Abstract
Enteropathogen contamination of groundwater, including potable water sources, is a global concern. The spreading on land of animal slurries and manures, which can contain a broad range of pathogenic microorganisms, is considered a major contributor to this contamination. Some of the pathogenic microorganisms applied to soil have been observed to leach through the soil into groundwater, which poses a risk to public health. There is a critical need, therefore, for characterization of pathogen movement through the vadose zone for assessment of the risk to groundwater quality due to agricultural activities. A lysimeter experiment was performed to investigate the effect of soil type and condition on the fate and transport of potential bacterial pathogens, using Escherichia coli as a marker, in four Irish soils (n = 9). Cattle slurry (34 tonnes per ha) was spread on intact soil monoliths (depth, 1 m; diameter, 0.6 m) in the spring and summer. No effect of treatment or the initial soil moisture on the E. coli that leached from the soil was observed. Leaching of E. coli was observed predominantly from one soil type (average, 1.11 ± 0.77 CFU ml−1), a poorly drained Luvic Stagnosol, under natural rainfall conditions, and preferential flow was an important transport mechanism. E. coli was found to have persisted in control soils for more than 9 years, indicating that autochthonous E. coli populations are capable of becoming naturalized in the low-temperature environments of temperate maritime soils and that they can move through soil. This may compromise the use of E. coli as an indicator of fecal pollution of waters in these regions.
The contamination of groundwater, including potable water supplies, with microbial pathogens continues to be a global concern (52, 59). Of particular importance in developed countries are the high levels of contamination associated with small-scale and very-small-scale drinking water supplies (5, 19, 57), often groundwater, which serve an estimated 10% of the total population in the European Union (13). The high numbers of these water supplies found to be contaminated with fecal bacteria and thus considered to be unfit for human consumption are worrying because the water from them is often untreated or inadequately treated prior to consumption. Microbial pathogens are known to survive for considerable periods of time in groundwater (29), which increases the health risk due to utilization of contaminated supplies. There are various sources of contamination, but evidence suggests that contamination from the spreading of animal slurries and manures on land can be a significant contributor (3, 33, 53). Spreading of agricultural slurries and manures on land is used by the agricultural sector as a means of nutrient recycling. The health risks associated with the spreading of animal and human wastes containing enteric pathogens have been recognized for a long time (10, 18). Animal manure and wastewaters may contain a broad range of pathogenic microorganisms, including Escherichia coli O157:H7, Campylobacter, Cryptosporidium, Salmonella spp., and pathogenic viruses, which are released into the environment during spreading (15, 22, 55). The levels and incidence of pathogens present in animal manures and slurries are influenced by a number of factors, including herd health, age demographics, stress factors, diet, season, and manure management and storage (37, 39).
Soils (and subsoils) often act as a zone for mitigating microbial contamination of groundwater associated with the spreading of animal slurries and manures on land. Some of the pathogenic microorganisms applied to agricultural soils have, however, been observed to leach through the soil into groundwater, which can affect drinking water quality and pose a risk to public health (16, 26, 28, 42, 50), confirming that soil is not always a sufficient obstruction for protection of groundwater (16, 53). Consequently, characterization of the movement of pathogens through the unsaturated soil and subsoil zone (vadose zone) has become critical for assessment of the risk to groundwater posed by agricultural activities (8, 14, 42). The soil and subsoil type is believed to be a major factor influencing the potential transfer of pathogens through soil to groundwater (3, 34, 41, 50). The preapplication moisture status of a soil, which may be influenced by the season, also impacts pathogen survival, fate, and transport (2, 11, 43, 54).
E. coli is widely used as an indicator of fecal contamination of water, and certain strains are known to be pathogenic (12). Thus, characterizing this organism's transport through soil is important because of the health risk posed by the organism itself and with regard to its validity as an indicator of the fate of enteropathogens in the environment. E. coli strains have diverse properties and capabilities that affect their survival and transport in soils (9, 36, 56, 60). Consequently, data obtained by using total E. coli rather than individual surrogate strains can be more representative of the fate and transport of E. coli present in animal slurries. E. coli O157 die-off in soils has been reported to be the same as or quicker than total E. coli die-off, suggesting that data for total E. coli provide a conservative estimate of the survival potential (38, 56). Although many field and laboratory studies have investigated E. coli transport through soil columns (4, 6, 16, 43, 46, 47, 50, 51), most studies have investigated transport through soil to a depth of less than 30 cm. For assessment of the risk of transport to groundwater, such studies may not take into account the variation in soil physical and chemical characteristics with depth (e.g., the frequency and continuity of macropores, organic matter, and moisture contents) that affect bacterial transport. Furthermore, rainfall was often simulated in previous studies, which allows experimental conditions to be controlled but may not be representative of the risk due to variable natural rainfall events over time. In this study, we used intact soil monoliths that were 1 m deep to assess the risk of leaching of total E. coli in four representative Irish soil types under natural rainfall and environmental conditions.
The objective of this study was to quantitatively investigate the impact of soil type and season (soil moisture content) on the fate and transport of E. coli spread on four different temperate maritime soil types under natural rainfall conditions. We hypothesized that there would be a greater microbial risk to underlying groundwater with better-drained soil types than with relatively poorly drained soil types following the application of animal slurry. In addition, we hypothesized that E. coli cells spread on wetter spring soils would be transported in greater numbers than E. coli cells spread on drier soils in the summer.
MATERIALS AND METHODS
Lysimeter unit.
A lysimeter experiment was carried out using an established lysimeter unit in Johnstown Castle, Wexford, Ireland (6°30′W, 52°17′N), under maritime temperate climatic conditions. The establishment and experimental design of the lysimeter unit were described previously by Ryan and Fanning (45). Briefly, replicate undisturbed monoliths of soils (diameter, 0.6 m; depth, 1 m) encased in rigid fiberglass cylinders were removed from each of five grassland sites in the Republic of Ireland in 1990. The soils were chosen to encompass a representative range of soil types, drainage characteristics, and soil parent material. Following transport to the lysimeter unit at Johnstown Castle, the lysimeters were randomly installed on both sides of an open trench where, by gravity, pipes direct drainage water from each lysimeter into collecting vessels. The area surrounding the lysimeters was backfilled with soil and covered with loose gravel. The lysimeter unit was caged to prevent contamination by birds or small mammals. A sward of perennial ryegrass (Lolium perenne) was established on each of the lysimeters. The lysimeter unit was previously used to study nitrate leaching on Irish soils (45), but no fecal material had been spread on the lysimeters since February 1998.
Treatments and sampling.
Four of the five soils in the lysimeter unit (named Clonroche [CL], Elton [EL], Oakpark [OA], and Rathangan [RA] after the localities from which they were taken) were used in this experiment, and there were nine replicate lysimeters for each soil. Major properties of the soils are shown in Table 1. Three lysimeters for each soil type received an application of cattle slurry at an average concentration of 34 tonnes per ha in August 2006 (summer, low soil moisture) (day 0), while another three lysimeters received a similar application in February 2007 (spring, high soil moisture) (day 184). The remaining three lysimeters for each soil type were maintained as controls (no treatment) throughout the experimental period. Slurry was applied by hand in a manner replicating that of a splash plate spreader. Subsamples of slurry were analyzed to determine the dry matter (DM) content and E. coli load prior to spreading. The DM content was analyzed by drying samples for 48 h (or until a constant weight was obtained) at 100°C. Samples were analyzed to determine the E. coli content using the Idexx Colisure most-probable-number methodology (35) after dilution with phosphate-buffered saline.
TABLE 1.
Major properties of lysimeter soils
| Soil | Soil classification | Parent material | Drainage | Depth (cm) | pH | C/N ratio | % Organic matter | Cation exchange capacity (meq/liter) | % Sand | % Silt | % Clay | Texture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oakpark (OA) | Haplic Cambisol | Fluvioglacial gravels | Very good | 0-20 | 6.6 | 14.6 | 4.9 | 13.3 | 67 | 23 | 11 | Sandy loam |
| 20-45 | 7.8 | 10.4 | 3.4 | 14.9 | 68 | 20 | 12 | Sandy loam | ||||
| 45-100 | Gravelly sand | |||||||||||
| Clonroache (CL) | Haplic Cambisol | Glacial drift | Good | 0-20 | 6.5 | 10.1 | 8.4 | 11.4 | 44 | 39 | 17 | Loam |
| 20-45 | 7.3 | 11.8 | 4.1 | 5.2 | 38 | 37 | 25 | Loam | ||||
| 45-90 | 7.1 | 3.0 | 3.9 | 45 | 41 | 14 | Loam | |||||
| Elton (EL) | Cutanic Luvisol | Glacial drift | Good | 0-20 | 6.2 | 8.4 | 6.9 | 12.5 | 48 | 35 | 17 | Loam |
| 20-50 | 6.8 | 8.2 | 3.2 | 11.1 | 36 | 50 | 14 | Silt loam | ||||
| 50-90 | 6.9 | 10.4 | 1.9 | 7.9 | 47 | 30 | 23 | Loam | ||||
| Rathangan (RA) | Luvic Stagnosol | Glacial sea drift | Poor | 0-15 | 6 | 10.1 | 5.8 | 10.1 | 44 | 37 | 19 | Loam |
| 15-40 | 6.3 | 8.6 | 2.9 | 6.4 | 48 | 28 | 24 | Sandy clay loam | ||||
| 40-60 | 6.3 | 1.2 | 6.1 | 42 | 30 | 28 | Clay loam | |||||
| 60-90 | 6.7 | 1.2 | 8.9 | 32 | 39 | 29 | Clay loam |
The experiment was performed under natural rainfall conditions, and sampling was carried out regularly during the drainage period for 488 days. The volume of leachate obtained from each lysimeter was measured and expressed as mass (kg) throughout the experiment for each sampling event, while an onsite weather station next to the lysimeters recorded weather data, including rainfall, solar radiation, soil temperature, air temperature, and humidity. The following two types of sampling regimens were used. Throughout the experimental period, a subsample of the total drainage for each lysimeter was taken after each leachate-producing rainfall event for E. coli analysis. In addition, two rainfall events that occurred shortly after the first leachate emerged after application of the slurry were selected for intensive sampling. During this intensive sampling approximately every 250 ml of drainage water was sampled. For E. coli analysis 100-ml subsamples were taken aseptically and processed within 2 h, and organisms were enumerated using Idexx Colisure following incubation at 35°C for 24 h. Isolates obtained from a selection of positive Colisure wells on a number of sampling days were verified to be E. coli isolates by positive confirmation on MacConkey plates (Oxoid), UTI chromogenic plates (Oxoid), and API 20E (bioMérieux, Paris, France) strips.
Statistics.
An analysis of variance (ANOVA) was performed with log-transformed total lysimeter E. coli load data with Proc Mixed (SAS 9.1), using soil type as a blocking factor.
RESULTS
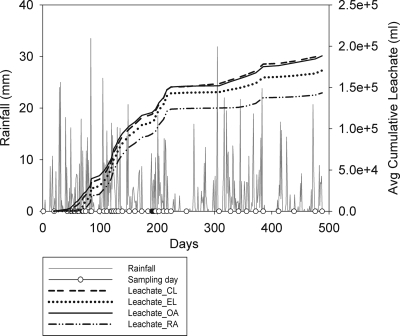
The slurry spread in the summer was found to have an average DM content of 8% ± 0.01% and an average E. coli load of 1.6 × 105 ± 2.4 × 104 CFU per g (wet weight) of slurry, while the slurry spread in the spring had an average DM content of 7.4% ± 0.2% and an average E. coli load of 1.2 × 105 ± 2.8 × 104 CFU per g (wet weight) of slurry. A summary of the climatic data recorded by the onsite weather station is shown in Table 2. The data show the low average temperature, as well as the frequent rainfall and relatively small variation in soil and air temperatures, which is characteristic of a temperate maritime climate. The daily rainfall data and the average cumulative amounts of leachate for the four soils are shown in Fig. 1. The RA and EL soils exhibited similar drainage patterns during the experimental period, while the OA and CL soils had similar drainage patterns.
TABLE 2.
Solar radiation, humidity, soil temperature, rainfall, and air temperature data for the lysimeter site during the period from September 2006 to August 2007
| Parameter | Avg | SD | Maximum | Minimum |
|---|---|---|---|---|
| Daily rainfall (mm) | 3.1 | 5.4 | 33.5 | 0.0 |
| Air temp (°C) | 11.0 | 3.6 | 18.9 | 2.0 |
| Solar radiation (J/cm2 day−1) | 938.9 | 672.6 | 2,563.9 | 29.3 |
| Relative humidity (%) | 85.1 | 7.0 | 98.3 | 7.0 |
| Soil temp (°C) at: | ||||
| 10 cm | 11.9 | 4.3 | 19.2 | 3.2 |
| 20 cm | 11.9 | 4.2 | 18.7 | 3.8 |
| 30 cm | 11.9 | 4.1 | 18.6 | 4.1 |
| 50 cm | 12.0 | 3.8 | 17.4 | 3.8 |
| 1 m | 12.1 | 3.1 | 16.4 | 3.1 |
FIG. 1.
Sampling days, rainfall, and average cumulative amounts of leachate (n = 9) for the 4 soils during the experimental period.
Treatments.
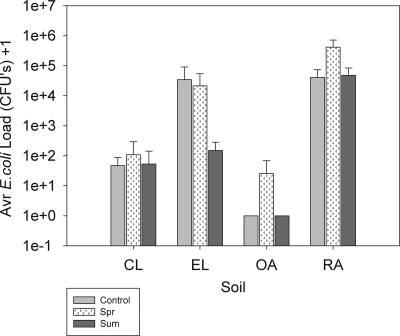
E. coli was detected in leachate from both lysimeters to which slurry was applied (spring and summer) and nontreated soil (control) lysimeters. As the slurry treatments were not applied at the same time, the comparison of treatments was based on the total amount of E. coli that leached in a fixed amount of leachate (45 kg) following application of the slurry or, in the case of the control treatment, after day 0 of the experiment. Although the time period required for this amount of drainage water to be leached varied between lysimeters and soils (from 49 to 297 days), this comparison was considered to be the most equitable comparison across all of the treatments. Box plots (not shown) suggested that the variance across soils was not equal. The heterogeneity was tested and modeled using the repeated statement in Proc Mixed. Residual checks showed that the assumptions of the tests were met. In addition, another analysis was carried out to compare the total amounts of E. coli leached from control lysimeters and from lysimeters to which slurry was applied in the summer for the whole experiment (488 days) and to compare the total amounts of E. coli leached from control lysimeters and from lysimeters to which slurry was applied in the spring for the experimental period after the spring application date (i.e., after day 184). All assumptions of the test were met. In both analyses treatment was not significant (α = 0.05), while the block effect suggested that there was a strong association with soil type; the F statistic values were 104.96, 9.41, and 16.14 for the 45-kg leachate, summer-versus-control, and spring-versus-control comparisons, respectively.
Soils.
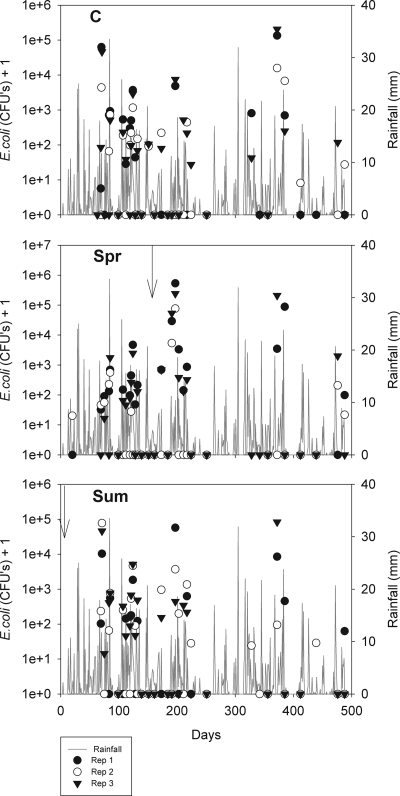
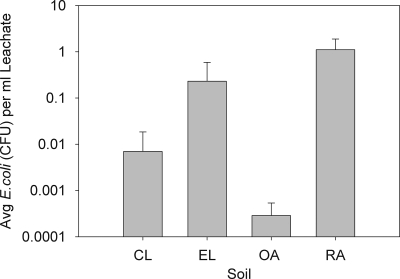
Under natural rainfall conditions, E. coli was detected in the drainage water from all four soil types during the experimental period (Fig. 2). Leaching of the bacterium was observed predominantly from the RA soil, and low levels of bacterial contamination were frequently observed for the drainage water from all 9 replicate lysimeters for this soil, including water from the untreated soil in control lysimeters and water from other lysimeter soils prior to treatment (Fig. 3). The total amount of E. coli leached from the RA soil lysimeters during the experiment was 9.5, 368, and 2,863 times the total amounts leached from the EL, CL, and OA soil lysimeters, respectively. E. coli also leached more frequently from the RA soil lysimeters than from the other soil lysimeters; during the experimental period 138 leachate samples from the RA soil were positive for E. coli (Fig. 3), while 46, 11, and 7 of the leachate samples from the EL, CL, and OA soils, respectively, were positive for E. coli. The results indicated that positive samples comprised 48, 16, 3, and 2.0% of the total leachate samples collected for the RA, EL, CL, and OA soils, respectively. When the risk of E. coli leaching from the soil types in the control lysimeters was assessed, the largest amount per gram of leachate was found to leach from the RA soil (Fig. 4) .
FIG. 2.
Average (n = 3) total E. coli loads leached for the first 45 kg of leachate after application to soil by soil type (and treatment). Spr. Spring; Sum, summer.
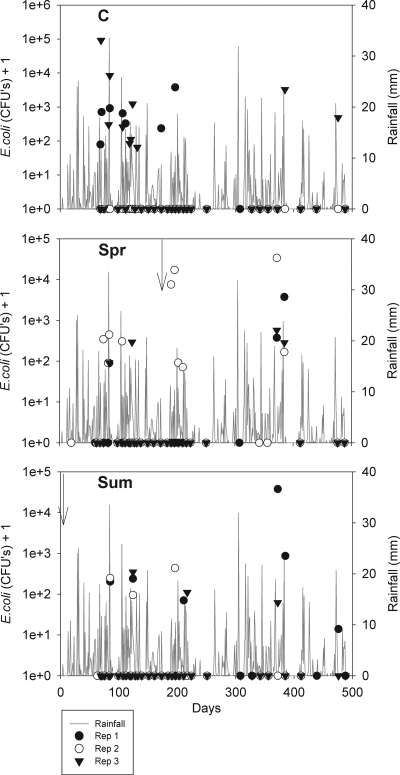
FIG. 3.
E. coli loads leached for the 3 replicates for each RA treatment (C, control; Spr, treated in the spring; Sum, treated in the summer) and daily rainfall over the experimental period. The arrows indicate slurry application dates.
FIG. 4.
Average amounts of E. coli leached per ml of leachate from the control lysimeters (n = 3) for each soil type. The error bars indicate standard deviations.
The presence of E. coli in the drainage water of the soils was often found to be related to rainfall events, and high numbers of bacteria were often associated with high-intensity or prolonged rainfall. For the RA soil, this was often associated with a change in the color (to brown) of the leachate. Variability in the occurrence and temporal variation of the E. coli leached was observed between replicate lysimeters. An example of this is the EL soil (Fig. 5). On some occasions, all replicate lysimeters of a treatment leached simultaneously, and this was often associated with a rainfall event. In other cases, leaching was more sporadic, with E. coli leaching from different replicates at different times. With the other soil types E. coli leaching was often confined to the drainage water of specific lysimeters, and E. coli never leached from other lysimeters (e.g., replicate 2 EL control soil [Fig. 5]).
FIG. 5.
Amounts of E. coli leached from the 3 replicates for each EL treatment (C, control; Spr, treated in the spring; Sum, treated in the summer) and daily rainfall over the experimental period. The arrows indicate slurry application dates.
DISCUSSION
This study was designed to quantitatively investigate the impact of soil type and application season on the fate and transport of E. coli spread on four different temperate maritime soils under natural rainfall conditions. We hypothesized that E. coli transport would be different in different soil types and that E. coli spread on land would be transported more quickly through better-drained soils. In addition, we hypothesized that E. coli spread on land in the spring would be transported in greater numbers than E. coli spread on land in the summer due to wetter soil conditions that facilitated transport and survival. The results demonstrate that there was a soil type effect on the transport of E. coli in our experiments. Contrary to our hypothesis, however, the lowest numbers of E. coli cells leached from the best-drained soil (OA), while the greatest numbers of E. coli cells leached from the most poorly drained soil (RA). The results indicate that the soil moisture at the time of application had no effect on the numbers of E. coli cells leached.
Filtration is believed to be the principal factor influencing the transport of bacteria through soil (17). The similar drainage patterns of the RA and EL soils, from which the highest numbers of E. coli cells leached during the experimental period, suggest that the physical properties of these soils may be similar, and this has been reported previously for these soils (31). In both soils the clay content increases with depth, and this may favor the formation of macropores, which are known to be important pathways for bacterial transport and to reduce the filtration capacity of soils (1, 6, 53). Aislabie et al. (4) reported that differences in soil structure between soils resulted in differences in microbial movement, with the coarse subsoil structure of poorly drained soils favoring preferential flow through macropores and the fine soil structure of well-drained soils favoring matrix flow. Smith (50) also found that the extent of E. coli transport in soils was related to soil structure, and transport through macropores was believed to be an important transport mechanism. Water travels at a higher velocity through macropores than through the soil matrix, and this may shear off bacteria attached to soil particles in pore channels. Guber et al. (20) asserted that differences in bacterial transport in soil could be attributed to variations in pore water velocity caused by the spatial variability of the soil structure, while Abu-Ashour and Abu-Zreig (1) reported that desorption of biotracer cells was favored at a higher interstitial velocity, which resulted in greater shear forces. The leaching of greater numbers of E. coli cells from the more poorly drained soils in this experiment suggests that bacterial transport occurs by means of preferential flow routes. The observation that E. coli leaching occurred only in certain lysimeters within soil types further supports the hypothesis that there is structural variation in soils between lysimeters, and this variation may include the number and continuity of preferential routes.
The increases in the levels of bacterial leaching associated with high-intensity or prolonged rainfall events indicated that microorganisms were flushed through the soils. The change in the color of the leachate for the RA soil accompanying these rainfall events supports this view. This was not unexpected as a number of studies have related numbers of bacteria and the hydraulic loading rate associated with major rainfall events (21, 23, 51). The leaching of E. coli between intensive rainfall events or during lower-intensity rainfall events may have occurred because the threshold value required for saturation of the lysimeter base necessary for leaching of the drainage water was reached. Saini et al. (46) found that the length of time between application of manure and the first rainfall event was the most important factor influencing the leaching of E. coli. Due to the large background amounts of E. coli that leached throughout the experimental period in our study, E. coli leaching could not be directly attributed to application of slurry. However, in both the RA and EL soils large amounts of E. coli leached shortly after the spring application of slurry, which may have been indicative of preferential flow events. The reduced moisture content of the soils during the summer application may have precluded transport after application due to the reduction in the number of conducting transport pathways.
The leaching of E. coli from control soils, and from amended soils prior to treatment, more than 9 years after the last application of fecal material indicates that this organism can survive for prolonged periods in the lysimeter soils. The survival of enteric bacteria in soils is dependent on a number of factors (3, 17). A principal factor is the moisture content of the soil environment, which is strongly influenced by the soil particle distribution and organic matter content of the soil (27). The clay content of soils is particularly important with respect to survival as clay particles provide a larger ecological niche that is protected against predators and greater availability of substrates and moisture than sand particles (32). Survival of enteric bacteria in soil is, therefore, strongly dependent on the soil type. The OA soil, from which the lowest numbers of E. coli cells leached, has a low water-holding capacity and the lowest clay content of the soils studied. Survival of enteric bacteria in this soil type would be expected to be limited and perhaps confined to organic material hotspots. In contrast, the RA soil is clay rich and has a compact soil structure below a depth of 50 cm, above which there are likely to be saturated soil conditions for a considerable portion of the year. The survival of E. coli, a facultative anaerobe, may be favored by the resulting anaerobic environment. Protozoan grazing, which is known to reduce E. coli populations in soils (44), may also be decreased under anaerobic conditions.
Die-off rates for E. coli in soil have been investigated often, and the majority of studies have reported a survival time of 2 to 4 months for enteric bacteria (27). Ohtomo et al. (40) reported that E. coli could survive for at least several months in grassland soils. Avery et al. (7) observed that E. coli O157:H7 could survive for at least 8 weeks in pasture. Sjogren (49) investigated E. coli survival in soils and produced model die-off curves that estimated probable survival times ranging from 20.7 to 23.3 months. Very few studies have shown long-term persistence of E. coli in grassland soils. Sjogren (48) reported that an antibiotic-resistant E. coli strain applied to Podzol field plots survived for 13 years after it was applied at high loading rates in a nutrient broth. In most studies, however, it has been observed that the majority of E. coli cells die rapidly once they are introduced into soil, and a key factor in this may be E. coli's inability to step down its metabolic rate to cope with the low availability of usable carbon in the soil environment (30). While in general the size of an E. coli population declines rapidly after this organism enters soil, elements of the population may exhibit enhanced survival due to advantageous physiological properties or colonization of more favorable sites (38). It has been proposed for a long time that bacteria can survive under inhospitable environmental conditions by entering a viable but nonculturable (VBNC) state (58). However, isolates recovered from lysimeter soils in this study were readily cultured and so were considered physiologically active.
The physiological status of the organisms, combined with the length of time since the last application of fecal material and the amount and frequency of E. coli leaching from all nine lysimeters with the RA soil, indicates that E. coli not only survives in this soil but also likely grows. Autochthonous, or naturalized, E. coli populations have previously been reported for soils in tropical and subtropical regions and, more recently, for soils in temperate and northern temperate regions (25), but to our knowledge this is the first report of the presence of autochthonous E. coli populations in relatively low-temperature maritime temperate soils. This raises interesting questions about how these organisms grow and compete for niche space with indigenous soil organisms at temperatures which are suboptimal for E. coli growth and demonstrates the importance of long-term survival studies. Ishii (24) demonstrated that naturalized E. coli was present and grew in northern temperate soils but observed that during laboratory incubation E. coli could grow only at higher temperatures and growth was followed by rapid die-off. This suggests that soilborne isolates survive in soils until the temperature rises enough to facilitate growth. In maritime temperate soils E. coli growth would have to occur at lower temperatures. This suggests that organisms may have phenotypic characteristics favorable for growth in this environment or that the soil environment is favorable in terms of substrate availability and protection from predation. Outside tropical and subtropical environments, where high temperatures and nutrient availability favor growth, autochthonous E. coli strains have been found mainly in wet or submerged environments (25), indicating that survival of E. coli in the environment may be favored by anaerobic or microaerobic conditions. E. coli may have the capacity to grow in anaerobic zones or in micropores (where the formation of biofilms may provide protection against predation) in the RA soil. Similar favorable sites in the EL soil may allow the presence of autochthonous E. coli populations.
In conclusion, the greatest numbers of E. coli cells leached from the poorly drained Luvic Stagnosol soil in our lysimeter study, while the smallest numbers of E. coli cells leached from the freely drained Haplic Cambisol soil over the experimental period. For all soil types, spatial variability in the soil structure was important in the transport of the bacterium. No effect of the soil moisture status prior to application was observed in this study. In this trial E. coli was found to leach from lysimeters for all four soil types at a wide range of concentrations under natural rainfall conditions, but the public health consequences of this finding are unclear. The potential for bacterial transport through subsoil below a depth of 1 m, in which there may be reduced macropore conductivity, is poorly understood and is an important consideration in assessing the risk to groundwater. In more poorly drained soil types, it is likely that artificial soil drainage schemes may facilitate the transport of E. coli in soil to surface water. The high levels of E. coli leaching from control soils that had not been amended with fecal material in over 9 years indicates that there is long-term persistence of E. coli in Irish soils, implying that the characteristics of a soil that influence survival are at least as important as the characteristics that influence transport in predicting potential risk. The high frequency of E. coli leaching, particularly in the Luvic Stagnosol soil, suggests that autochthonous E. coli populations are capable of becoming naturalized in the low-temperature environments of temperate maritime soils. This may compromise use of E. coli as the sole indicator of fecal pollution in waters in these regions.
Acknowledgments
This work was funded by the Irish Research Council for Science, Engineering and Technology (IRCSET) and was supported by Teagasc, Johnstown Castle.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Abu-Ashour, J., and M. Abu-Zreig. 2005. Effect of interstitial velocity on the adsorption of bacteria onto soil. Adsorp. Sci. Technol. 23:535-542. [Google Scholar]
- 2.Abu-Ashour, J., D. M. Joy, H. Lee, H. R. Whiteley, and S. Zelin. 1998. Movement of bacteria in unsaturated soil columns with macropores. Trans. ASAE 41:1043-1050. [Google Scholar]
- 3.Abu-Ashour, J., D. M. Joy, H. Lee, H. R. Whiteley, and S. Zelin. 1994. Transport of microorganisms through soil. Water Air Soil Pollut. 75:141-158. [Google Scholar]
- 4.Aislabie, J., J. J. Smith, R. Fraser, and M. McLeod. 2001. Leaching of bacterial indicators of faecal contamination through four New Zealand soils. Aust. J. Soil Res. 39:1397-1406. [Google Scholar]
- 5.Artz, R. R. E., and K. Killham. 2002. Survival of Escherichia coli O157:H7 in private drinking water wells: influences of protozoan grazing and elevated copper concentrations. FEMS Microbiol. Lett. 216:117-122. [DOI] [PubMed] [Google Scholar]
- 6.Artz, R. R. E., J. Townend, K. Brown, W. Towers, and K. Killham. 2005. Soil macropores and compaction control the leaching potential of Escherichia coli O157:H7. Environ. Microbiol. 7:241-248. [DOI] [PubMed] [Google Scholar]
- 7.Avery, L. M., P. Hill, K. Killham, and D. L. Jones. 2004. Escherichia coli O157 survival following the surface and subsurface application of human pathogen contaminated organic waste to soil. Soil Biol. Biochem. 36:2101-2103. [Google Scholar]
- 8.Balkwill, D. L., E. M. Murphy, D. M. Fair, D. B. Ringelberg, and D. C. White. 1998. Microbial communities in high and low recharge environments: implications for microbial transport in the vadose zone. Microb. Ecol. 35:156-171. [DOI] [PubMed] [Google Scholar]
- 9.Bolster, C. H., B. Z. Haznedaroglu, and S. L. Walker. 2009. Diversity in cell properties and transport behavior among 12 different environmental Escherichia coli isolates. J. Environ. Qual. 38:465-472. [DOI] [PubMed] [Google Scholar]
- 10.Burton, C. H., and C. Turner. 2003. Health risks from pathogens in livestocks manures, p. 109-157. In C. H. Burton and C. Turner (ed.), Manure management. Treatment strategies for sustainable agriculture, 2nd ed. Silsoe Research Institute, Bedford, United Kingdom.
- 11.Chu, Y., Y. Jin, T. Baumann, and M. V. Yates. 2003. Effect of soil properties on saturated and unsaturated virus transport through columns. J. Environ. Qual. 32:2017-2025. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Indicators for Waterborne Pathogens. 2004. Indicators for waterborne pathogens. National Academy of Sciences Press, Washington, DC.
- 13.Cortvriend, J., and A. Hulsmann. 2006. Europe paves the way for the revision of the drinking water directive. Water 21 August:17-19. [Google Scholar]
- 14.Darnault, C. J. G., T. S. Steenhuis, P. Garnier, Y.-J. Kim, M. B. Jenkins, W. C. Ghiorse, P. C. Baveye, and J. Y. Parlange. 2004. Preferential flow and transport of Cryptosporidium parvum oocysts through the vadose zone: experiments and modelling. Vadose Zone J. 3:262-270. [Google Scholar]
- 15.Duffy, G. 2003. Verocytoxigenic Escherichia coli in animal faeces, manures and slurries. J. Appl. Microbiol. 94:94S-103S. [DOI] [PubMed] [Google Scholar]
- 16.Gagliardi, J. V., and J. S. Karns. 2000. Leaching of Escherichia coli O157:H7 in diverse soils under various agricultural management practices. Appl. Environ. Microbiol. 66:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerba, C. P., and G. Bitton. 1984. Microbial pollutants: their survival and transport to groundwater, p. 65-88. In G. Bitton and C. P. Gerba (ed.), Groundwater pollution microbiology. John Wiley & Sons Inc., New York, NY.
- 18.Gerba, C. P., and J. E. Smith, Jr. 2005. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 34:42-48. [PubMed] [Google Scholar]
- 19.Goss, M. J., D. A. J. Barry, and D. L. Rudolph. 1998. Contamination in Ontario farmstead domestic wells and its association with agriculture. 1. Results from drinking water wells. J. Contam. Hydrol. 32:267-293. [Google Scholar]
- 20.Guber, A. K., D. R. Shelton, and Y. A. Pachepsky. 2005. Transport and retention of manure-borne coliforms in soil. Vadose Zone J. 4:828-837. [Google Scholar]
- 21.Hagedorn, C., D. T. Hansen, and G. H. Simonson. 1978. Survival and movement of fecal indicator bacteria in soil under conditions of saturated flow. J. Environ. Qual. 7:55-59. [Google Scholar]
- 22.Hutchison, M. L., L. D. Walters, S. M. Avery, B. A. Synge, and A. Moore. 2004. Levels of zoonotic agents in British livestock manures. Lett. Appl. Microbiol. 39:207-214. [DOI] [PubMed] [Google Scholar]
- 23.Huysman, F., and W. Verstraete. 1993. Water-facilitated transport of bacteria in unsaturated soil columns: influence of inoculation and irrigation methods. Soil Biol. Biochem. 25:91-97. [Google Scholar]
- 24.Ishii, S., W. B. Ksoll, R. E. Hicks, and M. J. Sadowsky. 2006. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 72:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii, S., and M. J. Sadowsky. 2008. Escherichia coli in the environment: implications for water quality and human health. Microbes Environ. 23:101-108. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, S. G., R. B. Goodbrand, R. P. Johnson, V. G. Odorico, D. Alves, K. Rahn, J. B. Wilson, M. K. Welch, and R. Khakhria. 1998. Escherichia coli O157:H7 diarrhoea associated with well water and infected cattle on an Ontario farm. Epidemiol. Infect. 120:17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamieson, R. C., R. J. Gordon, K. E. Sharples, G. W. Stratton, and A. Madani. 2002. Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: a review. Can. Biosyst. Eng. 44:1.1-1.9. [Google Scholar]
- 28.Jiang, G. 2005. Transport and deposition of Bacillus subtilis through an intact soil column. Aust. J. Soil Res. 43:695-703. [Google Scholar]
- 29.John, D. E., and J. B. Rose. 2005. Review of factors affecting microbial survival in groundwater. Environ. Sci. Technol. 39:7345-7356. [DOI] [PubMed] [Google Scholar]
- 30.Klein, D. A., and L. E. Casida, Jr. 1967. Escherichia coli die-out from normal soil as related to nutrient availability and the indigenous microflora. Can. J. Microbiol. 13:1461-1470. [DOI] [PubMed] [Google Scholar]
- 31.Kramers, G. 2009. Preferential flow in Irish grassland soils. Ph.D. thesis. University College Dublin, Dublin, Ireland.
- 32.Lang, N. L., and S. R. Smith. 2007. Influence of soil type, moisture content and biosolids application on the fate of Escherichia coli in agricultural soil under controlled laboratory conditions. J. Appl. Microbiol. 103:2122-2131. [DOI] [PubMed] [Google Scholar]
- 33.Mawdsley, J. L., R. D. Bardgett, R. J. Merry, B. F. Pain, and M. K. Theodorou. 1995. Pathogens in livestock waste, their potential for movement through soil and environmental pollution. Appl. Soil Ecol. 2:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mawdsley, J. L., A. E. Brooks, and R. J. Merry. 1996. Movement of the protozoan pathogen Cryptosporidium parvum through three contrasting soil types. Biol. Fertil. Soils 21:30-36. [Google Scholar]
- 35.McFeters, G. A., S. C. Broadaway, B. H. Pyle, M. Pickett, and Y. Egozy. 1997. Comparative performance of Colisure. J. Am. Water Works Assoc. 89:112-120. [PubMed] [Google Scholar]
- 36.Morrow, J. B., R. Stratton, H. H. Yang, B. F. Smets, and D. Grasso. 2005. Macro- and nanoscale observations of adhesive behavior for several E. coli strains (O157:H7 and environmental isolates) on mineral surfaces. Environ. Sci. Technol. 39:6395-6404. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson, F. A., S. J. Groves, and B. J. Chambers. 2005. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 96:135-143. [DOI] [PubMed] [Google Scholar]
- 38.Ogden, I. D., D. R. Fenlon, A. J. A. Vinten, and D. Lewis. 2001. The fate of Escherichia coli O157 in soil and its potential to contaminate drinking water. Int. J. Food Microbiol. 66:111-117. [DOI] [PubMed] [Google Scholar]
- 39.Ogden, I. D., M. MacRae, and N. J. C. Strachan. 2004. Is the prevalence and shedding concentrations of E. coli O157 in beef cattle in Scotland seasonal? FEMS Microbiol. Lett. 223:297-300. [DOI] [PubMed] [Google Scholar]
- 40.Ohtomo, R., K. Minato, and M. Saito. 2004. Survival of Escherichia coli in a field amended with cow feces slurry. Soil Sci. Plant Nutr. 50:575-581. [Google Scholar]
- 41.Paterson, E., J. S. Kemp, S. M. Gammack, E. A. Fitzpatrick, M. S. Cresser, C. E. Mullins, and K. Killham. 1993. Leaching of genetically modified Pseudomonas fluorescens through intact soil microcosms: influence of soil type. Biol. Fertil. Soils 15:308-314. [Google Scholar]
- 42.Powelson, D. K., and C. P. Gerba. 1995. Fate and transport of microorganisms in the vadose zone, p. 123-135. In L. G. Wilson, L. G. Everett, and S. J. Cullen (ed.), Handbook of vadose zone characterization and monitoring. CRC Press, Boca Raton, FL.
- 43.Powelson, D. K., and A. L. Mills. 2001. Transport of Escherichia coli in sand columns with constant and changing water contents. J. Environ. Qual. 30:238-245. [DOI] [PubMed] [Google Scholar]
- 44.Recorbet, G., C. Steinberg, and G. Faurie. 1992. Survival in soil of genetically engineered Escherichia coli as related to inoculum density, predation and competition. FEMS Microbiol. Lett. 101:251-260. [Google Scholar]
- 45.Ryan, M., and A. Fanning. 1996. Effects of fertiliser N and slurry on nitrate leaching—lysimeter studies on 5 soils. Irish Geogr. 29:126-136. [Google Scholar]
- 46.Saini, R., L. J. Halverson, and J. C. Lorimor. 2003. Rainfall timing and frequency influence on leaching of Escherichia coli RS2G through soil following manure application. J. Environ. Qual. 32:1865-1872. [DOI] [PubMed] [Google Scholar]
- 47.Saini, R., J. C. Lorimor, and L. Halverson. 2001. Effect of manure application and rainfall timing on the leaching of labeled bacteria through soil columns, paper no. 2195. In Proceedings of the ASAE Annual International Meeting, California. American Society of Agricultural and Biological Engineers, St. Joseph, MI.
- 48.Sjogren, R. E. 1995. 13-Year survival study of an environmental Escherichia coli in field mini-plots. Water Air Soil Pollut. 81:315-335. [Google Scholar]
- 49.Sjogren, R. E. 1994. Prolonged survival of an environmental Escherichia coli in laboratory soil microcosms. Water Air Soil Pollut. 75:389-403. [Google Scholar]
- 50.Smith, M. S. 1985. Transport of Escherichia coli through intact and disturbed soil columns. J. Environ. Qual. 14:87-91. [Google Scholar]
- 51.Stevik, T. K., G. Ausland, J. F. Hanssen, and P. D. Jenssen. 1999. The influence of physical and chemical factors on the transport of E. coli through biological filters for wastewater purification. Water Res. 33:3701-3706. [Google Scholar]
- 52.Theron, J., and T. E. Cloete. 2002. Emerging waterborne infections: contributing factors, agents, and detection tools. Crit. Rev. Microbiol. 28:1-26. [DOI] [PubMed] [Google Scholar]
- 53.Unc, A., and M. J. Goss. 2004. Transport of bacteria from manure and protection of water resources. Appl. Soil Ecol. 25:1-18. [Google Scholar]
- 54.Van Elsas, J. D., J. T. Trevors, and L. S. Van Overbeek. 1991. Influence of soil properties on the vertical movement of genetically-marked Pseudomonas fluorescens through large soil microcosms. Biol. Fertil. Soils 10:249-255. [Google Scholar]
- 55.Vernozy-Rozand, C., M. P. Montet, F. Lequerrec, E. Serillon, B. Tilly, C. Bavai, S. Ray-Gueniot, J. Bouvet, C. Mazuy-Cruchaudet, and Y. Richard. 2002. Prevalence of verotoxin-producing Escherichia coli (VTEC) in slurry, farmyard manure and sewage sludge in France. J. Appl. Microbiol. 93:473-478. [DOI] [PubMed] [Google Scholar]
- 56.Vinten, A. J. A., D. R. Lewis, D. R. Fenlon, K. A. Leach, R. Howard, I. Svoboda, and I. Ogden. 2002. Fate of Escherichia coli and Escherichia coli O157 in soils and drainage water following cattle slurry application at 3 sites in southern Scotland. Soil Use Manag. 18:223-231. [Google Scholar]
- 57.Vinten, A. J. A., J. Potts, L. Avery, and N. J. C. Strachan. 2009. Microbial pollution of water by livestock: approaches to risk assessment and mitigation. Animal 3:744-752. [DOI] [PubMed] [Google Scholar]
- 58.Winfield, M. D., and E. A. Groisman. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69:3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. 2006. Microbial aspects, p. 121-144. In Guidelines for drinking water quality, 3rd ed., 1st addendum. World Health Organization, Geneva, Switzerland.
- 60.Yang, H.-H., J. B. Morrow, D. Grasso, R. T. Vinopal, and B. F. Smets. 2006. Intestinal versus external growth conditions change the surficial properties in a collection of environmental Escherichia coli isolates. Environ. Sci. Technol. 40:6976-6982. [DOI] [PubMed] [Google Scholar]