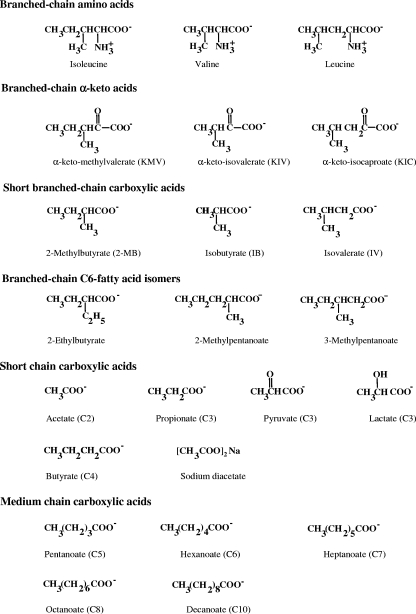
Abstract
Listeria monocytogenes is a food-borne pathogen that grows at refrigeration temperatures and increases its content of anteiso-C15:0 fatty acid, which is believed to be a homeoviscous adaptation to ensure membrane fluidity, at these temperatures. As a possible novel approach for control of the growth of the organism, the influences of various fatty acid precursors, including branched-chain amino acids and branched- and straight-chain carboxylic acids, some of which are also well-established food preservatives, on the growth and fatty acid composition of the organism at 37°C and 10°C were studied in order to investigate whether the organism could be made to synthesize fatty acids that would result in impaired growth at low temperatures. The results indicate that the fatty acid composition of L. monocytogenes could be modulated by the feeding of branched-chain amino acid, C4, C5, and C6 branched-chain carboxylic acid, and C3 and C4 straight-chain carboxylic acid fatty acid precursors, but the growth-inhibitory effects of several preservatives were independent of effects on fatty acid composition, which were minor in the case of preservatives metabolized via acetyl coenzyme A. The ability of a precursor to modify fatty acid composition was probably a reflection of the substrate specificities of the first enzyme, FabH, in the condensation of primers of fatty acid biosynthesis with malonyl acyl carrier protein.
Listeriosis is a severe and life-threatening human infection encompassing meningoencephalitis, meningitis, focal infections in the immunocompromised, and stillbirths and neonatal sepsis due to infection of pregnant women (2). The disease is caused by the Gram-positive food-borne pathogen Listeria monocytogenes, which is responsible for common-source and sporadic disease involving a variety of different foods (27). Listeriosis has a high fatality rate (24). The U.S. Department of Agriculture has a zero tolerance policy for L. monocytogenes in ready-to-eat products, and high costs are associated with product recalls.
L. monocytogenes has a remarkably low minimum growth temperature, e.g., −0.1°C (34), and thus the organism can multiply to dangerous levels when food is kept at refrigeration temperatures. We are interested in the molecular mechanisms of L. monocytogenes psychrotolerance, with a view to applying this knowledge to improve the control of the growth of the organism. Although the adaptations involved in low-temperature tolerance are global in scope, we have focused on changes in fatty acid composition that result in homeoviscous adjustments of membrane fluidity (31, 36). L. monocytogenes has a fatty acid composition that is dominated to an unusual extent (90% or more) by branched-chain fatty acids (BCFAs); the major fatty acids are anteiso-C15:0, anteiso-C17:0, and iso-C15:0. Numerous studies have shown that the major change in fatty acid composition when L. monocytogenes is grown at low temperatures is an increase in the content of anteiso-C15:0 fatty acid to 65% or more of the total (1, 12, 23, 25, 26, 28). Two cold-sensitive mutants with Tn917 insertions in the branched-chain α-keto acid dehydrogenase gene complex (bkd) were deficient in BCFAs, grew poorly at low temperatures, and had decreased membrane fluidity; all of these defects could be restored by growth in the presence of 2-methylbutyrate (2-MB), a precursor of odd-numbered anteiso fatty acids, including anteiso-C15:0 fatty acid (1, 7, 13, 37). We believe that anteiso-C15:0 fatty acid imparts fluidity to the cytoplasmic membrane, as revealed by its low phase transition temperature in model phospholipids (18) and disruption of the close packing of fatty acyl chains (21, 35).
The amino acids isoleucine, leucine, and valine are the starting points for the biosynthesis of odd-numbered anteiso, odd-numbered iso, and even-numbered iso fatty acids, respectively (18, 37). The amino acids are converted to their corresponding α-keto acid derivatives through the activity of branched-chain amino acid transaminase. Branched-chain α-keto acid dehydrogenase (Bkd) then converts these α-keto compounds to branched-chain acyl coenzyme A (acyl-CoA) primers of fatty acid biosynthesis (18). These primers are then used to initiate fatty acid biosynthesis through the activity of β-ketoacyl-acyl carrier protein synthase III (FabH), which prefers branched-chain acyl-CoAs to acetyl-CoA as substrates (4, 22, 32). β-Keto-acyl carrier protein synthase II (FabF) is responsible for subsequent rounds of elongation until the acyl chain reaches 14 to 17 carbon atoms (36).
We wished to ascertain whether we could manipulate the fatty acid composition of L. monocytogenes by feeding precursors that favored the production of fatty acids other than anteiso-C15:0 and thereby inhibit the growth of the organism, especially at low temperatures. Kaneda (15, 16) has grouped Bacillus subtilis fatty acids into four pairs based on the precursors from which they are generated, i.e., anteiso-C15:0 and C17:0 from isoleucine, iso-C15:0 and C17:0 from leucine, iso-C14:0 and C16:0 from valine, and n-C14:0 and n-C16:0 from acetate or butyrate. The proportions of the fatty acids could be modulated by precursor feeding. We have studied the effects of feeding the potential fatty acid precursors branched-chain amino acids, branched-chain α-keto acids, short branched-chain carboxylic acids, short straight-chain carboxylic acids, medium-length straight-chain carboxylic acids, branched-chain C6 carboxylic acids, and sodium diacetate (Fig. 1) on the growth and fatty acid composition of L. monocytogenes. Various short-chain carboxylic acids are used as food preservatives (5, 8, 29), and it was of interest to see whether any of them had an effect on the fatty acid composition of L. monocytogenes. Precursors giving rise to C5 and C6 branched-chain acyl-CoA derivatives, propionate, and butyrate had significant impacts on growth and fatty acid composition. Acetate and precursors that were metabolized to acetyl-CoA had minor effects on fatty acid composition, indicating that their preservative action is not due to effects on fatty acid composition.
FIG. 1.
Structures of potential fatty acid precursors.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
L. monocytogenes strain 10403S was grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI) at 37°C and 10°C. For growth studies and fatty acid analysis, a 5-ml overnight starter culture without supplements was prepared, and 0.5 ml of the overnight culture was used to inoculate 100 ml of BHI broth with and without supplements in a 250-ml Erlenmeyer flask. The culture was grown with shaking at 200 rpm. BHI broth was supplemented with a variety of amino acids (l-isoleucine, l-leucine, l-valine [100 mM each]), short branched-chain carboxylic acids (2-MB, isobutyrate [IB], isovalerate [IV] [100 mM each]), α-keto carboxylic acids (α-keto methylvalerate [KMV], α-keto isovalerate [KIV], α-keto isocaproate [KIC] [10 mM each]), short-chain carboxylic acids (sodium acetate [C2], sodium lactate [C3], sodium propionate [C3], sodium pyruvate [C3], sodium butyrate [C4], and valeric acid [C5] at 100 mM), medium straight-chain carboxylic acids (caproic acid [C6], enanthic acid [C7], caprylic acid [C8], and capric acid [C10] at 25 mM each), sodium diacetate alone at 7.04 mM, 21.2 mM, 35.21 mM, and 100 mM, and sodium diacetate at 100 mM plus 250 mM lactate (Fig. 1). One hundred millimolar concentrations of C6-branched-chain carboxylic acids (2-ethylbutyrate, 2-methylpentanoate, and 3-methylpentanoate) were studied for their effects on growth and fatty acid composition (Fig. 1). All supplements were added to the BHI medium as filter-sterilized solutions. The pH of BHI medium was adjusted to 7.0 with 10 M NaOH and 5 M HCl. Growth was monitored by measuring the optical density at 600 nm (OD600) using a Beckman DU-65 spectrophotometer. Growth experiments were carried out on a minimum of two separate occasions, and results of representative experiments are shown.
Fatty acid analysis.
Cells were harvested in the mid-exponential phase of growth (OD600, 0.5 to 0.7) by centrifugation at 3,000 × g and 4°C for 15 min, and the pellet was washed three times with distilled water. The fatty acids in the cells (30 to 40 mg [wet weight]) were saponified and methylated, and the methyl ester mixtures were separated using an Agilent 5890 dual tower gas chromatograph (29a). Fatty acids were identified by the MIDI microbial identification system (Sherlock 4.5 microbial identification system) (37, 38). This analysis was performed at Microbial ID (Newark, DE). Minor fatty acids (<0.3% of the total) are not reported in the tables.
Calculation of a theoretical lipid phase transition temperature.
An attempt was made to obtain an estimate of the fluidity of the various fatty acid compositions generated by the inclusion of fatty acid precursors in the medium through estimation of a theoretical lipid phase transition temperature, calculated as [[sum] (S × %)/100]/N, where S is the standard phase transition temperature (°C) of the specific fatty acid (−16.5 for anteiso-C15:0, 7.6 for anteiso-C17:0, 6.5 for iso-C14:0, 6.5 for iso-C15:0, 22 for iso-C16:0, 27 for iso-C17:0, 24 for n-C14:0, and 41.5 for n-C16:0) (18); % is the percentage (weight/weight) of the specific fatty acid in the fatty acid composition profile; and N is the number of standard fatty acids used in the calculation.
Chemicals.
All of the chemicals were obtained from commercial sources (Sigma-Aldrich, St. Louis, MO) and were used without further purification. Sodium diacetate was a generous gift from the Niacet Corporation, Niagara Falls, NY.
RESULTS
Branched-chain amino acids and short branched-chain carboxylic acids had similar impacts on fatty acid composition, but carboxylic acids were more growth inhibitory.
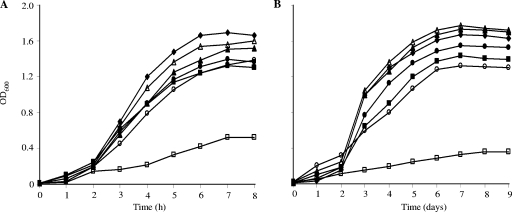
High concentrations of either l-isoleucine, l-leucine, or l-valine (100 mM) were used to supplement BHI medium at 37 and 10°C. At 37°C, each of the amino acids depressed the growth rate and the final turbidity achieved to a modest extent (Fig. 2). However, at 10°C, 100 mM l-isoleucine stimulated growth slightly, whereas growth in the presence of the other amino acids was slightly diminished from that in unsupplemented BHI broth.
FIG. 2.
Growth of L. monocytogenes in the presence and absence of branched-chain amino acids and short branched-chain carboxylic acids at 37°C (A) and 10°C (B), respectively. The supplements used are indicated as follows: ⧫, no supplement; ▪, leucine; ▴, isoleucine; •, valine; ▵, 2-MB; ○, IB; □, IV.
The fatty acid composition of cells grown in unsupplemented BHI medium at 37°C (Table 1) is compatible with previous reports from our laboratory (37) where the three major fatty acids were anteiso-C15:0 (46.6%), anteiso-C17:0 (34.7%), and iso-C15:0 (9.3%). Growth in the presence of 100 mM l-isoleucine, the precursor of odd-numbered anteiso fatty acids, increased the amounts of anteiso-C15:0 (50.5%) and anteiso-C17:0 (47.3%), such that these two fatty acids accounted for 97% of the total fatty acids, and increased the ratio of anteiso fatty acids to iso fatty acids (anteiso/iso ratio) from 5.1 to 109.
TABLE 1.
Fatty acid composition of 10403S grown with branched-chain amino acids and short branched-chain carboxylic acids at 37°C and 10°C
| Temp and supplementa | % (wt/wt) of total fatty acidsb |
Anteiso/iso ratio | % BCFA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anteiso-C15:0 | Anteiso-C17:0 | Sum | Iso-C15:0 | Iso-C17:0 | Sum | Iso-C14:0 | Iso-C16:0 | Sum | C14:0 | C16:0 | Sum | |||
| 37°C | ||||||||||||||
| No supplement | 46.6 ± 1.3 | 34.7 ± 1.5 | 81.3 | 9.3 ± 0.3 | 2.6 ± 0.4 | 11.9 | 0.8 ± 0.5 | 3.3 ± 0.2 | 4.1 | 0.4 ± 0.0 | 1.2 ± 0.0 | 1.6 | 5.1 | 97 |
| Isoleucine | 50.5 ± 0.5 | 47.3 ± 0.3 | 97.8 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.6 | ND | 0.3 ± 0.5 | 0.3 | 0.2 ± 0.0 | 0.9 ± 0.0 | 1.1 | 109 | 99 |
| Leucine | 30.2 ± 1.2 | 13.0 ± 1.7 | 43.2 | 40.0 ± 1.5 | 9.3 ± 0.5 | 49.3 | 1.2 ± 0.3 | 2.8 ± 0.6 | 4 | 0.6 ± 0.1 | 1.6 ± 0.2 | 2.2 | 0.8 | 97 |
| Valine | 35.5 ± 1.4 | 17.9 ± 1.5 | 53.4 | 5.6 ± 0.4 | 1.5 ± 0.0 | 7.1 | 8.5 ± 1.4 | 27.2 ± 0.8 | 36 | 0.5 ± 0.0 | 1.5 ± 0.1 | 2 | 1.3 | 96 |
| 2-MB | 46.0 ± 1.0 | 41.4 ± 1.3 | 87.4 | 4.8 ± 0.2 | 3.0 ± 0.3 | 7.8 | 0.3 ± 0.1 | 1.3 ± 0.2 | 1.6 | 0.3 ± 0.0 | 1.3 ± 0.0 | 1.6 | 9.3 | 97 |
| IV | 25.6 ± 1.2 | 13.8 ± 0.2 | 39.4 | 36.8 ± 0.9 | 11.7 ± 0.7 | 48.5 | 3.0 ± 0.3 | 7.2 ± 0.5 | 10 | 0.3 ± 0.0 | 1.5 ± 0.2 | 1.8 | 0.7 | 98 |
| IB | 21.9 ± 1.9 | 8.9 ± 0.3 | 30.8 | 6.7 ± 0.3 | 2.1 ± 0.4 | 8.8 | 22.0 ± 1.0 | 38.4 ± 1.1 | 60 | 0.4 ± 0.1 | 0.9 ± 0.0 | 1.3 | 0.4 | 99.9 |
| 10°C | ||||||||||||||
| No supplement | 66.7 ± 0.3 | 8.8 ± 0.5 | 75.5 | 16.1 ± 0.1 | 0.7 ± 0.0 | 16.8 | 2.3 ± 0.3 | 1.8 ± 0.2 | 4.1 | 0.9 ± 0.1 | 0.4 ± 0.0 | 1.3 | 3.6 | 96 |
| Isoleucine | 78.7 ± 1.3 | 16.8 ± 1.6 | 95.5 | 0.5 ± 0.1 | ND | 0.5 | ND | 0.2 ± 0.0 | 0.2 | 0.7 ± 0.2 | 0.4 ± 0.0 | 1.1 | 136 | 96 |
| Leucine | 41.9 ± 0.3 | 3.7 ± 0.5 | 45.6 | 38.1 ± 1.9 | 1.2 ± 0.2 | 39.3 | 4.5 ± 1.1 | 1.6 ± 0.5 | 6.1 | 1.2 ± 0.3 | 0.3 ± 0.0 | 1.5 | 1 | 91 |
| Valine | 36.4 ± 1.2 | 4.8 ± 1.2 | 41.2 | 7.9 ± 1.1 | 0.4 ± 0.1 | 8.3 | 30.2 ± 1.2 | 15.3 ± 1.2 | 46 | 1.1 ± 0.2 | 0.4 ± 0.0 | 1.5 | 0.7 | 97 |
| 2-MB | 68.7 ± 1.6 | 22.0 ± 0.9 | 90.7 | 6.1 ± 0.1 | 0.5 ± 0.2 | 6.6 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.6 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.9 | 13 | 98 |
| IV | 41.3 ± 0.7 | 5.2 ± 0.6 | 46.5 | 37.2 ± 1.6 | 2.0 ± 0.2 | 39.2 | 3.6 ± 0.2 | 1.3 ± 0.4 | 4.9 | 0.6 ± 0.1 | 0.7 ± 0.2 | 1.3 | 1 | 91 |
| IB | 39.6 ± 1.4 | 6.6 ± 0.4 | 46.2 | 9.3 ± 1.4 | 0.8 ± 0.2 | 10.1 | 21.9 ± 1.0 | 15.5 ± 0.8 | 37 | 0.7 ± 0.2 | 0.4 ± 0.0 | 1.1 | 0.9 | 94 |
All supplements were used at 100 mM.
Values are means for at least two independent experiments ± standard deviations; some minor fatty acid components are not shown. ND, not detected. Each “Sum” column gives the sum of the values in the two columns immediately to the left.
The major change in the fatty acid composition of cells grown in unsupplemented BHI medium at 10°C from that of cells grown at 37°C was fatty acid shortening (Table 1), so that the proportion of anteiso-C15:0 increased to 66.7% of the total fatty acids at the expense of anteiso-C17:0. Supplementation with l-isoleucine resulted in a further increase in the level of anteiso-C15:0 to 78.7%, and together anteiso-C15:0 and anteiso-C17:0 accounted for 95.5% of the total fatty acids. The anteiso/iso ratio increased to a remarkable 136.
In cultures supplemented with l-leucine at 37°C, the precursor of odd-numbered iso fatty acids, iso-C15:0, became the major fatty acid (40%), the level of total anteiso fatty acids decreased from 81.3 to 43.2%, and the anteiso/iso ratio decreased to 0.8, compared to cells grown in unsupplemented BHI medium. In cells supplemented with l-leucine at 10°C, there were increases in the level of anteiso-C15:0 from 30.2% at 37°C to 41.9%, a decrease in the level of total odd-numbered iso fatty acids, and an increase in the anteiso/iso ratio from 0.8 to 1.0.
Supplementation of BHI medium at 37°C with l-valine increased the proportion of total even-numbered iso fatty acids to 36% from 4.1% and decreased that of the total anteiso fatty acids to 53.4% from 81.3%. At 10°C, total even-numbered iso fatty acids constituted 46% of total fatty acids, and total anteiso fatty acids constituted 41.2%. It was surprising that cells with this fatty acid composition could grow as well as they did at 10°C. Probably the high concentration of l-valine results in the production of large amounts of IB-CoA via Bkd, which competes with 2-MB-CoA, thereby preventing the normal increase in the level of anteiso-C15:0 that is typically observed as an adaptation to low-temperature growth (37, 38). However, it should be noted that there was a dramatic shift toward the shorter iso-C14:0 fatty acid at 10°C compared to 37°C (Table 1). Thus, the increased membrane fluidity offered by the shorter iso-C14:0 may allow growth at low temperatures, albeit slower growth than that achieved when the anteiso-C15:0 level is high.
The influence of 100 mM concentrations of the short branched-chain carboxylic acids on growth is shown in Fig. 2. IB was inhibitory, and IV was strongly inhibitory, at 37 and 10°C. However, 2-MB was not inhibitory.
The inclusion of 2-MB in the medium at 37°C increased the proportion of total anteiso fatty acids to 87.4% and the anteiso/iso ratio to 9.3 (Table 1). The effects were similar to, but more modest than, those of isoleucine. Isoleucine feeds into fatty acid biosynthesis via Bkd, whereas 2-MB bypasses this step (18, 37, 38). At 10°C, the level of total anteiso fatty acids increased to 90.7% from 75.5% and the anteiso/iso ratio rose to 13 from 3.6.
At 37°C, IV, a precursor of odd-numbered iso fatty acids increased their level to 48.5% of the total from 11.9% and decreased the level of total anteiso fatty acids to 39.4% from 81.3% and the anteiso/iso ratio to 0.7 from 5.1. At 10°C, IV increased the level of odd-numbered iso fatty acids to 39.2% of the total from 16.8% and decreased the level of total anteiso fatty acids to 46.5% from 75.5% and the anteiso/iso ratio to 1.0 from 3.6. Again, these results were similar to those obtained with l-leucine. However, once again, there was a temperature-dependent shift toward shorter fatty acids (i.e., a decrease in the iso-C17 level from 11.7 to 2.0%) (Table 1). Thus, it appears that when the bacterium is grown at cold temperatures, it changes toward shorter fatty acids in order to maintain membrane fluidity.
A precursor of even-numbered iso fatty acids, IB, boosted the proportion of total even-numbered iso fatty acids to 60% from 4.1% at 37°C, and to 37% from 4.1% at 10°C, and decreased the level of total anteiso fatty acids and the anteiso/iso ratio in a manner similar to that of l-valine at 10°C.
Effects of short straight-chain carboxylic acids on fatty acid composition.
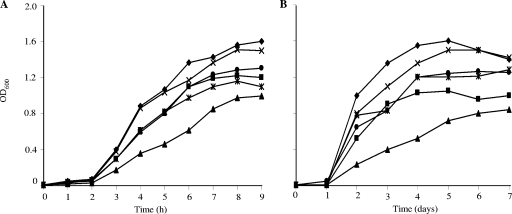
The effects of 100 mM concentrations of various short straight-chain carboxylic acids on growth were determined (Fig. 3). Acetate and lactate caused similar degrees of growth inhibition in that the growth rate was about three times lower in their presence than normal growth at 37°C, but they had lesser inhibitory effects at 10°C (Fig. 3). Diacetate, both alone and in combination with lactate, is used as a food preservative, and diacetate and lactate, alone and in combination, had inhibitory effects on growth at 37°C and 10°C (data not shown). Propionate was the most inhibitory straight-chain carboxylic acid both at 37°C and at 10°C. Pyruvate, an α-keto C3 carboxylic acid, was not very inhibitory.
FIG. 3.
Growth of L. monocytogenes in the presence and absence of short straight-chain carboxylic acids at 37°C (A) and 10°C (B). Symbols: ⧫, no supplement; ×, pyruvate; •, acetate; ▪, butyrate; ✴, lactate; ▴, propionate.
Acetate had only minor effects on fatty acid composition (Table 2). Diacetate and lactate, both alone and in combination, had only minor impacts on fatty acid composition (Table 2). Pyruvate, which, like lactate, is metabolized to acetyl-CoA, had minor impacts on fatty acid composition (data not shown). At 37°C, 100 mM propionate decreased the proportion of total anteiso fatty acids to 70% from 84.3% and resulted in the appearance of n-C13:0 at 10.6% (Table 2) in the fatty acid profile. In cells grown at 10°C in the presence of 100 mM propionate, the major change was an increase in the level of n-C13:0 to 23% (Table 2) of the total fatty acids. Again, it appears that shortening the fatty acid length may compensate somewhat for decreased levels of odd-numbered anteiso fatty acids for maintaining adequate membrane fluidity.
TABLE 2.
Influence of short straight-chain carboxylic acids on fatty acid composition
| Temp and supplementa | % (wt/wt) of total fatty acidsb |
Anteiso/iso ratio | % BCFA | % SCFA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anteiso-C15:0 | Anteiso-C17:0 | Sum | Iso-C15:0 | Iso-C17:0 | Sum | Iso-C14:0 | Iso-C16:0 | Sum | C13:0 | C17:0 | Sum | ||||
| 37°C | |||||||||||||||
| No supplement | 44.9 ± 0.0 | 39.4 ± 0.0 | 84.3 | 7.2 ± 0.0 | 3.1 ± 0.0 | 10.3 | 0.5 ± 0.0 | 2.7 ± 0.0 | 3.2 | ND | ND | ND | 6.2 | 97.8 | 2.2 |
| Acetate | 44.4 ± 1.4 | 27.6 ± 0.6 | 72 | 9.4 ± 1.3 | 2.9 ± 0.1 | 12 | 1.9 ± 0.5 | 6.9 ± 0.5 | 8.8 | ND | ND | ND | 3.4 | 93 | 7 |
| Propionate | 59.5 ± 0.3 | 10.0 ± 0.3 | 70 | 7.1 ± 0.2 | 0.7 ± 0.2 | 7.8 | 2.8 ± 0.2 | 2.1 ± 0.1 | 4.9 | 10.6 ± 1.7 | 2.9 ± 1.0 | 14 | 5.5 | 82 | 18 |
| Diacetate | 46.3 ± 1.3 | 28.3 ± 0.7 | 75 | 9.9 ± 0.5 | 3.1 ± 0.2 | 13 | 1.7 ± 0.3 | 6.5 ± 0.8 | 8.2 | ND | ND | ND | 3.5 | 96 | 4 |
| Diacetate + lactate | 50.7 ± 1.6 | 29.0 ± 1.2 | 80 | 8.9 ± 1.1 | 2.9 ± 0.8 | 12 | 1.3 ± 0.3 | 5.6 ± 1.5 | 6.9 | ND | ND | ND | 4.3 | 98 | 2 |
| 10°C | |||||||||||||||
| No supplement | 70.9 ± 0.1 | 5.7 ± 0.2 | 76.6 | 13.2 ± 1.0 | 0.4 ± 0.0 | 13.6 | 2.7 ± 0.0 | 1.4 ± 0.2 | 4.1 | ND | ND | ND | 5.2 | 94.3 | 5.7 |
| Acetate | 60.1 ± 1.8 | 5.7 ± 1.1 | 66 | 20.6 ± 1.3 | 0.7 ± 0.2 | 21 | 4.8 ± 0.2 | 2.4 ± 0.6 | 7.2 | ND | ND | ND | 2.3 | 94 | 6 |
| Propionate | 57.3 ± 0.1 | 3.9 ± 0.6 | 61 | 3.4 ± 0.6 | 0.3 ± 0.0 | 3.7 | 1.9 ± 0.2 | 0.5 ± 0.0 | 2.4 | 23.0 ± 1.2 | ND | 23.0 | 10 | 67 | 33 |
| Diacetate | 57.8 ± 1.8 | 5.9 ± 0.9 | 64 | 17.5 ± 1.9 | 1.0 ± 0.2 | 19 | 5.1 ± 0.2 | 2.2 ± 0.3 | 7.3 | ND | ND | ND | 2.5 | 90 | 11 |
| Diacetate + lactate | 58.6 ± 0.9 | 6.3 ± 0.5 | 65 | 17.2 ± 2.5 | 1.0 ± 0.1 | 18 | 4.7 ± 0.4 | 1.9 ± 0.3 | 6.6 | ND | ND | ND | 2.6 | 90 | 10 |
All supplements except lactate were used at 100 mM. Lactate was used at 250 mM.
Values are means for at least two independent experiments ± standard deviations; some minor fatty acid components are not shown. ND, not detected. Each “Sum” column gives the sum of the values in the two columns immediately to the left.
Butyrate was growth inhibitory and had the greatest impact on fatty acid composition of the short-chain carboxylic acids tested.
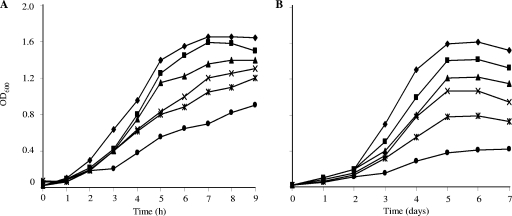
Butyrate inhibited growth and had the largest effect on fatty acid composition of the straight-chain carboxylic acids tested (Fig. 4; Table 3). At 37°C, 100 mM butyrate caused the level of total anteiso fatty acids to be reduced from 82.9% to 45% and that of total straight even-numbered fatty acids (n-C14:0 and n-C16:0) to increase from 1.3% to 37%. At 10°C, the inclusion of 100 mM butyrate in the medium decreased the level of total anteiso fatty acids from 76.2% to 58%, with n-C14:0 and n-C16:0 rising to 17% from 1%. However, the level of total straight-chain fatty acids (SCFAs) was about half of that at 37°C (37% at 37°C and 17% at 10°C), and the amount of n-C16:0 was much reduced (from 23.3% at 37°C to 1.8% at 10°C).
FIG. 4.
Growth of L. monocytogenes in the presence and absence (⧫) of different concentrations of butyrate at 37°C (A) and 10°C (B). Butyrate concentrations were 25 mM (▪), 50 mM (▴), 75 mM (×), 100 mM (✴), and 250 mM (•).
TABLE 3.
Influences of different concentrations of butyrate on fatty acid composition
| Temp and butyrate concn | % (wt/wt) of total fatty acidsa |
Anteiso/iso ratio | % BCFA | % SCFA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anteiso-C15:0 | Anteiso-C17:0 | Sum | Iso-C15:0 | Iso-C17:0 | Sum | Iso-C14:0 | Iso-C16:0 | Sum | C14:0 | C16:0 | Sum | ||||
| 37°C | |||||||||||||||
| None | 45.2 ± 0.0 | 37.7 ± 2.0 | 82.9 | 8.1 ± 0.9 | 3.0 ± 0.0 | 11.1 | 0.6 ± 0.0 | 2.9 ± 0.0 | 3.5 | 0.3 ± 0.0 | 1.0 ± 0.0 | 1.3 | 5.6 | 97.5 | 2.5 |
| 25 mM | 41.5 ± 1.1 | 28.9 ± 0.4 | 70 | 8.8 ± 0.4 | 3.0 ± 1.5 | 12 | 0.5 ± 0.1 | 2.7 ± 0.1 | 3.2 | 4.6 ± 1.5 | 8.2 ± 0.7 | 13 | 4.7 | 85.4 | 15 |
| 50 mM | 38.7 ± 1.7 | 23.6 ± 0.7 | 62 | 9.5 ± 0.5 | 3.2 ± 0.1 | 13 | 0.6 ± 0.2 | 2.9 ± 0.1 | 3.5 | 6.8 ± 1.5 | 12.6 ± 1.1 | 19 | 3.8 | 78.5 | 22 |
| 75 mM | 34.9 ± 1.9 | 17.2 ± 1.1 | 52 | 9.7 ± 0.8 | 2.8 ± 0.2 | 13 | 1.4 ± 0.2 | 3.1 ± 0.1 | 4.5 | 10.4 ± 1.8 | 18.3 ± 1.2 | 29 | 3 | 69.1 | 31 |
| 100 mM | 31.9 ± 1.8 | 13.2 ± 0.9 | 45 | 9.4 ± 0.8 | 2.5 ± 0.3 | 12 | 1.5 ± 0.0 | 2.9 ± 0.2 | 4.4 | 13.8 ± 1.7 | 23.2 ± 1.5 | 37 | 2.7 | 61.4 | 39 |
| 250 mM | 20.8 ± 0.9 | 4.4 ± 0.2 | 25 | 5.4 ± 1.0 | 0.8 ± 0.1 | 6.2 | 2.3 ± 0.1 | 1.8 ± 0.0 | 4.1 | 32.5 ± 0.9 | 27.8 ± 1.3 | 60 | 2.4 | 35.5 | 65 |
| 10°C | |||||||||||||||
| None | 68.6 ± 2.2 | 7.6 ± 1.6 | 76.2 | 15.2 ± 1.8 | 0.5 ± 0.0 | 15.7 | 2.3 ± 0.3 | 1.5 ± 0.1 | 4.1 | 0.7 ± 0.1 | 0.3 ± 0.0 | 1 | 3.8 | 96 | 4 |
| 25 mM | 58.8 ± 1.3 | 4.8 ± 0.4 | 64 | 16.3 ± 0.5 | 0.5 ± 0.2 | 17 | 2.3 ± 0.2 | 1.0 ± 0.2 | 3.3 | 7.5 ± 1.0 | 1.5 ± 0.1 | 9 | 3.2 | 83.7 | 16 |
| 50 mM | 54.9 ± 1.7 | 5.2 ± 0.2 | 60 | 17.0 ± 0.1 | 0.7 ± 0.2 | 18 | 2.5 ± 0.1 | 1.1 ± 0.3 | 3.6 | 11.0 ± 0.3 | 1.4 ± 0.5 | 12 | 2.8 | 80.9 | 19 |
| 75 mM | 53.8 ± 0.9 | 4.9 ± 0.4 | 59 | 12.8 ± 0.7 | 0.6 ± 0.1 | 13 | 2.4 ± 0.4 | 0.9 ± 0.0 | 3.3 | 13.1 ± 0.3 | 1.6 ± 0.2 | 15 | 3.5 | 75.4 | 25 |
| 100 mM | 52.1 ± 0.3 | 5.4 ± 0.6 | 58 | 11.7 ± 1.5 | 0.5 ± 0.0 | 12 | 2.0 ± 0.2 | 1.1 ± 0.1 | 3.1 | 15.0 ± 0.2 | 1.8 ± 0.5 | 17 | 3.8 | 72.8 | 27 |
| 250 mM | 50.9 ± 0.6 | 6.3 ± 0.8 | 57 | 9.4 ± 1.0 | 1.0 ± 0.2 | 10 | 1.6 ± 0.0 | 1.2 ± 0.3 | 2.8 | 18.6 ± 0.4 | 3.0 ± 0.0 | 22 | 4.3 | 70.4 | 30 |
Values are means for at least two independent experiments ± standard deviations; some minor fatty acid components are not shown. Each “Sum” column gives the sum of the values in the two columns immediately to the left.
The impact of a range of butyrate concentrations on growth and fatty acid composition was determined (Fig. 4; Table 3). A clear dose response was seen in growth inhibition at both 37 and 10°C. Also, a dose response was seen in the percentage of SCFAs, which rose from 2.5% in the absence of butyrate to 65% at 37°C in the presence of 250 mM butyrate (Table 3).
Butyrate supplementation provided a pronounced demonstration that when the organism is forced to utilize a less-than-desirable fatty acid precursor, membrane fluidity is maintained by shortening the chain length at lower temperatures. For example, at 10°C, the maximum level of total SCFAs was 30% in the presence of 250 mM butyrate. Little n-C16:0 was produced at 10°C (Fig. 4; Table 3). The level of total anteiso fatty acids decreased only to 57%, and that of anteiso-C15:0 from 68.6% to 50.9%, in cultures supplemented with 250 mM butyrate. Nevertheless, the organism was able to maintain surprisingly rapid growth with a significant amount of SCFAs at 10°C.
Medium-length straight-chain carboxylic acids were growth inhibitory but had minor effects on fatty acid composition.
The medium-chain-length straight-chain carboxylic acids (C5 to C10) were effective inhibitors of growth at both 37 and 10°C; inhibition was proportional to the number of carbon atoms, and C10 was the most inhibitory (data not shown). However, the medium-chain-length carboxylic acids (at a 25 mM concentration) had almost no effect on the fatty acid composition of the organism at either 37 or 10°C (data not shown).
Branched-chain C6 carboxylic acids were growth inhibitory and caused the production of “unnatural” fatty acids.
It is clear that the precursors of the natural BCFAs, 2-methylbutyryl-CoA, isobutyryl-CoA, and isovaleryl-CoA, which are normally produced through the activity of Bkd, can be produced from the corresponding short branched-chain carboxylic acids 2-methylbutyrate (C5), isobutyrate (C4), and isovalerate (C5), respectively. Consequently, it was of interest to study the effects of C6 branched-chain carboxylic acids on the growth and fatty acid composition of L. monocytogenes. The compounds tested were 2-ethylbutyrate, 3-methylpentanoate, and 2-methylpentanoate (Fig. 1). Previous studies have shown that new sets of BCFAs were produced after B. subtilis was fed with various branched-chain C6 carboxylic acids (17).
2-Ethylbutyrate (100 mM) was the least inhibitory of the compounds (the growth rate was 93 and 88% of the control rate at 37 and 10°C, respectively). 2-Methylpentanoate and 3-methylpentanoate gave growth rates 58 and 77% of the control growth rate, respectively, at 37°C, and 82 and 67% of the control growth rate at 10°C.
Growth in the presence of the C6 potential fatty acid precursors led to the appearance of novel fatty acids in the Glc traces at both 37°C and 10°C. The inclusion of 2-ethybutyrate at 37°C resulted in two new fatty acids with retention times of 2.72 min (18.9% of total) and 3.36 min (4.3% of total), which are postulated to be the methyl esters of 12-ethyltetradecanoic acid (ethyl-branched C16) and 14-ethylhexadecanoic acid (ethyl-branched C18), respectively (Table 4). Growth in the presence of 2-methylpentanoate at 37°C resulted in new peaks at 2.69 min (20.4% of total) and 3.32 min (2.2% of total), which are postulated to represent 12-methylpentadecanoic acid (methyl-branched C14) and 14-mehylheptadecanoic acid (methyl-branched C16), respectively. In cultures grown at 10°C, there was an increase in the level of anteiso-C15:0 fatty acid, as well as the predicted shift toward the shorter of the novel fatty acids, and a decrease in the total percentage of the novel fatty acids in the profile (Table 4).
TABLE 4.
Influence of branched-chain C6 carboxylic acid precursors on L. monocytogenes fatty acid composition
| Fatty acid (retention time [min]) | % of fatty acid composition in: |
|||||
|---|---|---|---|---|---|---|
| BHI |
BHI + 2-ethylbutyrate |
BHI + 2-methylpentanoate |
||||
| 37°C | 10°C | 37°C | 10°C | 37°C | 10°C | |
| Anteiso-C15:0 (2.42) | 45.0 | 71.0 | 25.0 | 55.8 | 25.4 | 49.2 |
| Anteiso-C17:0 (3.05) | 39.0 | 6.0 | 33.8 | 9.2 | 32.8 | 5.3 |
| Iso-C15:0 (2.34) | 7.0 | 12.0 | 7.2 | 13.1 | 7.9 | 20.9 |
| Iso-C17:0 (3.02) | 3.1 | 0.4 | 5.3 | 0.4 | 5.8 | 0.5 |
| Iso-C14:0 (2.09) | 1.0 | 3.0 | 0.4 | 1.0 | 0.4 | 2.6 |
| Iso-C16:0 (2.71) | 2.8 | 1.4 | 2.8 | 1.1 | 3.5 | 0.9 |
| 12-Ethyltetradecanoic acida (2.72) | NDb | ND | 18.9 | 14.8 | ND | ND |
| 14-Ethylhexadecanoic acida (3.36) | ND | ND | 4.3 | 0.4 | ND | ND |
| 10-Methyltridecanoic acida (2.07) | ND | ND | ND | ND | 0.25 | 7.3 |
| 12-Methylpentadecanoic acida (2.69) | ND | ND | ND | ND | 20.4 | 9.2 |
| 14-Methylheptadecanoic acida (3.32) | ND | ND | ND | ND | 2.2 | ND |
Tentative identification.
ND, not detected.
Insights into the impact of changes in fatty acid composition on membrane fluidity.
The calculated phase transition temperature of the fatty acids of cells grown at 37°C in BHI medium was −0.49°C and decreased to −1.42°C at 10°C, a decrease that was correlated with the increase in the proportion of anteiso-C15:0 (Table 5). The cold-sensitive, BCFA-deficient cld-1 and cld-2 mutants had considerably higher calculated phase transition temperatures of 2.80 and 3.08, respectively, when grown in BHI medium (1, 37), thus providing evidence for the validity of this approach. The increased phase transition temperature for the cold-sensitive mutants was reflective of the deficiency in anteiso fatty acids and the increased content of SCFAs and even-numbered iso fatty acids in these strains (1, 37). The inclusion of l-isoleucine or 2-MB in the medium led to a lower calculated phase transition temperature with strain 10403S (Table 5). Calculated phase transition temperatures were considerably higher for cells grown in the presence of l-leucine or IV. The expected lower fluidities of the membranes in cells grown under these conditions likely account for the lower growth rates observed when these compounds are included in the medium.
TABLE 5.
Calculated average phase transition temperatures of cell membrane fatty acid composition under different culture conditions
| Supplementa or mutant | Culture temp (°C) | Phase transition temp (°C) |
|---|---|---|
| No supplement | 37 | −0.49 |
| 10 | −1.42 | |
| cld-1 mutant (bkdB) | 37 | 2.80 |
| cld-2 mutant (lpd) | 37 | 3.08 |
| Isoleucine | 37 | −0.76 |
| 10 | −1.91 | |
| Leucine | 37 | 0.45 |
| 10 | 0.03 | |
| Valine | 37 | 0.29 |
| 10 | −0.53 | |
| 2-MB | 37 | −0.51 |
| 10 | −1.48 | |
| IV | 37 | 1.31 |
| 10 | −0.06 | |
| IB | 37 | 0.67 |
| 10 | −0.49 | |
| Butyrate | 37 | 1.31 |
| 10 | −0.32 |
For all supplements, the concentration was 100 mM.
Cultures supplemented with either l-valine or isobutyrate at 10°C had a calculated phase transition temperature of −0.53 or −0.49°C, respectively. This is higher than the phase transition temperatures for unsupplemented or 2-methylbutyrate-supplemented cultures but lower than those for cultures supplemented with l-leucine (0.03°C) or isovalerate (−0.06°C), indicating a more-fluid membrane, which is compatible with the more-rapid growth observed in the presence of l-valine or IB than in the presence of l-leucine or IV. Growth in the presence of 100 mM butyrate at 37°C increased the calculated transition temperature to 1.31°C compared to that for unsupplemented cultures (−0.49°C). However, at 10°C, the calculated phase transition temperature was −0.32°C, compared to −1.42°C for cells from unsupplemented BHI medium. These data clearly show that the concomitant shift to shorter fatty acids observed at lower temperatures provides adequate compensation for the increased SCFA content.
DISCUSSION
Additions of branched-chain amino acids and short branched-chain carboxylic acids (100 mM) to BHI medium had similar effects on L. monocytogenes fatty acid composition. l-Isoleucine and 2-MB increased anteiso-C15:0 and anteiso-C17:0 levels; l-leucine and IV increased iso-C15:0 and iso-C17:0 levels; and l-valine and IB increased iso-C14:0 and iso-C16:0 levels. Kaneda (15, 16) pointed out that the fatty acids of B. subtilis could be grouped into four pairs (anteiso-C15:0 and anteiso-C17:0, iso-C15:0 and iso-C17:0, iso-C14:0 and iso-C16:0, C14:0 and C16:0). The addition of branched-chain amino acids or short branched-chain carboxylic acids to glucose-yeast extract medium increased the synthesis of the specific pairs of fatty acids structurally related to the added substrate, and decreased the synthesis of the other fatty acids, in B. subtilis. Also, the feeding of leucine or valine to Streptomyces glaucescens led to increases in the contents of the fatty acids for which they were precursors (9). Clearly, a similar situation exists in L. monocytogenes. The impact of low temperatures on this kind of feeding experiment was not examined in the studies of Kaneda (15, 16) or in those of Han et al. (9). The branched-chain amino acids and short branched-chain carboxylic acids produce branched-chain CoA fatty acid primers that are the substrates for FabH by different routes. FabH is the critical enzyme for the formation of acyl carrier protein derivatives (ACPs) from acyl-CoA precursors, and the ACPs serve as the primers for fatty acid biosynthesis (4, 36). Acyl-CoA precursors are formed via branched-chain α-keto acid dehydrogenase in the case of the branched-chain amino acids, and by the formation of CoA derivatives of the free short branched-chain carboxylic acids. l-Isoleucine had a greater impact on the anteiso/iso ratio than 2-MB. The impressive impact of l-isoleucine on the fatty acid composition of L. monocytogenes (38) and B. subtilis (20) has been noted previously. However, while the amino acids had moderate growth-inhibitory activity, the short branched-chain carboxylic acids had significantly more growth-inhibitory activity. In particular, IV was strongly inhibitory to growth, yet the fatty acid compositions produced by it and leucine were very similar. This indicates that the inhibitory effect of IV is not due primarily to its effect on fatty acid composition.
All indications are that in the absence of abnormal culture conditions, L. monocytogenes increases its content of anteiso-C15:0 fatty acid at low temperatures (Table 1) (1, 37, 38). However, by including high concentrations of leucine and valine (and of IV and IB) in the growth medium, the organism could be “forced” to modify its fatty acid compositions in such a way as to dramatically increase the levels of odd-numbered and even-numbered iso fatty acids, respectively. This is expected to be due to the increase in the concentration of isovaleryl-CoA or isobutyryl-CoA over that of the preferred substrate of FabH, 2-methylbutyryl-CoA (4, 32). The content of anteiso-C15:0 dropped to 41.9% in the presence of 100 mM l-leucine at 10°C, and to 36.4% in the presence of 100 mM l-valine, from 66.7% in unsupplemented BHI medium. Nevertheless, the cultures were able to maintain a growth rate nearly 50% of that in unsupplemented medium under these conditions. The use of the calculated average phase transition temperatures for these fatty acid compositions (Table 5)—−1.42°C for growth in BHI medium alone, 0.03°C for BHI medium plus 100 mM leucine, and −0.53°C for BHI medium plus 100 mM valine—shows that these likely reductions in membrane fluidity are consistent with the lower growth rates observed but obviously are not substantial enough to abolish L. monocytogenes growth completely. l-Leucine was the most growth-inhibitory amino acid, consistent with the predicted least-fluid membrane (Table 5).
Acetate, sodium diacetate, lactate, and pyruvate are all food preservatives and inhibited growth significantly at both temperatures tested but had only minor impacts on fatty acid composition. These compounds are metabolized via acetyl-CoA. Although acetyl-CoA is a precursor of SCFA in Escherichia coli, it does not appear to be an effective precursor of SCFA in L. monocytogenes (32) or B. subtilis (19). This is most likely due to the very low utilization of acetyl-CoA by FabH from BCFA-containing bacteria (4, 32). Findings with these compounds suggest that their inhibitory effects on growth are only minimally associated with fatty acid composition, as the calculated phase transition temperatures shift slightly toward positive temperatures (data not shown). Short-chain carboxylic acids, such as acetate, propionate, and lactate, are well-known food preservatives (5, 8, 29). Their antimicrobial activity is highly pH dependent. It is believed that in the undissociated form these organic acids readily penetrate the cytoplasmic membrane. Once in the cytoplasm, the acid dissociates, releasing protons, which must be exported to avoid acidification of the cytoplasm. This is thought to deplete energy through consumption of ATP to extrude the protons. The short-chain carboxylic acids are also thought to dissipate the proton motive force and interfere with amino acid transport (5, 8, 29). Therefore, it may be a combination of effects that decreases growth rates in the presence of these short-chain carboxylic acids. Surprisingly, these potential uncoupling effects induced by organic acids appeared to be completely absent when 2-MB (which is also a short-chain, albeit a branched-chain carboxylic acid) was added to growth medium, where it actually enhanced growth (Table 1).
Tokarskyy and Marshall (33) have shown that exposure to monolaurin leads to the incorporation of C12:0 fatty acid into the L. monocytogenes membrane. Also, Juneja and Davidson (14) showed that L. monocytogenes could incorporate long-chain fatty acids, such as C14:0, C16:0, C18:0, and C18:1, into the membrane and that this altered the susceptibility of the organism to various antimicrobials. However, at one and two carbon atoms longer, respectively, than acetate, propionate and butyrate were both growth inhibitory and significantly impacted fatty acid composition, giving rise to n-C13:0 and to n-C14:0 and n-C16:0, respectively. The preferred substrate for L. monocytogenes FabH is 2-methylbutyryl-CoA; FabH also exhibits significant activities with the related compounds isovaleryl-CoA and isobutyryl-CoA (32). Given the observations in the present study, we can speculate that L. monocytogenes FabH can utilize butyryl-CoA and, to a lesser extent, propionyl-CoA as substrates. The presence of 100 mM butyrate in the culture medium had considerable impact on the fatty acid composition of L. monocytogenes, particularly at 37°C, increasing the SCFA level to 37% from 1.3% in its absence. Interestingly, butyrate had significantly less impact on fatty acid composition at 10°C. Compared to that with growth at 37°C in the presence of 100 mM butyrate, the percentage of SCFA dropped to 17% and the percentage of anteiso fatty acids increased to 58% from 45%. This is probably a reflection of the increased preference of FabH for 2-methylbutyrate at lower temperatures, which we have described previously (32); also, a concomitant shift toward shorter-chain fatty acids can be observed at 10°C. In addition, C6 branched-chain carboxylic acids could serve as primers of fatty acid biosynthesis, resulting in the incorporation of “unnatural” fatty acids into the membrane. Similar findings have been reported by Kaneda (17) for B. subtilis, and the relative activities were highest for 2-ethylbutyrate, intermediate for 2-methylpentanoate, and lowest for 3-methylpentanoate.
Clearly, the CoA derivatives of the various carboxylic acids described above can be utilized by FabH. Inclusion of propionate or butyrate in the growth medium led to the production of odd-numbered or even-numbered SCFAs derived from these precursors, respectively. In order to participate in fatty acid biosynthesis, the carboxylic acids must be converted to CoA derivatives. Butyrate kinases have been described for a number of bacterial species (6), and they catalyze the phosphorylation of butyrate at the expense of ATP. Butyryl phosphate is then converted to butyryl-CoA by phosphotransbutyrylase. Butyrate kinase is closely related to acetate and propionate kinases (3, 30), and these enzymes are members of the ASKHA superfamily of phosphotransferases. There is a gene, buk, that appears to encode butyrate kinase in the bkd gene cluster of L. monocytogenes (37). The fatty acid composition of L. monocytogenes is clearly modified by the short branched-chain carboxylic acids. It is possible that butyrate kinase is responsible for the phosphorylation of these substrates in L. monocytogenes, given that Clostridium acetobutylicum butyrate kinase can phosphorylate isobutyrate, isovalerate, and propionate, among other substrates (10).
Furthermore, these short-chain carboxylic acid fatty acid precursors must gain entry into L. monocytogenes. Recently, Jolkver et al. (11) have described a novel transport system in Corynebacterium glutamicum with high affinity for acetate and propionate and low affinity for pyruvate. This monocarboxylic acid transporter, MtcC, is a sodium solute symporter driven by the electrochemical proton gradient. It is not known whether a similar transporter is present in L. monocytogenes. Also, no transport systems for butyrate and related branched-chain carboxylic acids have been described, but given the effects observed by addition of these carboxylic acids to the growth medium, we can postulate that they must exist.
In conclusion, certain branched-chain amino acids and branched-chain carboxylic acids could significantly impact the fatty acid composition of L. monocytogenes to a similar extent when included in media at high concentrations. However, short branched-chain carboxylic acids were significantly more inhibitory than branched-chain amino acids. The only straight-chain carboxylic acids found to have significant effects on fatty acid composition were propionate and butyrate. However, all short-chain carboxylic acids tested had significant growth-inhibitory effects. The simultaneous presence of a fatty acid precursor compound resulting in growth-dependent modification of the fatty acid composition toward one less compatible with low-temperature growth and a growth-inhibitory short-chain carboxylic acid may be a potent antilisterial combination.
Acknowledgments
This work was supported by award 2006-35201-17386 from the National Research Initiative Competitive Grant Program of the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broome, C. V. 1993. Listeriosis: can we prevent it? ASM News 59:444-446. [Google Scholar]
- 3.Buss, K. A., D. R. Cooper, C. Ingram-Smith, J. G. Ferry, D. A. Sanders, and M. S. Hasson. 2001. Urkinase: structure of acetate kinase, a member of the ASKHA superfamily of phosphotransferases. J. Bacteriol. 183:680-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, K. H., R. J. Heath, and C. O. Rock. 2000. β-Ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 182:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson, P. M. 2001. Chemical preservatives and natural antimicrobial compounds, p 593-627. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. American Society for Microbiology, Washington, DC.
- 6.Diao, J., and M. S. Hasson. 2009. Crystal structure of butyrate kinase 2 from Thermotoga maritima, a member of the ASKHA superfamily of phosphotransferases. J. Bacteriol. 191:2521-2529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Edgcomb, M. R., S. Sirimanne, B. J. Wilkinson, P. Drouin, and P. D. Morse II. 2000. Electron paramagnetic resonance studies of the membrane fluidity of the foodborne pathogenic psychrotroph Listeria monocytogenes. Biochim. Biophys. Acta 1463:31-42. [DOI] [PubMed] [Google Scholar]
- 8.Freese, E., C. W. Sheu, and E. Galliers. 1973. Function of lipophilic acids as antimicrobial food additives. Nature 241:321-325. [DOI] [PubMed] [Google Scholar]
- 9.Han, L., S. Lobo, and K. A. Reynolds. 1998. Characterization of β-ketoacyl-acyl carrier protein synthase III from Streptomyces glaucescens and its role in initiation of fatty acid biosynthesis. J. Bacteriol. 180:4481-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmanis, M. G. N. 1987. Butyrate kinase from Clostridium acetobutylicum. J. Biol. Chem. 262:617-621. [PubMed] [Google Scholar]
- 11.Jolkver, E., D. Emer, S. Ballan, R. Kramer, B. J. Eikmanns, and K. Marin. 2009. Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J. Bacteriol. 191:940-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, C. E., G. Shama, D. Jones, I. S. Roberts, and P. W. Andrew. 1997. Physiological and biochemical studies on psychrotolerance in Listeria monocytogenes. J. Appl. Microbiol. 83:31-35. [DOI] [PubMed] [Google Scholar]
- 13.Jones, S. L., P. Drouin, B. J. Wilkinson, and P. D. Morse II. 2002. Correlation of long-range membrane order with temperature-dependent growth characteristics of parent and a cold-sensitive, branched-chain-fatty-acid-deficient mutant of Listeria monocytogenes. Arch. Microbiol. 177:217-222. [DOI] [PubMed] [Google Scholar]
- 14.Juneja, V. K., and P. M. Davidson. 1993. Influence of altered fatty acid composition on resistance of Listeria monocytogenes to antimicrobials. J. Food Prot. 56:302-305. [DOI] [PubMed] [Google Scholar]
- 15.Kaneda, T. 1963. Biosynthesis of branched chain fatty acids. II. Microbial synthesis of branched long chain fatty acids from certain short chain fatty acid substrates. J. Biol. Chem. 238:1229-1235. [PubMed] [Google Scholar]
- 16.Kaneda, T. 1966. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can. J. Microbiol. 12:501-514. [DOI] [PubMed] [Google Scholar]
- 17.Kaneda, T. 1971. Incorporation of branched-chain C6-fatty acid isomers into the related long-chain fatty acids by growing cells of Bacillus subtilis. Biochemistry 10:340-347. [DOI] [PubMed] [Google Scholar]
- 18.Kaneda, T. 1991. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneda, T., and E. J. Smith. 1980. Relationship of primer specificity of fatty acid de novo synthetase to fatty acid composition in 10 species of bacteria and yeasts. Can. J. Microbiol. 26:893-898. [DOI] [PubMed] [Google Scholar]
- 20.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legendre, S., L. Letellier, and E. Shechter. 1980. Influence of lipids with branched-chain fatty acids on the physical, morphological and functional properties of Escherichia coli cytoplasmic membrane. Biochim. Biophys. Acta 602:491-505. [DOI] [PubMed] [Google Scholar]
- 22.Lu, Y. J., Y.-M. Zhang, and C. O. Rock. 2004. Product diversity and regulation of type II fatty acid synthases. Biochem. Cell Biol. 82:145-155. [DOI] [PubMed] [Google Scholar]
- 23.Mastronicolis, S. K., N. Arvanitis, A. Karaliota, C. Litos, G. Stavroulakis, H. Moustaka, A. Tsakirakis, and G. Heropoulos. 2005. Cold dependence of fatty acid profile of different lipid structures of Listeria monocytogenes. Food Microbiol. 22:213-219. [Google Scholar]
- 24.Mead, P. S., L. Slutsker, V. Dietz, F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neunlist, M. R., M. Federighi, M. Laroche, D. Sohier, G. Delattre, C. Jacquet, and N. E. Chihib. 2005. Cellular lipid fatty acid pattern heterogeneity between reference and recent food isolates of Listeria monocytogenes as a response to cold stress. Antonie Van Leeuwenhoek 88:199-206. [DOI] [PubMed] [Google Scholar]
- 26.Nichols, D. S., K. A. Presser, J. Olley, T. Ross, and T. A. McMeekin. 2002. Variation of branched-chain fatty acids marks the normal physiological range for growth in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2809-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nightingale, K. K., K. Windham, and M. Wiedmann. 2005. Evolution and molecular phylogeny of Listeria monocytogenes isolated from human and animal listeriosis cases and foods. J. Bacteriol. 187:5537-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Püttman, M., N. Ade, and H. Hof. 1993. Dependence of fatty acid composition of Listeria spp. on growth temperature. Res. Microbiol. 144:279-283. [DOI] [PubMed] [Google Scholar]
- 29.Ricke, S. C. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 82:632-639. [DOI] [PubMed] [Google Scholar]
- 29a.Sasser, M. February 2001. Identification of bacteria by gas chromatography of cellular fatty acids. Technical note 101. Microbial ID, Inc., Newark, DE. http://www.microbialid.com/PDF/TechNote_101.pdf.
- 30.Simanshu, D. K., H. S. Savithri, and M. R. Murthy. 2005. Crystal structures of ADP and AMPPNP-bound propionate kinase (TdcD) from Salmonella typhimurium: comparison with members of acetate and sugar kinase/heat shock cognate 70/actin superfamily. J. Mol. Biol. 352:876-892. [DOI] [PubMed] [Google Scholar]
- 31.Sinensky, M. 1974. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 71:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh, A. K., Y.-M. Zhang, K. Zhu, C. Subramanian, C. Gatto, Z. Li, R. K. Jayaswal, C. O. Rock, and B. J. Wilkinson. 2009. FabH selectivity for anteiso branched-chain fatty acid precursors in low-temperature adaptation in Listeria monocytogenes. FEMS Microbiol. Lett. 301:188-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokarskyy, O., and D. L. Marshall. 2008. Mechanism of synergistic inhibition of Listeria monocytogenes growth by lactic acid, monolaurin, and nisin. Appl. Environ. Microbiol. 74:7126-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 35.Willecke, K., and A. B. Pardee. 1971. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain α-keto acid dehydrogenase. J. Biol. Chem. 246:5264-5272. [PubMed] [Google Scholar]
- 36.Zhang, Y.-M., and C. O. Rock. 2008. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 6:222-233. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, K., D. O. Bayles, A. Xiong, R. K. Jayaswal, and B. J. Wilkinson. 2005. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain α-keto acid dehydrogenase. Microbiology 151:615-623. [DOI] [PubMed] [Google Scholar]
- 38.Zhu, K., X. Ding, M. Julotok, and B. J. Wilkinson. 2005. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl. Environ. Microbiol. 71:8002-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]