. Forsmark, G. Rossi, C. Konopka, M. Oyen, M. Goksor, H. Ronne, P. Brennwald, and L. Adler. 2006. The yeast tumor suppressor homologue Sro7p is required for targeting of the sodium pumping ATPase to the cell surface. Mol. Biol. Cell 17:4988-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Watanabe, Y., S. Miwa, and Y. Tamai. 1995. Characterization of Na+/H(+)-antiporter gene closely related to the salt-tolerance of yeast Zygosaccharomyces rouxii. Yeast 11:829-838. [DOI] [PubMed] [Google Scholar]
- 285.Watanabe, Y., Y. Shimono, H. Tsuji, and Y. Tamai. 2002. Role of the glutamic and aspartic residues in Na+-ATPase function in the ZrENA1 gene of Zygosaccharomyces rouxii. FEMS Microbiol. Lett. 209:39-43. [DOI] [PubMed] [Google Scholar]
- 286.Wells, K. M., and R. Rao. 2001. The yeast Na+/H+ exchanger Nhx1 is an N-linked glycoprotein. Topological implications. J. Biol. Chem. 276:3401-3407. [DOI] [PubMed] [Google Scholar]
- 287.Wiebe, C. A., C. Rieder, P. G. Young, P. Dibrov, and L. Fliegel. 2003. Functional analysis of amino acids of the Na+/H+ exchanger that are important for proton translocation. Mol. Cell. Biochem. 254:117-124. [DOI] [PubMed] [Google Scholar]
- 288.Wieland, J., A. M. Nitsche, J. Strayle, H. Steiner, and H. K. Rudolph. 1995. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 14:3870-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Withee, J. L., R. Sen, and M. S. Cyert. 1998. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA1. Genetics 149:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wright, M. B., J. Ramos, M. J. Gomez, K. Moulder, M. Scherrer, G. Munson, and R. F. Gaber. 1997. Potassium transport by amino acid permeases in Saccharomyces cerevisiae. J. Biol. Chem. 272:13647-13652. [DOI] [PubMed] [Google Scholar]
- 291.Ye, T., K. Elbing, and S. Hohmann. 2008. The pathway by which the yeast protein kinase Snf1p controls acquisition of sodium tolerance is different from that mediating glucose regulation. Microbiology 154:2814-2826. [DOI] [PubMed] [Google Scholar]
- 292.Ye, T., R. Garcia-Salcedo, J. Ramos, and S. Hohmann. 2006. Gis4, a new component of the ion homeostasis system in the yeast Saccharomyces cerevisiae. Eukaryot. Cell 5:1611-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yenush, L., S. Merchan, J. Holmes, and R. Serrano. 2005. pH-responsive, posttranslational regulation of the Trk1 potassium transporter by the type 1-related Ppz1 phosphatase. Mol. Cell. Biol. 25:8683-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yenush, L., J. M. Mulet, J. Arino, and R. Serrano. 2002. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 21:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:31079-31088. [DOI] [PubMed] [Google Scholar]
- 296.Zhou, X. L., B. Vaillant, S. H. Loukin, C. Kung, and Y. Saimi. 1995. YKC1 encodes the depolarization-activated K+ channel in the plasma membrane of yeast. FEBS Lett. 373:170-176. [DOI] [PubMed] [Google Scholar]