Abstract
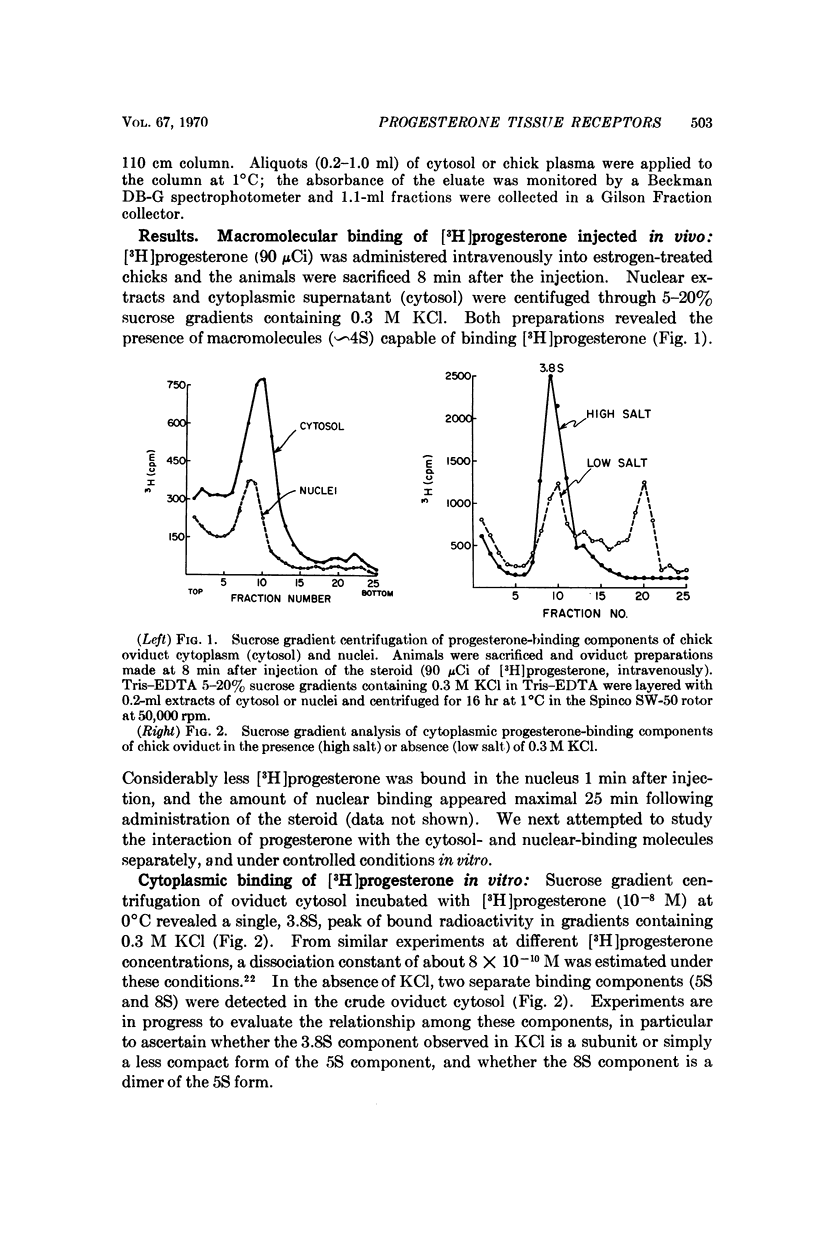
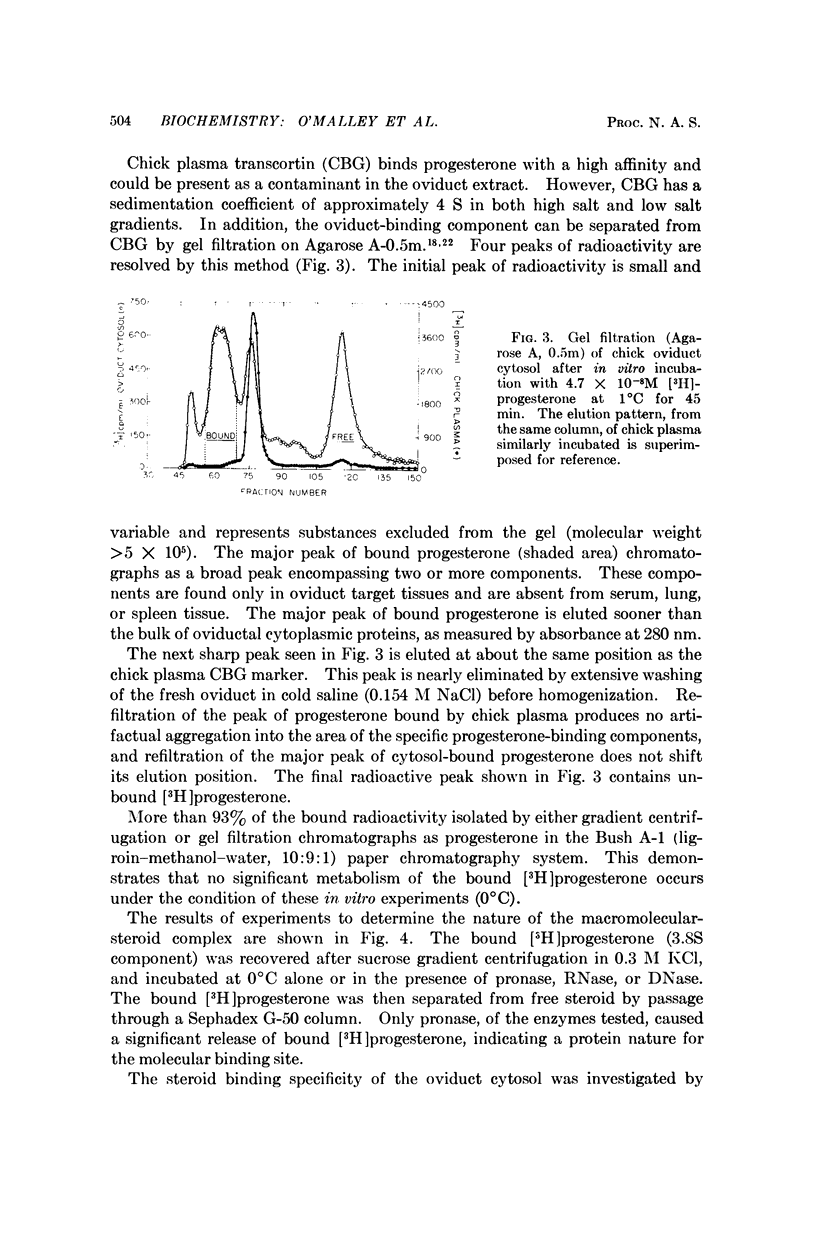
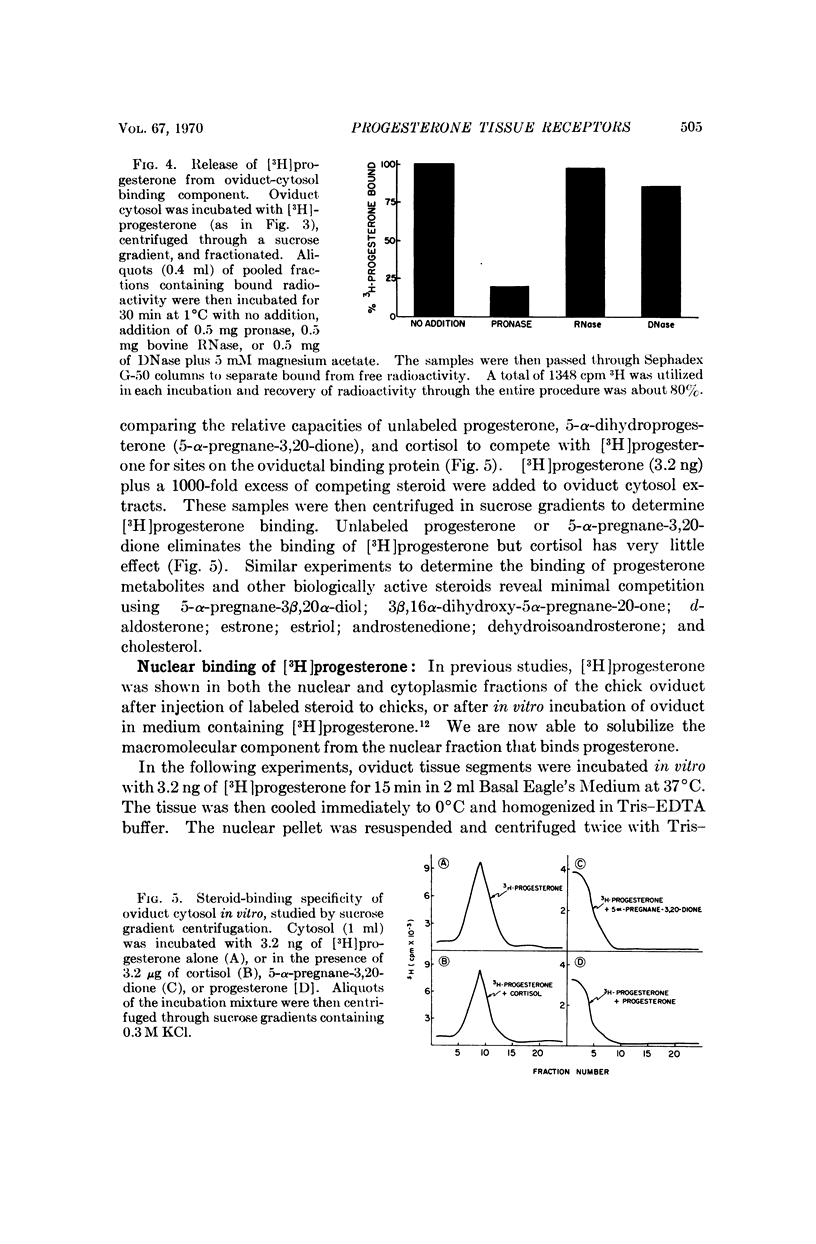
This report demonstrates that the chick oviduct, a specific target organ for progesterone, contains both cytoplasmic and nuclear macromolecules which bind progestins. These binding molecules can be clearly distinguished from transcortin by centrifugation through sucrose gradients of low ionic strength and by agarose gel filtration. The cytoplasmic progesterone-binding molecules also bind 5-α-pregnane-3,20-dione, but have significantly lower affinity for cortisol, estrone, or aldosterone. They are absent from blood and nontarget organs such as lung and spleen. The tissue-specific binding components appear to be heat-labile proteins with an average dissociation constant for progesterone of about 8 × 10-10 M at 2°C. These results are consistent with the identification of the progesterone-binding molecules as the functional hormone receptors. In further support of this concept is the finding that treatment of the chicks with estrogen coordinately induces a 20-fold increase in the number of progesterone-binding molecules and enhances the capacity of progesterone to induce avidin synthesis.
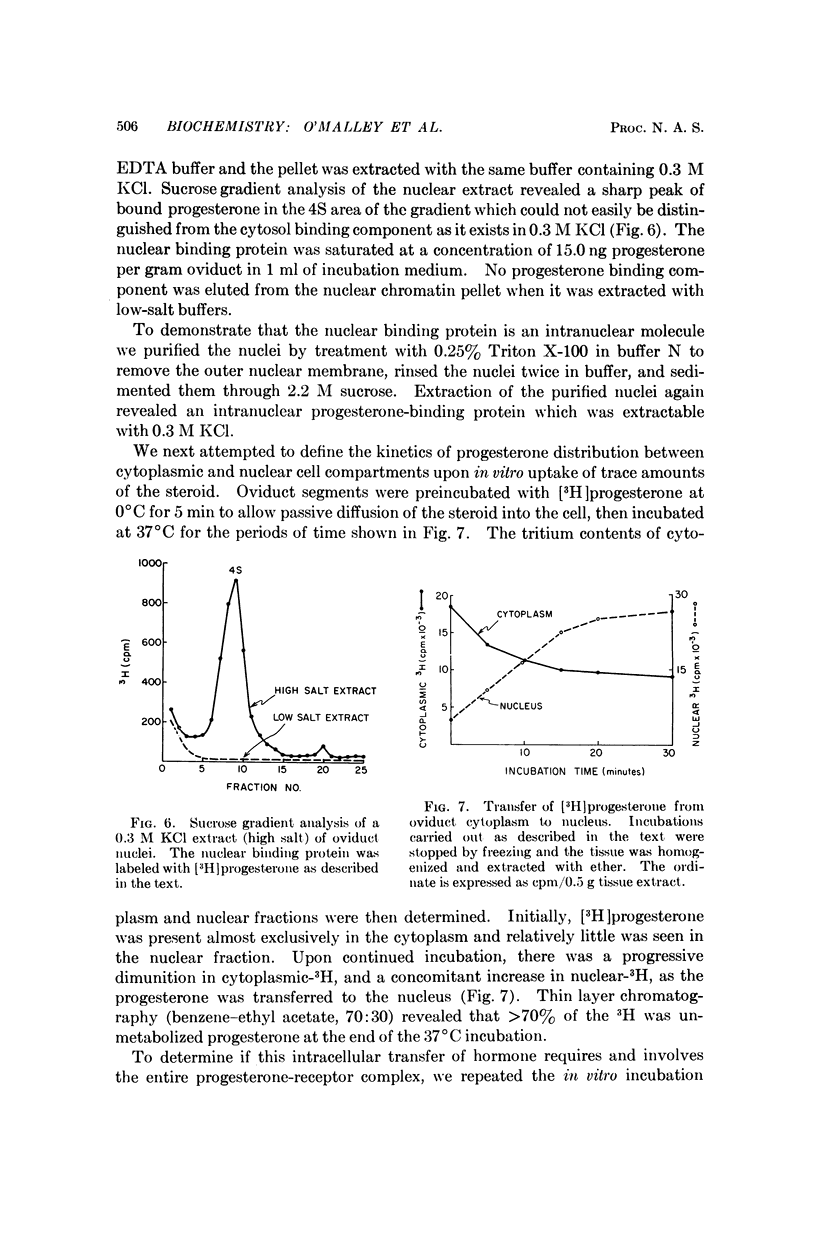
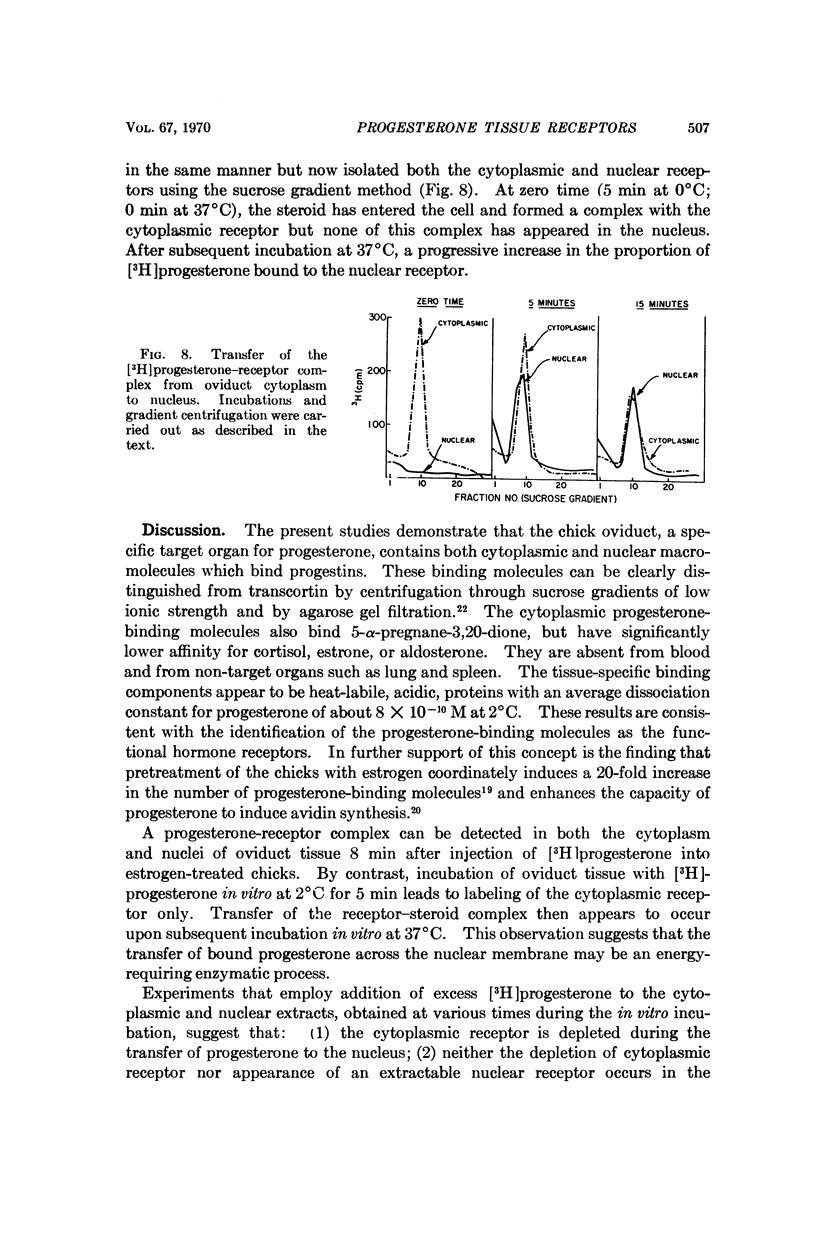
A progesterone-„receptor” complex can be detected in both the cytoplasm and nuclei of oviduct tissue after an injection of [3H]progesterone to estrogen-treated chicks. By contrast, incubation of oviduct tissue with [3H]progesterone in vitro at 2°C for 5 min leads to labeling of the cytoplasmic „receptor” only. Transfer of the „receptor”-steroid complex into the nucleus then appears to occur upon subsequent incubation in vitro at 37°C. This observation suggests that the transfer of bound progesterone across the nuclear membrane may be an energy-requiring enzymatic process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Edelman I. S., Fimognari G. M. On the biochemical mechanism of action of aldosterone. Recent Prog Horm Res. 1968;24:1–44. doi: 10.1016/b978-1-4831-9827-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Fang S., Anderson K. M., Liao S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-beta-hydroxy-5-alpha-androstan-3-one by rat ventral prostate in vivo and in vitro. J Biol Chem. 1969 Dec 25;244(24):6584–6595. [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Herman T. S., Fimognari G. M., Edelman I. S. Studies on renal aldosterone-binding proteins. J Biol Chem. 1968 Jul 25;243(14):3849–3856. [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenman S. G., O'Malley B. W. Progesterone action: regulation of avidin biosynthesis by hen oviduct in vivo and in vitro. Endocrinology. 1968 Jul;83(1):11–17. doi: 10.1210/endo-83-1-11. [DOI] [PubMed] [Google Scholar]
- Li R. T., Sato Y. Synthesis of the isomeric 3 beta-acetoxy-20-chloro-5 alpha-pregnanes. Steroids. 1969 Apr;13(4):451–456. doi: 10.1016/0039-128x(69)90055-5. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969 Dec;45(4):531–541. doi: 10.1677/joe.0.0450531. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W. In vitro hormonal induction of a specific protein (avidin) in chick oviduct. Biochemistry. 1967 Aug;6(8):2546–2551. doi: 10.1021/bi00860a036. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Korenman S. G. Estrogen stimulation of synthesis of specific proteins and RNA polymerase activity in the immature chick oviduct. Biochim Biophys Acta. 1967 Aug 22;145(1):204–207. doi: 10.1016/0005-2787(67)90679-x. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L. Progesterone-induced synthesis of a new species of nuclear RNA. Endocrinology. 1969 Jan;84(1):63–68. doi: 10.1210/endo-84-1-63. [DOI] [PubMed] [Google Scholar]
- Shyamala G., Gorski J. Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. J Biol Chem. 1969 Mar 10;244(5):1097–1103. [PubMed] [Google Scholar]
- Toft D., Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D., Shyamala G., Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1740–1743. doi: 10.1073/pnas.57.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]