Abstract
A total of 32 strains of Legionella pneumophila were used to optimize pulsed-field gel electrophoresis (PFGE) for subtyping of L. pneumophila. Twenty-six isolates of L. pneumophila with various origins and 11 isolates from five different water systems were used as the panels. For optimization of electrophoretic parameters (EPs) of SfiI PFGE, 26 isolates were analyzed with SfiI digestion, using four EPs yielding the same D value. The EP of a switch time of 5 to 50 s for 21 h had the smallest similarity coefficients and was declared the optimal EP for SfiI PFGE of L. pneumophila. By software analysis and pilot study, AscI was chosen as another PFGE enzyme. AscI PFGE could cluster the isolates from each water system into the same or very similar patterns and had a high degree of typing concordance with other molecular methods. In evaluating the discriminatory power of AscI with the panel of 26 isolates, AscI PFGE gave one single pattern and a D value of 100%. AscI PFGE had a high discriminatory power and a high degree of consistency with epidemiological data and other molecular typing methods for L. pneumophila subtyping, and hence, AscI could be used as a restriction enzyme in PFGE subtyping of L. pneumophila.
Legionella pneumophila is an environmental organism that can cause disease in humans and is increasingly recognized as an important pathogen causing nosocomial pneumonia. Potable water systems (14, 26), spa water (28), and cooling towers (7, 13) are among the sources implicated in outbreaks of Legionnaires’ disease. Transmission of bacteria from the environment to humans occurs via inhalation or aspiration of Legionella-containing aerosols (3, 5). Strain differentiation is necessary for the identification of sources of contamination and determination of routes of transmission; this could in turn enable us to more accurately detect outbreaks and limit the spread of L. pneumophila infections. A variety of subtyping techniques have been used to identify and characterize L. pneumophila strains, including monoclonal antibody (MAb) analysis (16, 19), ribotyping (4), amplified fragment length polymorphism (AFLP) analysis (9, 22), PCR-based methods (15, 24), sequence-based typing (SBT) (9, 16), and pulsed-field gel electrophoresis (PFGE) (1, 6).
Preliminary reports demonstrated that PFGE is a highly discriminative epidemiological marker for subtyping of L. pneumophila (6, 11, 23, 25), and a number of L. pneumophila PFGE protocols have been described in the literature (1, 2, 4, 14); however, most laboratories that use PFGE to subtype L. pneumophila cannot compare their results because the protocols differ from each other in critical parameters, such as the restriction enzymes and electrophoresis conditions used to generate the DNA fingerprints. To enhance our ability to monitor this pathogen, there is an urgent need for a standardized L. pneumophila PFGE protocol which can readily be implemented in different laboratories for information interpretation.
An optimal PFGE protocol produces a suitable number of restriction fragments and gives distinct patterns by agarose gel electrophoresis, with these determined by the restriction enzymes and the electrophoretic parameters (EPs) used. SfiI is the most frequently used enzyme in conventional PFGE protocols for L. pneumophila, and there are several different EPs for SfiI digestion used by investigators for characterization and epidemiological studies. For a certain restriction enzyme, selection of the EP with the smallest similarity coefficients will increase the discriminatory power of PFGE. As the first phase of this study, we compared the similarity coefficients obtained for four EPs with SfiI digestion and determined the one with the maximal discriminatory power.
There were some problems found in practical applications of epidemiological investigation of L. pneumophila by PFGE with single SfiI digestion, such as having epidemiologically unrelated strains exhibit the same patterns (30) and the appearance of “ghost” or “phantom” bands. Combination use of two enzymes would give a higher discriminatory power and more accurate results (10, 29). Thus, as the second phase of this study, we selected another suitable enzyme and compared it with SfiI to evaluate the possibility of its use in characterization and epidemiological studies of L. pneumophila.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Thirty-two strains of L. pneumophila, isolated in China and including at least four serogroups, were used in this study (Table 1). All isolates were obtained from water systems in China and were stored in our lab. They were revived from lyophilized isolates and 0.7% semisolid culture medium. The bacteria were streaked onto buffered charcoal yeast extract (BCYE) agar plates, and typical colonies were picked up, identified by serotyping, and inoculated onto BCYE agar plates. The bacteria were grown at 35°C in 2.5% CO2 for 48 h for preparation of PFGE plugs. A panel of 26 strains of various origins was selected first from the test strains to optimize the EPs of SfiI digestion and to evaluate the discriminatory power of the enzyme. Three strains were used in the pilot test to choose the enzyme. Eleven strains associated with five independent water systems were included to evaluate the concordance between PFGE and four other molecular typing methods and the epidemiologic concordance of PFGE. Another 11 strains were selected randomly to test the reproducibility of the protocol.
TABLE 1.
Characterization of the 32 test strains in this study
| Strain | Serogroup | Year | Area | Source |
|---|---|---|---|---|
| SZ026 | 1 | 2004 | Guangdong | Cooling tower |
| SZ029 | 1 | 2004 | Guangdong | Cooling tower |
| SZ114 | 1 | 2004 | Guangdong | Cooling tower |
| SZ117 | 1 | 2004 | Guangdong | Cooling tower |
| FS24 | 1 | 2005 | Beijing | Spa water |
| FS25 | 1 | 2005 | Beijing | Spa water |
| FS27 | 1 | 2005 | Beijing | Spa water |
| FS28 | 1 | 2005 | Beijing | Spa water |
| Yu329 | 1 | 2005 | Chongqing | Cooling tower |
| Yu281 | 1 | 2005 | Chongqing | Cooling tower |
| GX4-1 | 1 | 2006 | Guangxi | Cooling tower |
| GX3-5 | 1 | 2006 | Guangxi | Cooling tower |
| ZJ050065 | 1 | 2006 | Zhejiang | Cooling tower |
| ZJ050051 | 1 | 2006 | Zhejiang | Cooling tower |
| NX0702 | 1 | 2007 | Ningxia | Cooling tower |
| NX0703 | 1 | 2007 | Ningxia | Cooling tower |
| Qin1 | 1 | 2008 | Hebei | Cooling tower |
| Qin5 | 1 | 2008 | Hebei | Cooling tower |
| JX6 | 1 | 2008 | Jiangxi | Cooling tower |
| JX1 | 1 | 2008 | Jiangxi | Cooling tower |
| JX2 | 1 | 2008 | Jiangxi | Cooling tower |
| JX3 | 1 | 2008 | Jiangxi | Cooling tower |
| JX4 | 1 | 2008 | Jiangxi | Cooling tower |
| JX5 | 1 | 2008 | Jiangxi | Cooling tower |
| Hu3 | 1 | 2008 | Neimenggu | Cooling tower |
| Hu6 | 1 | 2008 | Neimenggu | Cooling tower |
| JX7 | 5 | 2008 | Jiangxi | Cooling tower |
| BJ-6 | 6 | 2004 | Beijing | Household warm water system |
| BJ-7 | 6 | 2004 | Beijing | Household warm water system |
| FS6B | 6 | 2005 | Beijing | Spa water |
| Hu1 | Nonea | 2008 | Neimenggu | Cooling tower |
| Hu5 | Nonea | 2008 | Neimenggu | Cooling tower |
Not in serogroups 1 to 6.
Salmonella serotype Braenderup H9812 was used as a DNA size marker, as recommended by PulseNet (18).
PFGE protocol.
The PFGE protocol used was based on the PulseNet 1-day standardized PFGE protocol for Vibrio cholerae (10, 31). A cell suspension in a polystyrene tube (Falcon; 12 by 75 mm) was adjusted to an optical density of 3.8 to 4.0, using bioMérieux Densimat. L. pneumophila slices were digested with 50 U of SfiI per slice (Roche Diagnostics, Mannheim, Germany) or with a corresponding amount of other enzymes (New England Biolabs) for 4 h at 50°C or 37°C. Electrophoresis was performed with a CHEF-DRIII system (Bio-Rad Laboratories, Hercules, CA). Images were captured on a Gel Doc 2000 system (Bio-Rad) and converted to TIFF files for computer analysis. Plugs of strain H9812 were prepared and digested along with the test strains. Slices of H9812 were digested with 40 U/slice XbaI (TaKaRa Bio, Dalian, China). All electrophoresis steps were run with a voltage gradient of 6 V/cm, an included angle of 120°, and a linear ramp.
This protocol was similar to the improved PFGE protocol for L. pneumophila developed by Chang et al. (8).
Computer analysis of PFGE patterns.
The PFGE patterns were analyzed using the BioNumerics software package (version 4.0; Applied Maths, Inc.). Similarity analysis was performed by calculating Dice coefficients (SD) (12), with customized tolerance for each EP. SD was calculated as follows: SD = [2(nxy)]/(nx + ny), where nxy is the number of bands common to isolates x and y, nx is the total number of bands for isolate x, and ny is the total number of bands for isolate y. The tolerance was determined according to the value when all the H9812 patterns obtained with the same EP were defined to be indistinguishable. Clustering was created using the unweighted-pair group method using average linkages (UPGMA). Fragments smaller than 20.5 kbp were not analyzed.
Optimization of electrophoretic parameters for SfiI digestion.
Twenty-six isolates were analyzed with SfiI digestion, using four EPs, which were named EP-a, EP-b, EP-c, and EP-d (Table 2). EP-a was the parameter used most in the literature (8), while EP-b, -c, and -d were selected from a pilot study. The Simpson diversity index (D value) (17) and similarity coefficients (31) were used to compare the discriminatory powers under each parameter.
TABLE 2.
Features of four electrophoretic parameters with SfiI digestion
| EP | Switch time (s) | Total run time (h) |
|---|---|---|
| EP-a | 5-50 | 21 |
| EP-b | 6.8-63.8 | 20 |
| EP-c | 6.8-63.8 for 15 h, 1-16 for 6 h | 21 |
| EP-d | 6.8-63.8 for 10 h, 1-10 for 10 h | 20 |
The D value was determined by the equation D = 1 − {∑[nj(nj − 1)]}/[N (N − 1)], where nj is the number of strains belonging to the jth type and N is the number of strains in the population. The similarity coefficients of every two PFGE patterns were compared. Two-tailed probability was calculated using the Friedman test by SPSS 11.5 for multigroup comparisons. The Friedman test is a nonparametric test for analyzing randomized complete block designs, testing the null hypothesis that the treatments have identical effects. If significance was achieved among groups, the Friedman test was performed for two-group comparisons, with an adjusted significance level of 0.007. EPs with higher discriminatory power can distinguish patterns better, thus yielding smaller similarity coefficients. Accordingly, the EP with minimal similarity coefficients was optimal for distinguishing strains.
The EP with high D values and minimal similarity coefficients was optimal for distinguishing strains and was considered the standard for evaluating the discriminatory power of another enzyme, selected later.
Enzyme selection.
The preliminary enzymes tested were selected using DNASTAR 5.01 software (DNASTAR, Inc., WI), based on whole nucleotide sequences (GenBank accession no. NC002942, NC006368, NC006369, and NC009494) published in GenBank. The primary enzymes were then selected. A pilot test using three strains was conducted for further evaluation, and the optimal enzyme was selected based on the distribution of the bands. This optimal enzyme was further evaluated for use in PFGE of L. pneumophila.
Evaluation of optimal enzyme, focusing on typeability, discriminatory power, reproducibility, epidemiologic concordance, and correction with other molecular typing methods.
Discriminatory power was compared using the Simpson diversity index and similarity coefficients as described above. Typeability was calculated as the percentage of distinct bacterial strains which could be assigned a pattern (27). Reproducibility was evaluated by repeat analysis of 11 isolates. They were analyzed on three different CHEF-DRIII machines, and the patterns obtained on different runs were clustered. Epidemiologic concordance and correction with other molecular typing methods were evaluated by analyzing 11 strains associated with five independent water systems.
SBT, ribotyping, randomly amplified polymorphic DNA (RAPD) analysis, and multiple-locus variable-number-tandem-repeat analysis (MLVA).
The SBT protocols used here were recommended by the European Working Group for Legionella Infections (version 4.2), using seven specific gene loci (flaA, pilE, asd, mip, mompS, proA, and neuA).
Ribotyping was performed as previously reported, using an automated RiboPrinter system (4) following the manufacturer's recommendations (Qualicon, Wilmington, DE). All consumables and equipment used in further analyses were supplied by the manufacturer. EcoRI (Qualicon) was used as the restriction enzyme.
RAPD analysis was performed with primer SK2 (5′-CGGCGGCGGCGG-3′). All PCR and electrophoresis conditions used were as previously reported (21).
For MLVA, nine loci (Lp1, Lp3, Lp13, Lp17, Lp19, Lp31, Lp33, Lp34, and Lp35) were selected from the MLVA genotyping databases for L. pneumophila (http://minisatellites.u-psud.fr/). The protocols used here are available online (http://mlva.u-psud.fr/Legionella2006/PROTOCOL.htm).
RESULTS
Optimization of electrophoretic parameters of SfiI digestion.
For every enzyme, an EP could be recommended by the CHEF Mapper equipment, based on the sizes of restriction fragments. The EP recommended for SfiI digestion of L. pneumophila was a switch time of 6.8 to 63.8 s for 20 h (EP-b). However, with this EP, the bands were not well distributed, and it was not sufficient to distinguish fragments of less than 200 kbp. We fine-tuned the EP to provide the best possible resolution, and two more EPs were obtained (EP-c and EP-d). These three EPs were selected for comparison with EP-a, which was the parameter used most in the literature (Table 2).
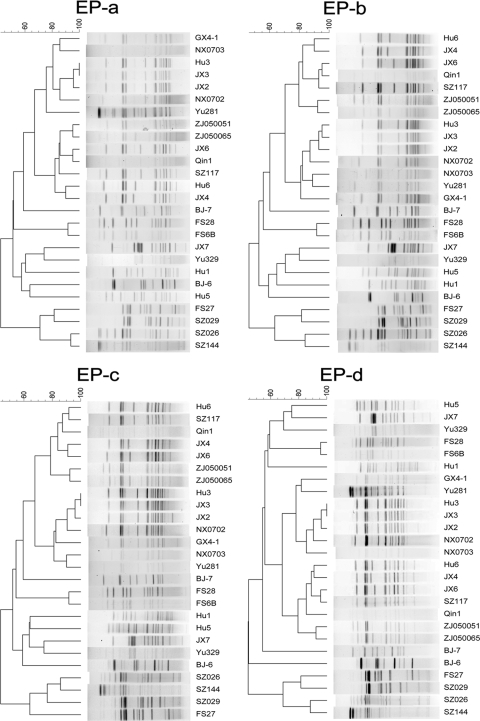
For the 26 L. pneumophila strains, the slight difference between the patterns given with the four EPs emerged because two strains (Hu3 and JX3) were defined as different with EP-b but indistinguishable with EP-a, -c, and -d (Fig. 1). The differences between patterns of strains Hu3 and JX3 were so small that they could not be distinguished under the tolerance values used by EP-a, -c, and -d, but they could be distinguished by visual observation using the BioNumerics software package. The four EPs gave the same D value, 100%, based on the types obtained by visual observation. At the 100% similarity breakpoint given by the software, the D value was 99.69% under EP-a, -c, and -d and 100% under EP-b.
FIG. 1.
Clustering results of patterns obtained using four EPs with SfiI digestion. Charts are shown for 26 L. pneumophila strains.
To compare the similarity coefficients, multigroup comparisons were made using SPSS 11.5. Nonparametric tests were performed because one-sample Kolmogorov-Smirnov tests demonstrated that the data were not normally distributed. The Friedman test showed that for the 26 test isolates (n = 351; chi-square = 14.286; df = 3), there were significant differences among the four groups (asymptotic significance, 0.003). Two-group comparisons were performed using the Friedman test, with an adjusted alpha value of 0.007 (Table 3). For the 26 isolates, similarity coefficients generated with EP-c and EP-d were significantly smaller than those obtained with EP-b. However, there was no significant difference between similarity coefficients generated by EP-a and those obtained with EP-b, -c, and -d, but EP-a exhibited smaller calculated similarity coefficients. Thus, EP-a was declared the optimal EP.
TABLE 3.
Two-group comparisons with the Friedman test for significant differences in similarity coefficientsa
| EP groups for comparison | Mean rankc |
Chi-square (n = 351; df = 1) | Asymp. sig.b | |
|---|---|---|---|---|
| Former | Latter | |||
| a-b | 1.43 | 1.57 | 6.412 | 0.011 |
| a-c | 1.49 | 1.51 | 0.303 | 0.582 |
| a-d | 1.46 | 1.54 | 2.333 | 0.127 |
| b-c | 1.58 | 1.42 | 8.836 | 0.003 |
| b-d | 1.57 | 1.43 | 7.167 | 0.007 |
| c-d | 1.44 | 1.45 | 3.084 | 0.079 |
Significance level was adjusted to 0.007.
The significance level based on the asymptotic distribution of a test statistic.
Former, the first EP group listed; latter, the second EP group listed.
Selection of another enzyme.
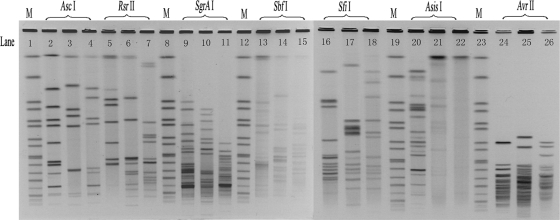
A theoretical enzyme selection using DNASTAR 5.01 software was based on four whole nucleotide sequences to include all satisfactory enzymes. Because the number of bands required for analysis should not be too large, many of the enzymes had too many bands. Therefore, beside SfiI, six enzymes (AscI, RsrII, SgrAI, SbfI, AsisI, and AvrII) were chosen as candidate enzymes for the pilot study. We obtained three images using these six enzymes and SfiI. The data showed that the image restriction cuts by AscI and SfiI were clear enough to meet our needs (Fig. 2).
FIG. 2.
PFGE images of three L. pneumophila isolates restricted with AscI (lanes 2 to 4), RsrII (lanes 5 to 7), SgrAI (lanes 9 to 11), SbfI (lanes 13 to 15), SfiI (lanes 16 to 18), AsisI (lanes 20 to 22), and AvrII (lanes 24 to 26). The size standard (M) was loaded in lanes 1, 8, 12, 19, and 23.
Typeability, reproducibility, and discriminatory power.
PFGE with AscI digestion had the ability to type all L. pneumophila strains and achieved the same level of typeability (100%) as that with SfiI digestion. Eleven randomly selected isolates were repeatedly analyzed three times with AscI digestion, and the patterns of the same isolate from different runs were defined to be indistinguishable, proving the good reproducibility of AscI digestion (see Fig. S1 in the supplemental material).
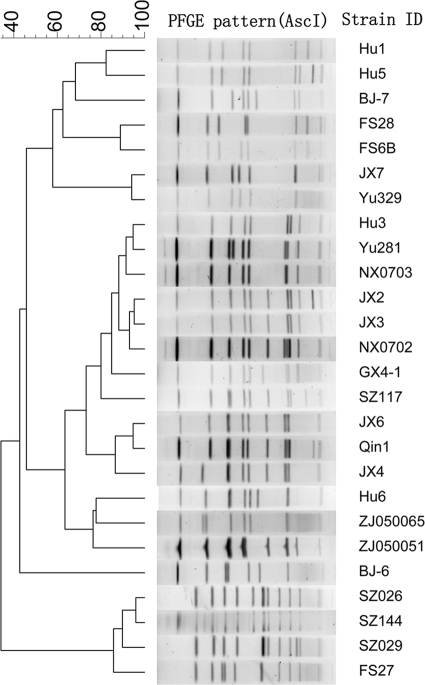
We analyzed the 26 strains with the EP of a switch time of 6.8 to 54.2 s for 19 h, which was recommended by the CHEF Mapper equipment and could provide reasonable patterns. PFGE with AscI digestion divided the 26 strains of L. pneumophila into 26 different pulsotypes and gave a D value of 100%, equivalent to the results of SfiI digestion (Fig. 3). Two-group comparisons using the Friedman test were also performed to compare the similarity coefficients generated by AscI and SfiI digestion. The results showed that for the 26 test isolates (n = 351; chi-square = 8.142), AscI digestion generated significantly smaller similarity coefficients than did SfiI digestion (asymptotic significance, 0.004). AscI PFGE had a higher discriminatory power than that of SfiI PFGE in this study.
FIG. 3.
Clustering results of patterns obtained with AscI digestion. Charts are shown for 26 L. pneumophila strains.
Concordance with epidemiologic data and other molecular typing methods.
We selected 11 isolates from five different water systems in different locations to evaluate the concordance of PFGE data with epidemiological data and data obtained by four other molecular typing methods. All of the isolates from the same water systems had identical SBT types, ribotypes, RAPD types, and MLVA types, except for the strains isolated from source 4 (Table 4). The two isolates from source 4 had different SBT types, with one base difference in the mip locus, and different MLVA types, with two loci showing differences. These two strains had the same PFGE type when digested with AscI and very similar patterns with SfiI digestion. The five isolates from sources 1 and 2 had the same SBT types, ribotypes, RAPD types, and MLVA types. However, PFGE with both AscI and SfiI digestion could distinguish all of the isolates from the different sources (Table 4).
TABLE 4.
Molecular typing characters of 11 isolates from five different water systems
| Strain | Water system | PFGE type |
SBT | Ribotype (RiboPrinter) | RAPD type | MLVA type (Lp1-Lp3-Lp13-Lp17-Lp19-Lp31-Lp33-Lp34-Lp35) | |
|---|---|---|---|---|---|---|---|
| AscI | SfiI | ||||||
| JX1 | 1 | PAT1 | PST1 | 1(1-4-3-1-1-1-1) | EcoRI 413-147-S-1 | RT1 | MT1 (7-7-10-2-4-9.5-4-2-17) |
| JX4 | 1 | PAT1 | PST1 | 1(1-4-3-1-1-1-1) | EcoRI 413-147-S-1 | RT1 | MT1 (7-7-10-2-4-9.5-4-2-17) |
| JX5 | 1 | PAT2 | PST2 | 1(1-4-3-1-1-1-1) | EcoRI 413-147-S-1 | RT1 | MT1 (7-7-10-2-4-9.5-4-2-17) |
| Qin1 | 2 | PAT3 | PST3 | 1(1-4-3-1-1-1-1) | EcoRI 413-147-S-1 | RT1 | MT1 (7-7-10-2-4-9.5-4-2-17) |
| Qin5 | 2 | PAT3 | PST3 | 1(1-4-3-1-1-1-1) | EcoRI 413-147-S-1 | RT1 | MT1 (7-7-10-2-4-9.5-4-2-17) |
| GX3-5 | 3 | PAT4 | PST4 | 630(1-4-3-1-1-1-10) | EcoRI 413-147-S-1 | RT1 | MT2 (7-7-10-2-4-9.5-4-2-18) |
| GX4-1 | 3 | PAT4 | PST4 | 630(1-4-3-1-1-1-10) | EcoRI 413-147-S-1 | RT1 | MT2 (7-7-10-2-4-9.5-4-2-18) |
| FS24 | 4 | PAT5 | PST5 | 149(17-10-17-3-2-14-11) | EcoRI 413-147-S-5 | RT2 | MT3 (8-8-10-2-4-14-2.5-3-6) |
| FS28 | 4 | PAT5 | PST6 | 155(17-10-17-28-2-14-11) | EcoRI 413-147-S-5 | RT2 | MT4 (8-8-8-2-5-14-2.5-3-6) |
| FS25 | 5 | PAT6 | PST7 | 154(11-14-16-16-15-13-2) | EcoRI 413-147-S-6 | RT3 | MT5 (8-8-3-0-0-16-4-0-8) |
| FS27 | 5 | PAT6 | PST8 | 154(11-14-16-16-15-13-2) | EcoRI 413-147-S-6 | RT3 | MT5 (8-8-3-0-0-16-4-0-8) |
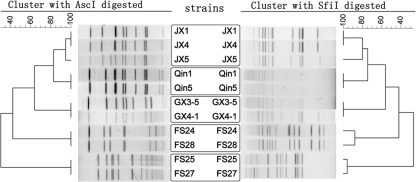
The analysis of isolates from various sources demonstrated that PFGE with AscI digestion was able to group isolates from all different water systems into clusters representing different pulsotypes, except for one system. The three isolates from one water system showed similar patterns (one-fragment difference; SD = 0.9474), with one isolate found to be different. This result of AscI digestion was nearly the same as that of SfiI digestion (Fig. 4). The difference was that the isolates within the other two water systems gave very similar, but not identical, patterns (one-fragment difference [SD = 0.9600] and three-fragment difference [SD = 0.8800]).
FIG. 4.
Clustering results of patterns obtained with SfiI digestion and AscI digestion of 11 L. pneumophila strains from five different water systems. The strains in each frame were from the same water system.
DISCUSSION
Previous studies have investigated the factors influencing PFGE results, including plug preparation, lysis of cells, and enzymatic digestion (8). In the initial stage of this study, we also optimized these procedures (data not shown). Our results were similar to the protocol developed by Chang et al. (8). The concentrated form (40 units/μl) of SfiI is especially recommended. Failure to follow these recommendations may lead to high background of the PFGE patterns, partial digestion, and poor resolution. The same situation also appeared in PFGE experiments with Vibrio parahaemolyticus (20). However, “ghost” or “phantom” bands were still observed in some SfiI PFGE patterns obtained with the concentrated form of SfiI. Another enzyme would give clearer patterns. At the same time, there were some problems found in the epidemiological investigation of L. pneumophila by PFGE with single SfiI digestion, and a combination of two restriction enzymes may increase the discriminatory power of the method. Thus, another enzyme was needed for use in PFGE for subtyping of L. pneumophila.
The principle for the selection of the restriction enzyme was to find one that can produce clear patterns with uniformly distributed bands. By means of software analysis and a pilot study, AscI was chosen as the other enzyme, based on clear patterns, the appropriate number of bands, and the distribution of bands.
Several criteria have been proposed for evaluating the performance of typing systems, including typeability, reproducibility, discriminatory power, and epidemiologic concordance (27). SfiI was the most frequently used enzyme in conventional PFGE protocols for typing of L. pneumophila. In spite of its slightly poor reproducibility, PFGE with SfiI digestion gave good typeability, discriminatory power, and epidemiologic concordance. The requirements of another enzyme were that it not only give clear patterns and good reproducibility but also have the same level of typeability, discriminatory power, and epidemiologic concordance with SfiI.
The discriminatory power of a method is the ability to distinguish between unrelated isolates, ideally assigning each to a different type. Discrimination indices and similarity coefficients are the indices usually used to compare discriminatory powers. In this study, both enzymes gave the same D value. However, with SfiI digestion, there were two isolates in the test panel with very similar patterns which could not be distinguished by BioNumerics software, and in contrast, the same test panel could easily be divided into types containing one strain each by AscI digestion. At the same time, similarity coefficients generated by AscI digestion were significantly smaller than those obtained with SfiI digestion. Thus, AscI PFGE had a higher discriminatory power than did SfiI.
In this study, we evaluated the epidemiologic concordance of AscI PFGE with SfiI PFGE. AscI PFGE was able to group isolates from all different water systems into clusters, except for one water system, containing three isolates, which gave two patterns with one different fragment; however, the similarity coefficient of these two patterns was quite high. The same situation applied to three water systems with SfiI digestion. It was understood that strains may undergo slight variation during dissemination in the environment, and in addition, the “ghost” or “phantom” bands caused by poor reproducibility could lead to this appearance.
There are several reports comparing different molecular typing methods for L. pneumophila subtyping (6, 23, 25). Their results show that PFGE has a high degree of consistency with other molecular typing methods for L. pneumophila subtyping, such as RAPD analysis, ribotyping, and SBT. In this study, we also compared the results of PFGE to those of other molecular typing methods, including SBT, ribotyping, RAPD analysis, and MLVA. Our results show that PFGE has a high degree of consistency with SBT, ribotyping, RAPD analysis, and MLVA for subtyping of isolates from the same source and a higher discriminatory power for subtyping of isolates from various sources.
PFGE is often considered the gold standard of molecular typing methods because of its high degree of reproducibility and unprecedented resolving power. Early reports of standardized PFGE protocols demonstrated that the combination of two restriction enzymes increased the discriminatory power of the method (10, 29). In this study, indistinguishable SfiI PFGE profiles of L. pneumophila strains could be differentiated further by the use of AscI, so the combination of two enzymes was used for subtyping of L. pneumophila by PFGE. Taking into account the more easily obtained satisfactory results of AscI digestion, we recommend the use of AscI as the primary enzyme, with SfiI used when further differentiation is needed.
Supplementary Material
Acknowledgments
This study was supported by grants from the Ministry of Health (200802016 and 2008ZX10004-008) and the Ministry of Science and Technology (2008ZX10004-008), People's Republic of China.
Footnotes
Published ahead of print on 8 January 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amemura-Maekawa, J., F. Kura, B. Chang, and H. Watanabe. 2005. Legionella pneumophila serogroup 1 isolates from cooling towers in Japan form a distinct genetic cluster. Microbiol. Immunol. 49:1027-1033. [DOI] [PubMed] [Google Scholar]
- 2.Bernander, S., K. Jacobson, J. H. Helbig, P. C. Lück, and M. Lundholm. 2003. A hospital-associated outbreak of Legionnaires’ disease caused by Legionella pneumophila serogroup 1 is characterized by stable genetic fingerprinting but variable monoclonal antibody patterns. J. Clin. Microbiol. 41:2503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatt, S. P., M. D. Parkinson, E. Pace, P. Hoffman, D. Dolan, P. Lauderdale, R. A. Zajac, and G. P. Melcher. 1993. Nosocomial Legionnaires’ disease: aspiration as a primary mode of disease acquisition. Am. J. Med. 95:16-22. [DOI] [PubMed] [Google Scholar]
- 4.Boccia, S., A. Stenico, R. Amore, L. Moroder, M. Orsini, V. Romano-Spica, and G. Ricciardi. 2005. Molecular epidemiology of Legionella pneumophila environmental isolates representing nine different serogroups determined by automated ribotyping and pulsed-field gel electrophoresis. Epidemiol. Infect. 133:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breiman, R. F., W. Cozen, B. S. Fields, T. D. Mastro, S. J. Carr, J. S. Spika, and L. Mascola. 1990. Role of air sampling in investigation of an outbreak of Legionnaires’ disease associated with exposure to aerosols from an evaporative condenser. J. Infect. Dis. 161:1257-1261. [DOI] [PubMed] [Google Scholar]
- 6.Casini, B., P. Valentini, A. Baggiani, F. Torracca, C. Lorenzini, S. Frateschi, B. Matteoli, and G. Privitera. 2008. Comparison of two molecular methods used for subtyping of Legionella pneumophila 1 strains isolated from a hospital water supply. Water Sci. Technol. 58:683-688. [DOI] [PubMed] [Google Scholar]
- 7.Castilla, J., A. Barricarte, J. Aldaz, M. García-Cenoz, T. Ferrer, C. Pelaz, S. Pineda, B. Baladrón, I. Martín, B. Goñi, P. Aratajo, J. Chamorro, F. Lameiro, L. Torroba, I. Dorronsoro, V. Martínez-Artola, M. J. Esparza, M. A. Gastaminza, P. Fraile, and P. Aldaz. 2008. A large Legionnaires’ disease outbreak in Pamplona, Spain: early detection, rapid control and no case fatality. Epidemiol. Infect. 136:823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, B., J. Amemura-Maekawa, and H. Watanabe. 2009. An improved protocol for the preparation and restriction enzyme digestion of pulsed-field gel electrophoresis agarose plugs for the analysis of Legionella isolates. Jpn. J. Infect. Dis. 62:54-56. [PubMed] [Google Scholar]
- 9.Chiarini, A., C. Bonura, D. Ferraro, R. Barbaro, C. Calà, S. Distefano, N. Casuccio, S. Belfiore, and A. Giammanco. 2008. Genotyping of Legionella pneumophila serogroup 1 strains isolated in Northern Sicily, Italy. New Microbiol. 31:217-228. [PubMed] [Google Scholar]
- 10.Cooper, K. L., C. K. Luey, M. Bird, J. Terajima, G. B. Nair, K. M. Kam, E. Arakawa, A. Safa, D. T. Cheung, C. P. Law, H. Watanabe, K. Kubota, B. Swaminathan, and E. M. Ribot. 2006. Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio cholerae. Foodborne Pathog. Dis. 3:51-58. [DOI] [PubMed] [Google Scholar]
- 11.De Zoysa, A. S., and T. G. Harrison. 1999. Molecular typing of Legionella pneumophila serogroup 1 by PFGE with SfiI and comparison of this method with restriction fragment-length polymorphism analysis. J. Med. Microbiol. 48:269-278. [DOI] [PubMed] [Google Scholar]
- 12.Dice, L. R. 1945. Measures of the amount of ecological association between species. Ecology 26:297-302. [Google Scholar]
- 13.Dondero, T. J., R. C. Rendtorff, G. F. Mallison, R. M. Weeks, J. S. Levy, E. W. Wong, and W. Schaffner. 1980. An outbreak of Legionnaires’ disease associated with a contaminated air-conditioning cooling tower. N. Engl. J. Med. 302:365-370. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Nuñez, M., N. Sopena, S. Ragull, M. L. Pedro-Botet, J. Morera, and M. Sabria. 2008. Persistence of Legionella in hospital water supplies and nosocomial Legionnaires’ disease. FEMS Immunol. Med. Microbiol. 52:202-206. [DOI] [PubMed] [Google Scholar]
- 15.Georghiou, P. R., A. M. Doggett, M. A. Kielhofner, J. E. Stout, D. A. Watson, J. R. Lupski, and R. J. Hamill. 1994. Molecular fingerprinting of Legionella species by repetitive element PCR. J. Clin. Microbiol. 32:2989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison, T. G., B. Afshar, N. Doshi, N. K. Fry, and J. V. Lee. 2009. Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000-2008). Eur. J. Clin. Microbiol. Infect. Dis. 28:781-791. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joly, J. R., R. M. McKinney, J. O. Tobin, W. F. Bibb, I. D. Watkins, and D. Ramsey. 1986. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J. Clin. Microbiol. 23:768-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kam, K. M., C. K. Luey, M. B. Parsons, K. L. Cooper, G. B. Nair, M. Alam, M. A. Islam, D. T. Cheung, Y. W. Chu, T. Ramamurthy, G. P. Pazhani, S. K. Bhattacharya, H. Watanabe, J. Terajima, E. Arakawa, O. A. Ratchtrachenchai, S. Huttayananont, E. M. Ribot, P. Gerner-Smidt, B. Swaminathan, and Vibrio parahaemolyticus PulseNet PFGE Protocol Working Group. 2008. Evaluation and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping Vibrio parahaemolyticus: an international multicenter collaborative study. J. Clin. Microbiol. 46:2766-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Presti, F., S. Riffard, F. Vandenesch, and J. Etienne. 1998. Identification of Legionella species by random amplified polymorphic DNA profiles. J. Clin. Microbiol. 36:3193-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavridou, A., E. Smeti, G. Mandilara, O. Pappa, S. Plakadonaki, E. Grispou, and M. Polemis. 2008. Prevalence study of Legionella spp. contamination in Greek hospitals. Int. J. Environ. Health Res. 18:295-304. [DOI] [PubMed] [Google Scholar]
- 23.Pruckler, J. M., L. A. Mermel, R. F. Benson, C. Giorgio, P. K. Cassiday, R. F. Breiman, C. G. Whitney, and B. S. Fields. 1995. Comparison of Legionella pneumophila isolates by arbitrarily primed PCR and pulsed-field gel electrophoresis analysis from seven epidemic investigations. J. Clin. Microbiol. 33:2872-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qasem, J. A., A. S. Mustafa, and Z. U. Khan. 2008. Legionella in clinical specimens and hospital water supply facilities: molecular detection and genotyping of the isolates. Med. Princ. Pract. 17:49-55. [DOI] [PubMed] [Google Scholar]
- 25.Schoonmaker, D., T. Heimberger, and G. Birkhead. 1992. Comparison of ribotyping and restriction enzyme analysis using PFGE for distinguishing Legionella pneumophila isolates obtained during a nosocomial outbreak. J. Clin. Microbiol. 30:1791-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shands, K. N., J. L. Ho, R. D. Meyer, G. W. Gorman, P. H. Edelstein, G. F. Mallison, S. F. Finegold, and D. W. Fraser. 1985. Potable water as a source of Legionnaires’ disease. JAMA 253:1412-1416. [PubMed] [Google Scholar]
- 27.Struelens, M. J. 1998. Molecular epidemiologic typing systems of bacterial pathogens: current issues and perspectives. Mem. Inst. Oswaldo Cruz 93:581-585. [DOI] [PubMed] [Google Scholar]
- 28.Su, H. P., L. R. Tseng, S. C. Tzeng, C. Y. Chou, and T. C. Chung. 2006. A legionellosis case due to contaminated spa water and confirmed by genomic identification in Taiwan. Microbiol. Immunol. 50:371-377. [DOI] [PubMed] [Google Scholar]
- 29.Swaminathan, B., T. J. Barrett, S. B. Hunter, R. V. Tauxe, and the CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thouverez, M., C. Godard, R. Leprat, and D. Talon. 2003. Is pulsed-field gel electrophoresis a valuable tool to identify nosocomial cases of Legionella pneumophila disease? J. Hosp. Infect. 55:254-259. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, J., B. Diao, N. Zhang, Z. Cui, L. Zhang, J. Xu, and B. Kan. 2007. Comparison of different electrophoretic parameters of pulse-field gel electrophoresis for Vibrio cholerae subtyping. J. Microbiol. Methods 71:15-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.