Abstract
Since some strains of Brucella species may require carbon dioxide for growth, a multilaboratory study was conducted to compare broth microdilution susceptibility results using ambient air (AA) and 5% CO2 incubation conditions. Six antimicrobial agents were tested against 39 Brucella isolates. Aminoglycoside MICs tended to be 1 log2 dilution higher in CO2 than in AA; tetracycline-class MICs to be 1 log2 dilution lower in CO2.
Routine susceptibility testing of Brucella spp. is not recommended since the susceptibility pattern of wild-type Brucella spp. is fairly predictable, the isolates are fastidious, and the organisms are a potential cause of laboratory-acquired infection (3, 12, 17). In addition, Brucella spp. are intracellular pathogens, and like other intracellular pathogens, in vitro susceptibility may not always correlate with clinical outcome (1, 3, 28). Typically, brucellosis is treated with dual-antimicrobial therapy to lower the possibility of relapse. The most common combinations are streptomycin (or gentamicin) and doxycycline and doxycycline combined with rifampin (4, 12, 21, 28). Trimethoprim-sulfamethoxazole is recommended as alternative therapy (28), but use of fluoroquinolone therapy is controversial (12, 18, 25). Although there has been little or no resistance reported to routinely prescribed antimicrobials for brucellosis, relapse is still common (3, 5, 22, 25), and development of laboratory-confirmed rifampin resistance has been reported (11).
Brucella suis, Brucella melitensis, and Brucella abortus are considered potential agents of bioterrorism (6, 24). As with other potential bacterial agents of bioterrorism, engineered antimicrobial resistance is a concern. Antimicrobial susceptibility testing of Brucella spp. to identify effective therapeutic and prophylactic agents would be an important response effort in a bioterrorism event. Although no antibiotic regimen has been precisely studied for prophylaxis of brucellosis in humans, combining doxycycline and rifampin or using trimethoprim-sulfamethoxazole alone (for children and pregnant women) has been used successfully in preventing laboratory-acquired disease, although side effects can occur (20, 23, 27).
Many different methods of antimicrobial susceptibility testing, using a variety of media and incubation conditions, have been described for testing Brucella spp. (1, 3-5, 12-14, 16, 21, 22, 25, 26). Jevitt et al. (15) developed a standardized method for susceptibility testing of Brucella spp. using brucella broth, a method that was adopted by the Clinical and Laboratory Standards Institute (CLSI) in 2006 (9). This initial CLSI method for Brucella spp. described a broth microdilution (BMD) procedure using incubation at 35 ± 2°C for 48 h in ambient air (AA). The present study describes a multicenter examination to evaluate incubation in ambient air supplemented with 5% CO2. Incubation in CO2-supplemented air is particularly important for some strains of Brucella spp. that are especially fastidious, such as B. abortus (19). Since many laboratories may not have a dedicated CO2 incubator in a biosafety level 3 (BSL3) space, incubation using a CO2-generating system, such as BBL GasPak CO2 (BD, Sparks, MD) and the BBL Gaspak CO2 pouch capnophilic system (BD), was evaluated.
Thirty-nine strains of Brucella spp. from the Centers for Disease Control and Prevention (CDC) collection were used for this study: 20 B. melitensis, 11 B. suis, and 8 B. abortus. Antimicrobial susceptibility testing was performed in 4 laboratories at 3 institutions. Two laboratories tested both ambient air and CO2 incubation conditions, and two laboratories performed testing using only one of the incubation conditions. BMD panels were prepared at the CDC with brucella broth (BBL, Sparks, MD) at pH 7 to 7.2 and were shipped to participating laboratories, where they were stored at −70°C until ready for use; all panels were from the same preparation lot number. Antimicrobial powders were obtained from Sigma (St. Louis, MO). The antimicrobial agents and ranges tested were as follows: doxycycline, 0.015 to 8 μg/ml; gentamicin, 0.015 to 8 μg/ml; rifampin, 0.12 to 8 μg/ml; streptomycin, 0.12 to 64 μg/ml; tetracycline, 0.015 to 8 μg/ml; and trimethoprim-sulfamethoxazole, 0.015/0.285 to 8/152 μg/ml. The MIC incubation temperature was 35°C for both atmospheres. CO2 incubation was accomplished with a single-use gas-generating system which produces an atmosphere that contains 2.5 to 10% CO2 (BBL product insert). The BBL GasPak CO2 system was used in 2 laboratories (sites A and B), and the BBL GasPak CO2 pouch capnophilic system was used in laboratory test site D. Inocula were prepared by the direct colony suspension method in Mueller-Hinton broth from 24- to 48-h cultures grown on 5% sheep blood agar plates (BBL) and incubated in ambient air or in ambient air supplemented with CO2 by using a gas-generating system if the isolate was dependent upon CO2 for growth (8). The final volume of brucella broth in the MIC tray wells was 100 μl per well; 10 μl of diluted inoculum was delivered by a sterile, plastic commercial inoculator system (Dynex, Chantilly, VA). MIC panels were incubated for 48 h, and endpoints were recorded as the lowest concentration of drug demonstrating no macroscopic growth, except for trimethoprim-sulfamethoxazole, where the endpoint was interpreted as the lowest drug concentration inhibiting 80% of the growth when compared to the growth control well.
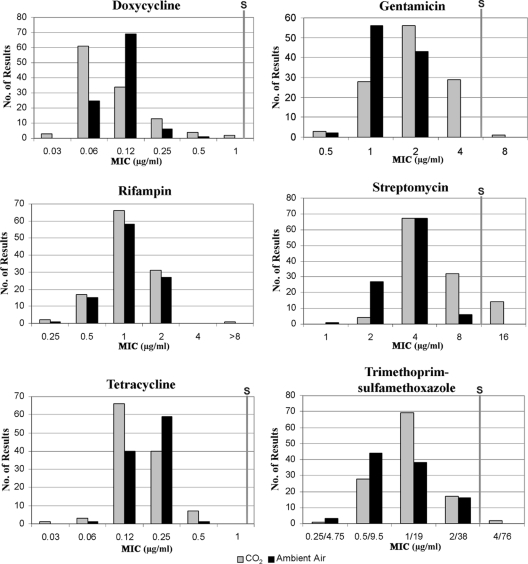
BMD MIC results from AA incubation and CO2 incubation for the 39 Brucella isolates are shown in Fig. 1. AA MIC results were not available for 4 of the 39 isolates because these isolates would not grow in AA; CO2 was required for growth in broth and on agar media. The MIC modes for tetracycline and doxycycline were 1 log2 dilution lower in CO2 than in AA, while gentamicin and trimethoprim-sulfamethoxazole modes were 1 log2 dilution higher in CO2 than in AA (Table 1). The modes for streptomycin MICs in AA and CO2 were the same (4 μg/ml), but there were 40 more results for which the MIC was 8 or 16 μg/ml in CO2 than in AA. Fourteen of these 40 streptomycin results were categorized as nonsusceptible (MIC of 16 μg/ml) in CO2, whereas no results fell into the nonsusceptible category for AA incubation.
FIG. 1.
Bar graphs of MICs under ambient air and CO2 conditions for six antimicrobial agents tested against 39 Brucella isolates at three test sites for each atmosphere. For one site, ambient air data were available for only 31 isolates, while the other two sites each had 35 ambient air results (4/39 isolates required CO2). S, susceptible. Category divisions are based on the original 2006 CLSI breakpoints; there are no published breakpoints for rifampin (9).
TABLE 1.
MIC modes and ranges for six antimicrobial agents tested against 39 Brucella isolates at three test sites for each incubation atmospherea
| Antimicrobial agent | MIC mode (μg/ml) |
MIC range (μg/ml) |
||
|---|---|---|---|---|
| CO2 | Ambient air | CO2 | Ambient air | |
| Doxycycline | 0.06 | 0.12 | 0.03-1 | 0.06-0.5 |
| Gentamicin | 2 | 1 | 0.5-8 | 0.5-2 |
| Rifampin | 1 | 1 | 0.25->8 | 0.25-2 |
| Streptomycin | 4 | 4 | 2-16 | 1-8 |
| Tetracycline | 0.12 | 0.25 | 0.03-0.5 | 0.06-0.5 |
| Trimethoprim-sulfamethoxazoleb | 1 | 0.5 | 0.25-4 | 0.25-2 |
For one site, ambient air data were available for only 31 isolates, while each of the other 2 sites had 35 ambient air results (4/39 isolates required CO2).
Only the trimethoprim portion is stated in the table.
Two types of nonparametric statistical methods were used to evaluate differences in MICs from AA incubation versus CO2 incubation for each of the antimicrobial agents by utilizing Statistical Analysis Software (SAS Institute, Inc., Cary, NC). The first method was the Wilcoxon rank sum test that compares the mean rank MICs for AA versus CO2 for a given agent. If the mean ranks are not statistically significantly different, the implication is that no significant shift in MIC has been observed based on this sample data. If, on the other hand, the mean rank is significantly higher for CO2 than for AA, this implies a positive MIC shift. The converse implies a positive MIC shift for AA incubation. The second statistical method, the Kuiper empirical distribution function test, was used to compare the empirical distribution of MICs for AA and CO2. The Kuiper test is used for two-sample data and compares the entire distribution of MICs for AA and CO2 that is as sensitive in the tails as at the median. Thus, it is possible to observe a significant shift in the mean rank, yet not necessarily to observe a significant shift in the entire distribution of MICs due to less difference in the tails of the distributions.
The results of the nonparametric analysis are shown in Table 2. Doxycycline and tetracycline showed a statistically significant shift to lower MICs under CO2 conditions, while gentamicin and streptomycin showed a significant shift to higher MICs in CO2; all were confirmed by the Kuiper test with P values of <0.05 (data not shown). Incubation in CO2 is expected to decrease the pH of the medium, which is known to decrease activity of aminoglycosides and to increase the activity of tetracyclines (2), so higher aminoglycoside MICs and lower tetracycline-class MICs in CO2 incubation were expected. Twelve streptomycin MICs resulted in a change from susceptible in AA to nonsusceptible when incubated in CO2. Two additional streptomycin MICs from different CO2-requiring isolates also had streptomycin MICs in the nonsusceptible range. Similarly, the MIC for one gentamicin result changed from susceptible in AA to nonsusceptible when incubated in CO2. As a result of these studies, CLSI made two new notations in the 2007 M100-S17 document for susceptibility testing of Brucella species (10). The first notation was an additional breakpoint for streptomycin if susceptibility testing is performed in CO2 incubation; the second notation warned that incubation of broth in CO2 may increase the MIC of aminoglycosides and decrease the MIC of tetracyclines, usually by 1 doubling dilution (10). Since tetracycline and doxycycline MICs in CO2 did not exceed 1 μg/ml, 2 log2 dilutions below the susceptible breakpoint of ≤4 μg/ml, CLSI deemed it unnecessary to provide alternate breakpoints for these drugs if CO2 incubation was used.
TABLE 2.
Nonparametric comparison of MICs for six antimicrobial agents tested against 39 Brucella isolates at three test sites for each incubation atmosphere
| Antimicrobial agent | Mean ranka |
P valueb | |
|---|---|---|---|
| Ambient air (n = 101) | CO2 (n = 117) | ||
| Doxycycline | 121.0 | 98.7 | 0.004 |
| Gentamicin | 85.6 | 130.2 | <0.001 |
| Rifampin | 108.4 | 109.6 | 0.876 |
| Streptomycin | 81.6 | 133.6 | <0.001 |
| Tetracycline | 119.4 | 101.0 | 0.016 |
| Trimethoprim-sulfamethoxazole | 98.3 | 119.2 | 0.008c |
n = the number of MIC results for each antimicrobial agent. There were 35 ambient air (4/39 isolates required CO2) MICs for 2 sites and only 31 at one site, for a total of 101 results. n = 100 available ambient air MICs for doxycycline and rifampin. The mean rank values were calculated by first converting the MICs to whole numbers in a linear fashion (e.g., MICs of 0.25, 0.5, 1, and 2 were converted to 1, 2, 3, and 4, respectively). These relative values were then used for statistical analysis.
Computed using the Wilcoxon rank sum test. A difference is significant if P is <0.05. If the mean ranks are not significantly different, the implication is that no significant shift in MIC has been observed based on these sample data. These results are supported by the Kuiper test for all agents except trimethoprim-sulfamethoxazole.
P = 0.110 by Kuiper test.
The interlaboratory MIC variability for the four drugs with different results in CO2 was examined (Table 3). Tetracycline and doxycycline did not show any obvious interlaboratory variation under either AA or CO2 incubation conditions. For gentamicin and streptomycin, test sites A and D tended to have higher MICs in CO2 than test site B but little or no variation between sites for AA incubation. This difference could not be explained by the CO2-generating system since test sites A and B used the same system and site D used another system. It is possible that these differences are the result of inoculum preparation or reader variability.
TABLE 3.
Comparison by laboratory test site of MICs for six antimicrobial agents incubated in ambient air and CO2 atmospheric conditions for 39 Brucella isolatesa
| Antimicrobial agent | Incubation condition | Test site | No. of occurrences at indicated MIC (μg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | |||
| Doxycycline | AA | A | 3 | 26 | 2 | |||||||
| B | 13 | 20 | 2 | |||||||||
| C | 9 | 23 | 2 | |||||||||
| CO2 | A | 26 | 11 | 2 | ||||||||
| B | 18 | 13 | 4 | 3 | 1 | |||||||
| D | 3 | 17 | 10 | 7 | 1 | 1 | ||||||
| Gentamicin | AA | A | 1 | 16 | 14 | |||||||
| B | 21 | 14 | ||||||||||
| C | 1 | 19 | 15 | |||||||||
| CO2 | A | 1 | 2 | 19 | 16 | 1 | ||||||
| B | 1 | 23 | 15 | |||||||||
| D | 1 | 3 | 22 | 13 | ||||||||
| Rifampin | AA | A | 1 | 3 | 19 | 7 | ||||||
| B | 3 | 21 | 11 | |||||||||
| C | 1 | 9 | 16 | 9 | ||||||||
| CO2 | A | 6 | 32 | 1 | ||||||||
| B | 1 | 5 | 19 | 14 | ||||||||
| D | 1 | 6 | 15 | 16 | 1b | |||||||
| Streptomycin | AA | A | 1 | 12 | 18 | |||||||
| B | 9 | 24 | 2 | |||||||||
| C | 6 | 25 | 4 | |||||||||
| CO2 | A | 14 | 15 | 10 | ||||||||
| B | 3 | 35 | 1 | |||||||||
| D | 1 | 18 | 16 | 4 | ||||||||
| Tetracycline | AA | A | 8 | 22 | 1 | |||||||
| B | 1 | 24 | 10 | |||||||||
| C | 8 | 27 | ||||||||||
| CO2 | A | 28 | 11 | |||||||||
| B | 21 | 13 | 5 | |||||||||
| D | 1 | 3 | 17 | 16 | 2 | |||||||
| Trimethoprim-sulfamethoxazolec | AA | A | 3 | 24 | 4 | |||||||
| B | 18 | 17 | ||||||||||
| C | 2 | 17 | 16 | |||||||||
| CO2 | A | 9 | 27 | 3 | ||||||||
| B | 1 | 15 | 18 | 5 | ||||||||
| D | 4 | 24 | 9 | 2 | ||||||||
For site A, ambient air (AA) data were available for only 31 isolates (30 for rifampin), while each of the other 2 AA testing sites had 35 AA results (4/39 isolates required CO2); site C had 34 AA results available for doxycycline.
One rifampin MIC was ≥16 μg/ml.
Only the trimethoprim portion of the 1/19 drug ratio is displayed for the MIC.
Trimethoprim-sulfamethoxazole demonstrated a shift to higher MICs in CO2 using the Wilcoxon rank sum test, but the Kuiper test did not confirm this, giving a P value of 0.110. Two trimethoprim-sulfamethoxazole MICs were in the nonsusceptible range when incubated in CO2: one from a CO2-requiring strain and one from a strain that did not require CO2 for growth. All trimethoprim-sulfamethoxazole MICs were in the susceptible range when incubated in AA. Changes in pH are not known to affect trimethoprim but can have a variable effect on sulfonamides (2); therefore, trimethoprim-sulfamethoxazole MICs may be affected by CO2. The interlaboratory variability of these results was examined (Table 3). Test site C tended to have slightly higher MICs in AA than the other two AA test sites, while test site D had higher MICs in CO2 than the other two sites for CO2 incubation. Since the endpoint of this drug is read at 80% inhibition, which is a subjective determination, reader variability is likely for trimethoprim-sulfamethoxazole MICs. Variability in endpoint determination may explain why the two statistical tests did not agree regarding a CO2 effect on trimethoprim-sulfamethoxazole MICs.
For quality control, MIC panels were tested with Staphylococcus aureus ATCC 29213, Streptococcus pneumoniae ATCC 49619, and Escherichia coli ATCC 25922 in AA and CO2 atmospheres, as applicable on each day of testing. MICs were read at 24 h and 48 h; all results were within acceptable ranges for Brucella susceptibility testing (7, 9), except for one rifampin MIC of 0.25 μg/ml at 24 h and 48 h in CO2 and one gentamicin MIC of >8 μg/ml at 48 h in AA. There appears to be no CO2 effect on quality control results, but this is difficult to assess with so few values (Table 4).
TABLE 4.
Comparison of MICs incubated in ambient air and CO2 atmospheric conditions for three quality control isolates at 48 h of incubation
| Antimicrobial agent | ATCC quality control isolate no.a | MIC (μg/ml) rangeb |
Acceptable AA MIC range (μg/ml) | |
|---|---|---|---|---|
| AA | CO2 | |||
| Doxycycline | 25922 | 1-2 | 1-2 | 1-4 |
| 29213 | 0.25-0.5 | 0.25-0.5 | 0.12-0.5 | |
| 49619 | 0.12 | 0.06-0.12 | 0.03-0.25 | |
| Gentamicin | 25922 | 4-≥8c | 4 | 1-8 |
| 29213 | NAd | NA | NA | |
| 49619 | NA | NA | NA | |
| Rifampin | 25922 | 8->8 | 8 | 4-16 |
| 29213 | NA | NA | NA | |
| 49619 | ≤0.12 | ≤0.12-0.25e | 0.008-0.06 | |
| Streptomycin | 25922 | 16-32 | 16 | 4-32 |
| 29213 | 8-32 | 16-32 | 8-64 | |
| 49619 | 32 | 32-64 | 16-128 | |
| Tetracycline | 25922 | 2 | 4 | 0.5-4 |
| 29213 | 0.5-1f | 0.5 | 0.25-1 | |
| 49619 | 0.25 | 0.12-1 | 0.06-0.5 | |
| Trimethoprim-sulfamethoxazoleg | 25922 | NA | NA | NA |
| 29213 | NA | NA | NA | |
| 49619 | 0.5-1 | 1-2 | 0.5-2 | |
The quality control isolates represent the following organisms: ATCC 25922, Escherichia coli; ATCC 29213, Staphylococcus aureus; and ATCC 49619, Streptococcus pneumoniae.
There were eight MICs in AA and four MICs under the CO2 conditions for each drug.
There was one quality control MIC of >8 μg/ml, there were six at 4 μg/ml, and there was one at 8 μg/ml.
NA, not applicable: there are no published breakpoints in brucella broth for this organism and drug.
The lowest attainable MIC for the rifampin dilution series was ≤0.12 μg/ml; one result was 0.25 μg/ml.
There were seven quality control MICs of 1 μg/ml.
Only the trimethoprim portion is displayed.
In summary, CO2 increased the aminoglycoside MICs for some Brucella isolates by 1 log2 dilution and lowered tetracycline and doxycycline MICs by 1 log2 dilution, but only affected the category interpretation of streptomycin. Rifampin MICs were not influenced by CO2 incubation. For trimethoprim-sulfamethoxazole, CO2 could not be conclusively proven to affect the MICs for the organisms tested. For MIC testing of Brucella spp. in CO2, additional comments and breakpoints have been approved by the CLSI and published in M100-S17 based upon the results from these investigations (10). A separate breakpoint (≤16 μg/ml susceptible instead of ≤8 μg/ml in ambient air) was given for interpreting streptomycin MICs when CO2 is used for BMD incubation and warning comments were given for gentamicin, tetracycline, and doxycycline MIC results for CO2 incubation. The use of CO2 should be used only for MIC testing of Brucella spp. when it is required for adequate growth, as it can affect the MIC results for aminoglycosides and tetracycline-class drugs.
(This study was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006.)
Acknowledgments
We thank the staff at CDC's Bacterial Zoonoses Branch and the Division of Food-Borne, Bacterial and Mycotic Diseases (National Center for Zoonotic, Vector-Borne, and Enteric Diseases) for providing many of the Brucella spp. used in this study. We thank Brandon Kitchel on the Antimicrobial Resistance Team, Division of Healthcare Quality Promotion at CDC, for making the figure graphics.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Use of trade names is for identification purposes and does not constitute endorsement by the Public Health Service or the U.S. Department of Health and Human Services.
Footnotes
Published ahead of print on 23 December 2009.
REFERENCES
- 1.Akova, M., D. Gur, D. M. Livermore, T. Kocagoz, and H. E. Akalin. 1999. In vitro activities of antibiotics alone and in combination against Brucella melitensis at neutral and acidic pHs. Antimicrob. Agents Chemother. 43:1298-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsterdam, D. 1996. Susceptibility testing of antimicrobials in liquid media, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD.
- 3.Ariza, J., J. Bosch, F. Gudiol, J. Linares, P. F. Viladrich, and R. Martin. 1986. Relevance of in vitro antimicrobial susceptibility of Brucella melitensis to relapse rate in human brucellosis. Antimicrob. Agents Chemother. 30:958-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baykam, N., H. Esener, O. Ergonul, S. Eren, A. K. Celikbas, and B. Dokuzoguz. 2004. In vitro antimicrobial susceptibility of Brucella species. Int. J. Antimicrob. Agents 23:405-407. [DOI] [PubMed] [Google Scholar]
- 5.Bosch, J., J. Linares, M. J. Lopez de Goicoechea, J. Ariza, M. C. Cisnal, and R. Martin. 1986. In-vitro activity of ciprofloxacin, ceftriaxone and five other antimicrobial agents against 95 strains of Brucella melitensis. J. Antimicrob. Chemother. 17:459-461. [DOI] [PubMed] [Google Scholar]
- 6.Bossi, P., A. Tegnell, A. Baka, F. Van Loock, J. Hendriks, A. Werner, H. Maidhof, and G. Gouvras. 2004. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Euro Surveill. 9:E15-E16. [PubMed] [Google Scholar]
- 7.Brown, S. D., and M. M. Traczewski. 2005. Broth microdilution susceptibility testing of Brucella species: quality control limits for ten antimicrobial agents against three standard quality control strains. J. Clin. Microbiol. 43:5804-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed., vol. 26, no. 2. Approved standard M7-A7. CLSI, Wayne, PA.
- 9.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing: 16th informational supplement, vol. 26, no. 3. M100-S16. CLSI, Wayne, PA.
- 10.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing: 17th informational supplement, vol. 27, no. 1. M100-S17. CLSI, Wayne, PA.
- 11.De Rautlin de la Roy, Y. M., B. Grignon, G. Grollier, M. F. Coindreau, and B. Becq-Giraudon. 1986. Rifampicin resistance in a strain of Brucella melitensis after treatment with doxycycline and rifampicin. J. Antimicrob. Chemother. 18:648-649. [DOI] [PubMed] [Google Scholar]
- 12.Falagas, M. E., and I. A. Bliziotis. 2006. Quinolones for treatment of human brucellosis: critical review of the evidence from microbiological and clinical studies. Antimicrob. Agents Chemother. 50:22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gur, D., S. Kocagoz, M. Akova, and S. Unal. 1999. Comparison of E test to microdilution for determining in vitro activities of antibiotics against Brucella melitensis. Antimicrob. Agents Chemother. 43:2337. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, W. H., and R. E. Manion. 1970. In vitro susceptibility of Brucella to various antibiotics. Appl. Microbiol. 20:600-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jevitt, L. A., L. M. Weigel, B. De, T. Popovic, and J. B. Patel. 2005. Development of a broth microdilution procedure for antimicrobial susceptibility testing of Brucella spp., abstr. C-357. Abstr. 105th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 16.Khan, M. Y., M. Dizon, and F. W. Kiel. 1989. Comparative in vitro activities of ofloxacin, difloxacin, ciprofloxacin, and other selected antimicrobial agents against Brucella melitensis. Antimicrob. Agents Chemother. 33:1409-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King, A. 2001. Recommendations for susceptibility tests on fastidious organisms and those requiring special handling. J. Antimicrob. Chemother. 48(Suppl. 1):77-80. [DOI] [PubMed] [Google Scholar]
- 18.Lang, R., R. Raz, T. Sacks, and M. Shapiro. 1990. Failure of prolonged treatment with ciprofloxacin in acute infections due to Brucella melitensis. J. Antimicrob. Chemother. 26:841-846. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist, D., M. C. Chu, and W. S. Probert. 2007. Francisella and Brucella, p. 815-834. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 20.Maley, M. W., K. Kociuba, and R. C. Chan. 2006. Prevention of laboratory-acquired brucellosis: significant side effects of prophylaxis. Clin. Infect. Dis. 42:433-434. [DOI] [PubMed] [Google Scholar]
- 21.Memish, Z., M. W. Mah, S. Al Mahmoud, M. Al Shaalan, and M. Y. Khan. 2000. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J. Infect. 40:59-63. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen, J. E., D. G. Moore, J. E. Clarridge, and E. J. Young. 1986. Antimicrobial susceptibility of clinical isolates of Brucella. Diagn. Microbiol. Infect. Dis. 5:163-169. [DOI] [PubMed] [Google Scholar]
- 23.Robichaud, S., M. Libman, M. Behr, and E. Rubin. 2004. Prevention of laboratory-acquired brucellosis. Clin. Infect. Dis. 38:e119-e122. [DOI] [PubMed] [Google Scholar]
- 24.Robinson-Dunn, B. 2002. The microbiology laboratory's role in response to bioterrorism. Arch. Pathol. Lab. Med. 126:291-294. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein, E., R. Lang, B. Shasha, B. Hagar, L. Diamanstein, G. Joseph, M. Anderson, and K. Harrison. 1991. In vitro susceptibility of Brucella melitensis to antibiotics. Antimicrob. Agents Chemother. 35:1925-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trujillano-Martin, I., E. Garcia-Sanchez, M. J. Fresnadillo, J. E. Garcia-Sanchez, J. A. Garcia-Rodriguez, and I. Montes Martinez. 1999. In vitro activities of five new antimicrobial agents against Brucella melitensis. Int. J. Antimicrob. Agents 12:185-186. [DOI] [PubMed] [Google Scholar]
- 27.Yagupsky, P., and E. J. Baron. 2005. Laboratory exposures to brucellae and implications for bioterrorism. Emerg. Infect. Dis. 11:1180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young, E. J. 1995. Brucellosis: current epidemiology, diagnosis, and management. Curr. Clin. Top. Infect. Dis. 15:115-128. [PubMed] [Google Scholar]