Abstract
The use of a specific peptide nucleic acid (PNA) probe demonstrated that Helicobacter pylori persisted inside biofilms exposed to low concentrations of chlorine (0.2 and 1.2 mg liter−1) for at least 26 days, although no culturable cells were recovered. Coupled with data obtained using viability stains in pure culture, this result suggests that H. pylori can survive chlorination but remain undetectable by culture methods, which can be effectively replaced by PNA hybridization.
Helicobacter pylori is a Gram-negative microorganism that colonizes the human stomach and can cause gastric ulcers that can degenerate into gastric carcinoma (5). The route of transmission for this pathogen is not well known, and even though culturable H. pylori has never been isolated from drinking water distribution systems (DWDS), molecular techniques such as PCR have detected the presence of H. pylori DNA in potable water (6, 15, 17), indicating that this environment could act as a reservoir for this bacterium.
Chlorine is the most commonly used disinfectant worldwide to ensure the safe distribution of water to the consumer (19). Although studies conducted by Johnson et al. (14) and Baker et al. (4) have shown that H. pylori is inactivated by chlorine, their conclusions were based on the lack of recovery using standard culture plating methods which fail to consider cells that have entered a viable but nonculturable (VBNC) state. Recently, Moreno et al. (16) applied molecular techniques to demonstrate that H. pylori can survive in low concentrations of chlorine in a VBNC state. However, all these studies were performed with pure cultures using suspended cells, and until now, there have been no studies reporting on the effect of chlorination on H. pylori when associated with heterotrophic biofilms where, as is well known, microorganisms become more resistant to the biocide effect of chlorine (10).
In a recent study, we demonstrated that H. pylori can be incorporated into drinking water biofilms and remain viable in the lower layers of these structures (11). It is therefore important to understand the ability of this pathogen to be incorporated and survive in heterotrophic biofilms formed in chlorinated waters. If this pathogen can remain viable under these conditions, it might therefore represent a risk to public health when released into the bulk fluid.
The aim of this work was to study the effect of low concentrations of chlorine on H. pylori cells both when associated with heterotrophic biofilms and when suspended in pure culture, to assess whether the biofilm can provide protection against disinfection.
Incorporation of H. pylori into heterotrophic biofilms.
The formation of biofilms was carried out using a two-stage chemostat model system as described elsewhere (12). The second stage consisted of three biofilm-growing vessels working in parallel. No chlorine was added to the first vessel, which served as a control, while to the other two vessels chlorine was continuously fed at a flow rate to maintain the concentrations of free chlorine at 0.2 and 1.2 mg liter−1. Due to the absence of autochthonous H. pylori, all the biofilm-growing vessels were inoculated with H. pylori NCTC 11637 at a final concentration of approximately 3.18 × 105 CFU ml−1 prior to the immersion of sterile unplasticized polyvinylchloride (uPVC) coupons (day 0). Coupons were removed after 1, 2, 4, 8, 16, and 26 days for cell quantification after neutralization of chlorine by the addition of sodium thiosulfate. Planktonic and sessile cells were quantified for total cells (by staining with Syto 9), heterotrophic plate count (HPC) (by plating onto R2A agar), and culturable H. pylori (by plating onto HP medium agar plates as described earlier [11]). In addition, sessile H. pylori was also quantified using a specific peptide nucleic acid (PNA) probe in a fluorescence in situ hybridization (FISH) assay, as described by Guimarães et al. (13). The differences between the parameters measured were compared by a one-way ANOVA followed by a Bonferroni post hoc test. Differences were considered relevant if the P value was <0.05.
The total number of cells in, and HPC for, the seed vessel did not change significantly with time (P > 0.05), showing that this vessel had stabilized. For the second-stage vessels, it was observed that the total numbers of cells were significantly lower (P = 0.001) in the chemostats where chlorine was added but similar between those vessels (P = 1.000). Concerning the HPC numbers, the values were statistically different when the three vessels were compared (P = 0.001), showing that chlorine affects the culturability of the heterotrophic bacteria.
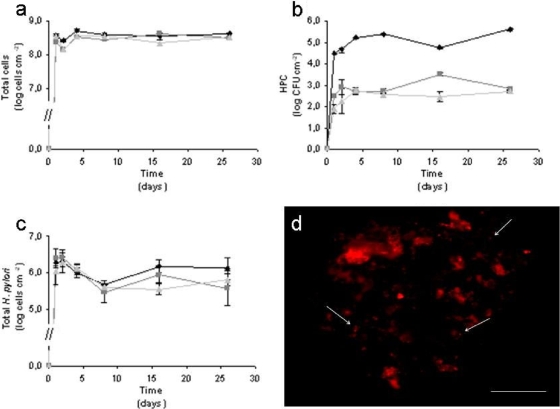
Figure 1a shows that most of the microorganisms adhered to the surface during the first day and that afterwards, the numbers did not significantly change (in general, P was >0.05). The total numbers of sessile cells in the absence and presence of chlorine were significantly different (P < 0.05) although similar for the two concentrations of chlorine used (P = 1.000). As expected, the HPC decreased significantly (P = 0.001) when the concentration of chlorine increased (Fig. 1b). In fact, the HPC numbers for the chlorinated biofilms were almost 3 log lower than that of the control. These results are supported by two studies conducted by Codony et al. (7, 8), where it was shown that under chlorinated conditions, the total number of cells was similar to that seen in the control study but that the cells were less culturable.
FIG. 1.
(a-c) Variation in the total cell number (a), HPC (b), and total numbers of H. pylori (c) in biofilms formed when no chlorine is added (⧫) and when chlorine is continuously added to final concentrations of 0.2 (▪) and 1.2 (▴) mg liter−1. (d) Epifluorescence microphotograph of a 2-day-old biofilm formed under 0.2 mg Cl2 liter−1 hybridized with the H. pylori specific PNA probe, using a tetramethyl rhodamine isocyanate (TRITC) filter. Bar, 20 μm.
The total numbers of H. pylori cells detected by the specific PNA probe did not change significantly under the three conditions tested (P > 0.05) (Fig. 1c). This result shows that H. pylori can incorporate into heterotrophic biofilms even in the presence of chlorine. It was also observed that during the first week, there was a decrease in the total number of H. pylori cells, which can be explained by the fact that the pathogen had just been inoculated at the beginning of the experiment with a 99% reduction of the planktonic cells in 24 h (14a). As such, after this time, the cells that detach from the biofilm cannot be replaced, and the detachment of biofilm that sloughs off H. pylori cells leads to a decrease in the total numbers of this pathogen. After 1 week of biofilm formation, the numbers of H. pylori cells are constant for all the conditions tested although slightly lower in the biofilms formed under chlorinated conditions. This might happen because the biofilms formed in the control chemostat were slimier than the biofilms formed under chlorinated conditions. The slimy aspect indicates the presence of exopolymeric substances (EPS) which surrounded the biofilm, protecting it from external stress conditions, such as exposure to biocides. On the other hand, when the biofilm is thicker, the detachment of biofilm portions occurs preferentially in the external layer, while internal layers remain intact. When chlorine was added, biofilms were thinner; the detachment could also occur in the internal layers to which H. pylori was adhered. Moreover, chlorine promotes biofilm detachment, which can also contribute to a decrease in H. pylori numbers.
Microscopy observation of H. pylori labeled with the 16S rRNA PNA probe showed that the morphology of the cells was mostly coccoid (Fig. 1d), in both the absence and the presence of chlorine. This is an important observation, as coccoid cells are known to retain for a longer time their viability and ability to cause infection (1, 20). Previous results have demonstrated that coccoid is the preferred shape at 15°C, while at 20°C, the cells are normally spiral shaped (11). This might indicate that at 15°C, cells are more resistant to chlorination, which is the most commonly used DWDS disinfectant method.
It was never possible to recover planktonic and sessile H. pylori on HP media. This could have been due to the overgrowth of other microorganisms as previously observed (11) but also could be due to the direct effect of chlorine on H. pylori cells, as observed for HPC.
Effect of chlorine on pure H. pylori cells in suspension.
The response of H. pylori to chlorination could have been influenced by two different factors: the presence of other microorganisms, which could influence the behavior of the pathogen, and inclusion into biofilms, where H. pylori is more protected from the biocide effect of chlorine. To try to understand the effect of this disinfectant on H. pylori cells, studies of suspended cells using a pure culture were carried out. The studies were performed with suspended cells, because other authors have already demonstrated the inability of H. pylori to form homogeneous monospecies biofilms under most conditions when suspended in water (1, 3, 9). For these experiments, chlorine was added to suspensions containing approximately 106 H. pylori CFU ml−1 to give final concentrations of 0.2, 0.7, and 1.2 mg liter−1. A control assay where no chlorine was added was also performed. After 0, 10, 20, and 30 min of chlorine exposure, samples were taken and chlorine was neutralized by the addition of sodium thiosulfate prior to quantification. Culturability of H. pylori was assessed by plating proper dilutions onto Columbia blood agar (CBA) plates, while membrane integrity was assessed by the use of a live/dead BacLight bacterial viability kit. At time zero and 30 min, the concentration of free chlorine was measured by the N,N-dimethyl-p-phenylenediamine (DPD) method.
Unlike in the biofilm experiments, chlorine was added only at the beginning of the experiment; as such, it was completely consumed after 30 min of contact time when the concentrations of free chlorine used were 0.2 and 0.7 mg liter−1, whereas for the highest concentration of chlorine (1.2 mg liter−1), there was still 0.1 mg liter−1 of chlorine remaining in solution.
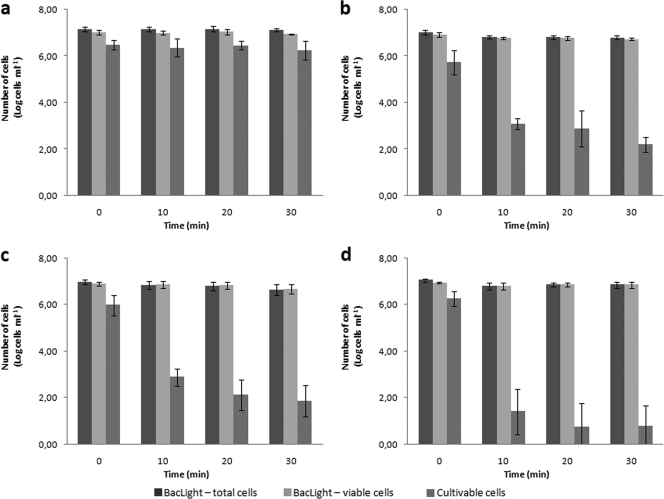
In the control experiment, in which no chlorine was added to the cell suspension, the total number of cells (sum of viable and nonviable) and number of culturable cells remained constant with time (P > 0.95), meaning that at 20°C, there was no effect of water exposure during the 30 min of the experiment (Fig. 2a). For all concentrations of chlorine added, there was no decrease in the number of viable cells, although some of the stained cells fluoresced yellow and orange instead of bright green. The morphology of the majority of cells was spiral shaped and did not change during the duration of the experiment under any of the conditions tested (Fig. 3a and b). This result is confirmed by the fact that cells did not completely lose culturability, even for the highest concentration of chlorine. This could have been due to the combination of organic matter that was introduced in the suspension with the cells, as suggested by Johnson and colleagues (14); however, chlorine was measured at the end of the experiments, and it was observed that for the experiment at 1.2 mg liter−1, there was still chlorine in the suspension, which means that there was enough chlorine to react with the cells. On the other hand, the results presented in this study are in contrast with those obtained in other studies (4, 14, 16), in which H. pylori completely lost culturability even at concentrations lower than 1.2 mg liter−1. Although in some of the studies the strain and the initial concentration of H. pylori were different from those used in this work, the differing results suggest that other parameters, such as temperature, light, and water characteristics, are important in chlorination efficiency (2).
FIG. 2.
Variation in the numbers of viable cells, nonviable cells, and cultivable cells of H. pylori after exposure to chlorine at a final concentration of 0.0 (a), 0.2 (b), 0.7 (c), and 1.2 (d) mg liter−1. Error bars represent standard deviations from at least three experiments.
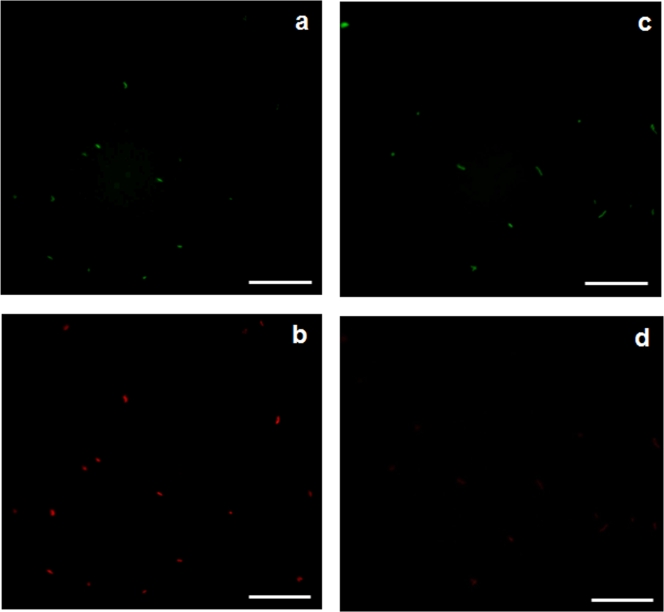
FIG. 3.
Epifluorescence microphotographs showing H. pylori cells treated with 1.2 mg liter−1 of free chlorine and stained with live/dead BacLight bacterial viability kit at time zero using an FITC filter (a) and a TRITC filter (b) and after 30 min using an FITC filter (c) and a TRITC filter (d). Bars, 20 μm.
An interesting result was the fact that the nonviable cells (cells that fluoresce red) (Fig. 3c) disappeared when the concentration of chlorine was 0.7 and 1.2 mg liter−1 (Fig. 3d). Therefore, a DNA electrophoresis assay was carried out, and results showed that after 30 min of exposure time there were no cuts in the DNA, as only one band appeared in the gel for all the conditions tested. However, the bands became faint at chlorine concentrations of 0.7 and 1.2 mg liter−1, meaning that the concentration of DNA decreased. Saby and colleagues have shown previously that chlorine can penetrate into E. coli cells and degrade the DNA, although the concentration that these authors used in their work was 20 times higher than the one used here (18). Combining these results suggests that less-well-adapted H. pylori cells are more susceptible to chlorine than stronger cells, and consequently, chlorine preferentially penetrates less-well-adapted cells, becoming unavailable to the strongest ones.
Conclusions.
The results obtained using pure culture provide new insights for what happened in the experiments with biofilms. The failure to recover culturable H. pylori was likely due to the fact that the isolation medium was not completely selective for H. pylori, allowing other microorganisms to grow. As H. pylori is a fastidious microorganism that grows very slowly, other species present may easily overgrow it, making it impossible to obtain culturable data which could give important information. The disappearance of nonviable cells and loss of brightness of the DNA bands suggest that the decrease in the total numbers of H. pylori in biofilms was due not only to the detachment of the cells but also to the direct action of chlorine on less-well-adapted cells. The detection of rRNA after 26 days of inoculation, as shown by the strong signal obtained by PNA FISH analysis, is indicative of the presence of viable cells. It has been shown that cells can maintain bright RNA fluorescence some hours after cellular death (21), but after 26 days, it would be expected that the RNA degradation had already occurred. These two results indicate that most dead cells disappeared in the first days of chlorine exposure; the cells that were detected afterwards by PNA-FISH could be considered viable, although probably in a VBNC state, which was corroborated by the fact that most of the cells were coccoid in shape.
The fact that H. pylori in pure culture never completely lost its culturability shows the resistance of this strain to chlorine and the extra protection of biofilms which can lead to the presence of viable H. pylori in DSDW, even if the cells are nonculturable. This work strongly supports the view that chlorine in water might inhibit the activity of H. pylori but that it fails to eliminate the pathogen from DWDS biofilms which survive in a VBNC state.
Acknowledgments
We thank Nuno Guimarães for technical assistance with the PNA FISH method.
This work was supported by the Portuguese Institute Fundação para a Ciência e Tecnologia (Ph.D. grant SFRH/BD/17088/2004 and postdoctoral grant SFRH/BPD/20484/2004).
Footnotes
Published ahead of print on 4 December 2009.
REFERENCES
- 1.Azevedo, N. F., C. Almeida, L. Cerqueira, S. Dias, C. W. Keevil, and M. J. Vieira. 2007. Coccoid form of Helicobacter pylori as a morphological manifestation of cell adaptation to the environment. Appl. Environ. Microbiol. 73:3423-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azevedo, N. F., C. Almeida, I. Fernandes, L. Cerqueira, S. Dias, C. W. Keevil, and M. J. Vieira. 2008. Survival of gastric and enterohepatic Helicobacter spp. in water: implications for transmission. Appl. Environ. Microbiol. 74:1805-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo, N. F., A. P. Pacheco, C. W. Keevil, and M. J. Vieira. 2006. Adhesion of water stressed Helicobacter pylori to abiotic surfaces. J. Appl. Microbiol. 101:718-724. [DOI] [PubMed] [Google Scholar]
- 4.Baker, K. H., J. P. Hegarty, B. Redmond, N. A. Reed, and D. S. Herson. 2002. Effect of oxidizing disinfectants (chlorine, monochloramine, and ozone) on Helicobacter pylori. Appl. Environ. Microbiol. 68:981-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunn, J. E. G., W. G. Mackay, J. E. Thomas, D. C. Reid, and L. T. Weaver. 2002. Detection of Helicobacter pylori DNA in drinking water biofilms: implications for transmission in early life. Lett. Appl. Microbiol. 34:450-454. [DOI] [PubMed] [Google Scholar]
- 7.Codony, F., J. Morato, and J. Mas. 2005. Role of discontinuous chlorination on microbial production by drinking water biofilms. Water Res. 39:1896-1906. [DOI] [PubMed] [Google Scholar]
- 8.Codony, F., J. Morato, F. Ribas, and J. D. Mas. 2002. Effect of chlorine, biodegradable dissolved organic carbon and suspended bacteria on biofilm development in drinking water systems. J. Basic Microbiol. 42:311-319. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. P., J. Harwood, R. Lee, R. She, and D. G. Guiney. 2004. Characterization of monospecies biofilm formation by Helicobacter pylori. J. Bacteriol. 186:3124-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Beer, D., R. Srinivasan, and P. S. Stewart. 1994. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 60:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gião, M. S., N. F. Azevedo, S. A. Wilks, M. J. Vieira, and C. W. Keevil. 2008. Persistence of Helicobacter pylori in heterotrophic drinking water biofilms. Appl. Environ. Microbiol. 74:5898-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gião, M. S., S. A. Wilks, N. F. Azevedo, M. J. Vieira, and C. W. Keevil. 2009. Incorporation of natural uncultivable Legionella pneumophila into potable water biofilms provides a protective niche against chlorination stress. Biofouling 25:345-351. [DOI] [PubMed] [Google Scholar]
- 13.Guimarães, N., N. F. Azevedo, C. Figueiredo, C. W. Keevil, and M. J. Vieira. 2007. Development and application of a novel peptide nucleic acid probe for the specific detection of Helicobacter pylori in gastric biopsy specimens. J. Clin. Microbiol. 45:3089-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, C. H., E. W. Rice, and D. J. Reasoner. 1997. Inactivation of Helicobacter pylori by chlorination. Appl. Environ. Microbiol. 63:4969-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Keevil, C. W. 2001. Continuous culture models to study pathogens in biofilms. Methods Enzymol. 337:104-122. [DOI] [PubMed] [Google Scholar]
- 15.Mackay, W. G., L. T. Gribbon, M. R. Barer, and D. C. Reid. 1998. Biofilms in drinking water systems—a possible reservoir for Helicobacter pylori. Water Sci. Technol. 38:181-185. [DOI] [PubMed] [Google Scholar]
- 16.Moreno, Y., P. Piqueres, J. L. Alonso, A. Jiménez, A. González, and M. A. Ferrús. 2007. Survival and viability of Helicobacter pylori after inoculation into chlorinated drinking water. Water Res. 41:3490-3496. [DOI] [PubMed] [Google Scholar]
- 17.Park, S. R., W. G. Mackay, and D. C. Reid. 2001. Helicobacter sp. recovered from drinking water biofilm sampled from a water distribution system. Water Res. 35:1624-1626. [DOI] [PubMed] [Google Scholar]
- 18.Saby, S., I. Sibille, L. Mathieu, J. L. Paquin, and J. C. Block. 1997. Influence of water chlorination on the counting of bacteria with DAPI (4′,6-diamidino-2-phenylindole). Appl. Environ. Microbiol. 63:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenen, D. 2002. Role of disinfection in suppressing the spread of pathogens with drinking water: possibilities and limitations. Water Res. 36:3874-3888. [DOI] [PubMed] [Google Scholar]
- 20.She, F. F., J. Y. Lin, J. Y. Liu, C. Huang, and D. H. Su. 2003. Virulence of water induced coccoid Helicobacter pylori and its experimental infection in mice. World J. Gastroenterol. 9:516-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheridan, G. E. C., C. I. Masters, J. A. Shallcross, and B. M. Mackey. 1998. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl. Environ. Microbiol. 64:1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]