Abstract
Endocarditis due to Actinomyces neuii is a rare disease, with only 14 reported cases. Recently, A. neuii was added to the list of species implicated in endocarditis of native valves. We now report the first case of prosthetic valve endocarditis and the first successful control of endocarditis caused by this organism without surgical intervention.
CASE REPORT
A 66-year-old retiree with a 2-month history of dyspnea, general weakness, and recurrent fever was referred to our university hospital with three independent consecutive blood cultures that were positive for Actinomyces neuii.
Gram-stained preparations from blood culture bottles had shown short Gram-positive rods.
Growth of small nonpigmented colonies was detected after the sixth day of incubation in one bottle and after the third day of incubation in two bottles. Positive blood cultures were subcultured on blood agar and incubated at 37°C anaerobically, aerobically, and on chocolate agar under 5% CO2 atmosphere. Poor growth was observed under anaerobic conditions, whereas a good growth rate was detected under aerobic and CO2 atmosphere conditions. Subcultured bottles showed tiny colonies on the second day of incubation.
Catalase production was tested positive, and Gram staining confirmed nonbranching Gram-positive rods. Due to the relative urgency of our case, we opted for a sequencing approach for further identification of the isolate. Three independent colonies of the cultured isolate were sequenced. Using the forward primer 5′-AGAGTTTGATCHTGGYTYAG-3′ (13) and the reverse primer 5′-CTTTACGCCCARTRAWTCCG-3 (3) (bold letters indicate degenerate primer sequences), we amplified a 570-bp template of the 16S rRNA gene. Sequencing was performed on an ABI Prism 3130xl in the forward direction using the BigDye Terminator kit and resulted in a 500-bp sequence. Comparison of sequences using Michigan 16S BLAST and NCBI BLAST showed a 100% homology to Actinomyces neuii subsp. neuii (strain DSM8576).
On admission, the patient had been on intravenous antibiotic therapy, with a combination of penicillin G, meropenem, and erythromycin for 2 weeks in a peripheral hospital (Table 1). He reported a weight loss of 6 kg during the past 2 months (current body weight, 90 kg), headaches, and sweating at night but no fever. He further described having injured his left hand about 2 weeks before the beginning of symptoms while working in his garden. While picking up foliage, he punctured the palm of his left hand on a hidden spike, resulting in the painful swelling of the left palm for several days. These symptoms disappeared spontaneously within a few days, and the patient did not seek medical advice.
TABLE 1.
Summary of the clinical course for the patient
| Time (day) | Antibiotic(s) (dosage)a | Results obtained from: |
|
|---|---|---|---|
| Echocardiography | Blood cultures | ||
| 1 | Valvular vegetations, paravalvular cavity | 3/3 positive | |
| 1-20 | Penicillin G (5 million units 4 times/day, i.v.), meropenem (1 g 2 times/day, i.v.), erythromycin (1 g 4 times/day) | Negative | |
| 21-46 | Penicillin G (5 million units 4 times/day, i.v.) | Fewer vegetations, constant paravalvular cavity | Negative |
| 47-365 | Amoxicillin (2 × 1 g/day, p.o.) | No vegetations, constant paravalvular cavity | Negative |
| 366-427 | None | No vegetations, constant paravalvular cavity | Negative |
i.v., intravenously; p.o., orally.
Four years previously, the patient had undergone aortic valve replacement (23-mm Medtronic-Hall composite prosthesis) and implantation of an aortic conduit due to thoracic aortic aneurysm and aortic valve insufficiency. The last routine echocardiographic control had shown regular function of the prosthetic valve and normal left and right ventricular function. His medical history included diabetes mellitus type II and traumatic amputation of the left lower leg in early childhood.
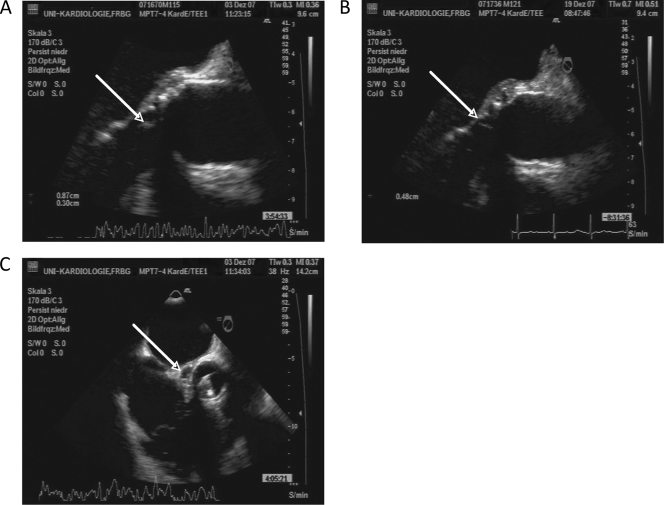
On admission to our hospital, the patient appeared well, afebrile, and normotensive, and his heart rate was 78 beats/min. Careful clinical examination revealed no signs of Osler's nodes, splinter hemorrhages, or Janeway lesions. Cardiac examination revealed normal mechanic heart sounds without murmurs and no signs of heart failure. Laboratory testing showed normocytic anemia (hemoglobin value, 5.7 mmol/liter), an elevated C-reactive protein (CRP) level of 66 mg/liter (normal value, <5 mg/liter), a white blood count of 5,600 cells/μl, and no further abnormalities. A transesophageal echocardiogram (TEE) revealed free-floating vegetations attached to the aortic valve prosthesis and a perivalvular cavity of approximately 22 by 9 mm, connected to the left ventricular outflow tract and compatible with a washed-out perivalvular abscess (Fig. 1). The aortic valve prosthesis showed normal function.
FIG. 1.
Transesophageal echocardiography (TEE). TEE imaging showed a regression of the valvular vegetations under antibiotic therapy. (A) At the time of diagnosis; (B) after 2 weeks of antibiotic therapy; (C) perivalvular cavity at the time of diagnosis, consistent with a washed-out abscess formation.
Computer tomography of the chest and abdominal sonography were unremarkable, and cerebral magnetic resonance imaging showed no signs of septic embolization. Ear, nose, and throat test results were unremarkable and showed no chronic focus of infection.
Regarding the modified Duke criteria (9), the patient therefore fulfilled two major criteria (a TEE demonstrating an oscillating mass on the prosthetic valve and a valve ring abscess-like formation and three independent blood cultures positive for an organism consistent with endocarditis) and one minor criterion (a predisposing heart condition, namely, an artificial valve).
Due to the stable clinical condition of the patient, the stable function of the prosthesis, and the markedly increased operative risk due to the aortic conduit, we opted for a trial of conservative management. Since the isolated strain of A. neuii showed excellent sensitivity to penicillin, the antibiotic regimen was switched to monotherapy with intravenous penicillin G, 5 million units four times daily.
Under continuous intravenous antibiotic therapy, the patient stayed afebrile and well, blood cultures remained negative, and CRP levels decreased to normal values at day 28 of antibiotic therapy.
Transesophageal echocardiographic reevaluation showed a regression of the valvular vegetations, which were no longer detectable after 6 weeks of antibiotic therapy. At this time point, the perivalvular cavity appeared with a constant size.
Antibiotic therapy was switched from an intravenous to an oral regimen, with amoxicillin at 2 g twice daily. The patient was discharged into ambulatory care and instructed to document his body temperature daily and to report any recurrence of symptoms.
Echocardiographic follow-ups at 8 weeks and at 11 months showed a stable valvular status, without any signs of actively progressing disease. The persistently asymptomatic patient stayed on oral antibiotic therapy for 11 months, which was well tolerated. After this time period, the antibiotic treatment was discontinued. A transesophageal echocardiographic follow-up at 2 months after the discontinuation of therapy showed no signs of disease progression. The patient still reports good health at 13 months following the initial diagnosis, with persistent negative blood cultures and normal values for CRP and leukocytes.
Endocarditis due to Actinomyces infection is a very uncommon condition with heterogeneous manifestations. A. neuii subsp. neuii was defined by Funke et al. in 1994 during a revision of the CDC grouping of coryneform organisms, corresponding to the CDC group 1 coryneform bacteria (11). These organisms are characterized as irregularly shaped Gram-positive rods that grow well under aerobic conditions (7, 10). A. neuii does not display the typical branching filaments observed in most other actinomyces species (11). Although the natural habitat of this species is not known, coryneform bacteria are, however, frequently observed as part of the normal oropharyngeal and sometimes urogenital flora (5). This is generally regarded as a nonpathogenic colonization, and the pathogenic potential of this particular species remains largely unclear. A. neuii does not cause typical actinomycosis, with granulomas and discharge of sulfur granules, but it is frequently isolated from abscesses and infected atheromas (11). Clinical reports include cases of chronic postoperative endophthalmitis after cataract surgery (15, 16), neonatal sepsis probably caused by A. neuii in the maternal genital tract (14), ventriculoperitoneal shunt infection (18), and infection of a mammary prosthesis caused by a coagulase-negative strain of A. neuii (6).
The only other report in the literature of endocarditis caused by A. neuii recently described a destruction of the native aortic valve of a 68-year-old male patient that required surgical management (8). In this case, the patient underwent urgent aortic valve replacement on day 9 of triple antibiotic therapy with ampicillin, gentamicin, and ceftriaxone. The antibiotic treatment was continued postoperatively with intravenous ampicillin, switched to intravenous ceftriaxone due to interstitial nephritis after 3 weeks, and continued for a total of 12 weeks. Oral doxycycline was given for at least 9 months after discharge, and the patient remained well during the follow-up.
To the best of our knowledge, we now report the first case of prosthetic valve endocarditis due to A. neuii and the first description of the successful conservative treatment of A. neuii endocarditis.
Since surgical intervention was associated with an intolerably high risk due to a previously implanted aortic conduit, the patient was treated with a 6-week intravenous regimen of penicillin, during which the valvular vegetations regressed and the patient remained free of symptoms. This treatment was followed by oral amoxicillin for 11 months.
The port of entry in our case remains unknown. Although the patient reported an abscess after injuring his left hand during garden work 2 weeks before the beginning of symptoms, A. neuii does not typically occur in topsoil or foliage. Actinomyces could not be isolated from superficial throat swabs, and no focus of infection was found.
Due to the small number of cases, the optimal choice and duration of therapy still remain unknown. Actinomyces species are generally highly susceptible to beta-lactam antibiotics, so penicillins (MIC, <0.03 mg/liter) or cephalosporins are the agents of first choice. Alternatives are erythromycin, clindamycin, tetracyclines, or vancomycin, whereas gentamicin and ciprofloxacin show only limited activity (11). Since beta-lactams are usually included in the first-line treatment of suspected endocarditis (1), no change of standard protocols before isolation of the causative organism is necessary.
Treatment guidelines for prosthetic valve endocarditis by the American Heart Association and the European Society of Cardiology generally recommend a duration of treatment for at least 6 weeks with an agent that is bactericidal for the isolated microorganism (2, 4, 12). We followed these guidelines, which are also consistent with the usual recommendations for the treatment of actinomycosis in noncardiac organs, such as cervicofacial or abdominal actinomycosis (17). The clinical outcome demonstrated the efficacy of this strategy in our case, followed by an oral treatment period with amoxicillin for 11 months.
If the perioperative risk is highly increased, as in our case due to a previously implanted aortic conduit, conservative management by using intravenous antibiotic therapy followed by a long-term oral treatment regimen seems to be a valid treatment option for prosthetic valve endocarditis caused by Actinomyces neuii. Furthermore, we suggest including A. neuii in the list of pathogens possibly causing endocarditis in both native and prosthetic valves.
Footnotes
Published ahead of print on 23 December 2009.
REFERENCES
- 1.Abrutyn, E., C. H. Cabell, V. G. Fowler, B. Hoen, J. M. Miro, C. A. Mestres, D. J. Sexton, and G. R. Corey. 2007. Medical treatment of endocarditis. Curr. Infect. Dis. Rep. 9:271-282. [DOI] [PubMed] [Google Scholar]
- 2.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newburger, T. J. Pallasch, M. Takahashi, and K. A. Taubert. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394-e434. [DOI] [PubMed] [Google Scholar]
- 3.Bergmans, A. M., J. W. Groothedde, J. F. Schellekens, J. D. van Embden, J. M. Ossewaarde, and L. M. Schouls. 1995. Etiology of cat scratch disease: comparison of polymerase chain reaction detection of Bartonella (formerly Rochalimaea) and Afipia felis DNA with serology and skin tests. J. Infect. Dis. 171:916-923. [DOI] [PubMed] [Google Scholar]
- 4.Bonow, R. O., B. A. Carabello, C. Kanu, A. C. de Leon, Jr., D. P. Faxon, M. D. Freed, W. H. Gaasch, B. W. Lytle, R. A. Nishimura, P. T. O'Gara, R. A. O'Rourke, C. M. Otto, P. M. Shah, J. S. Shanewise, S. C. Smith, Jr., A. K. Jacobs, C. D. Adams, J. L. Anderson, E. M. Antman, V. Fuster, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, R. Nishimura, R. L. Page, and B. Riegel. 2006. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Dis.): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114:e84-e231. [DOI] [PubMed] [Google Scholar]
- 5.Bowden, G. 1996. Actinomycosis. .In S. Baron (ed.), Baron's medical microbiology, 4th ed. University of Texas Medical Branch, Galveston, TX. [PubMed]
- 6.Brunner, S., S. Graf, P. Riegel, and M. Altwegg. 2000. Catalase-negative Actinomyces neuii subsp. neuii isolated from an infected mammary prosthesis. Int. J. Med. Microbiol. 290:285-287. [DOI] [PubMed] [Google Scholar]
- 7.Clarridge, J. E., III, and Q. Zhang. 2002. Genotypic diversity of clinical Actinomyces species: phenotype, source, and disease correlation among genospecies. J. Clin. Microbiol. 40:3442-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, E., J. Bishara, B. Medalion, A. Sagie, and M. Garty. 2007. Infective endocarditis due to Actinomyces neuii. Scand. J. Infect. Dis. 39:180-183. [DOI] [PubMed] [Google Scholar]
- 9.Durack, D. T., A. S. Lukes, and D. K. Bright. 1994. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am. J. Med. 96:200-209. [DOI] [PubMed] [Google Scholar]
- 10.Funke, G., G. M. Lucchini, G. E. Pfyffer, M. Marchiani, and A. von Graevenitz. 1993. Characteristics of CDC group 1 and group 1-like coryneform bacteria isolated from clinical specimens. J. Clin. Microbiol. 31:2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funke, G., S. Stubbs, A. von Graevenitz, and M. D. Collins. 1994. Assignment of human-derived CDC group 1 coryneform bacteria and CDC group 1-like coryneform bacteria to the genus Actinomyces as Actinomyces neuii subsp. neuii sp. nov., subsp. nov., and Actinomyces neuii subsp. anitratus subsp. nov. Int. J. Syst. Bacteriol. 44:167-171. [DOI] [PubMed] [Google Scholar]
- 12.Horstkotte, D., F. Follath, E. Gutschik, M. Lengyel, A. Oto, A. Pavie, J. Soler-Soler, G. Thiene, A. von Graevenitz, S. G. Priori, M. A. Garcia, J. J. Blanc, A. Budaj, M. Cowie, V. Dean, J. Deckers, E. Fernandez Burgos, J. Lekakis, B. Lindahl, G. Mazzotta, J. Morais, O. A. Smiseth, A. Vahanian, F. Delahaye, A. Parkhomenko, G. Filipatos, J. Aldershvile, and P. Vardas. 2004. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European Society of Cardiology. Eur. Heart J. 25:267-276. [DOI] [PubMed] [Google Scholar]
- 13.Kotilainen, P., J. Jalava, O. Meurman, O. P. Lehtonen, E. Rintala, O. P. Seppala, E. Eerola, and S. Nikkari. 1998. Diagnosis of meningococcal meningitis by broad-range bacterial PCR with cerebrospinal fluid. J. Clin. Microbiol. 36:2205-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann, C., S. Dertinger, G. Hartmann, R. Schurz, and B. Simma. 2002. Actinomyces neuii and neonatal sepsis. Infection 30:178-180. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Santonja, J. J., E. Campos-Mollo, E. Fuentes-Campos, J. Samper-Gimenez, and J. L. Alio. 2007. Actinomyces neuii subspecies anitratus chronic endophthalmitis after cataract surgery. Eur. J. Ophthalmol. 17:445-447. [DOI] [PubMed] [Google Scholar]
- 16.Raman, V. S., N. Evans, B. Shreshta, and R. Cunningham. 2004. Chronic postoperative endophthalmitis caused by Actinomyces neuii. J. Cataract Refract. Surg. 30:2641-2643. [DOI] [PubMed] [Google Scholar]
- 17.Smego, R. A., Jr., and G. Foglia. 1998. Actinomycosis. Clin. Infect. Dis. 26:1255-1261, 1262-1263. [DOI] [PubMed] [Google Scholar]
- 18.Watkins, R. R., K. Anthony, S. Schroder, and G. S. Hall. 2008. Ventriculoperitoneal shunt infection caused by Actinomyces neuii subsp. neuii. J. Clin. Microbiol. 46:1888-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]