Abstract
Sodium polyanethole sulfonate (SPS; trade name, Liquoid) is a constituent in culture media used to grow bacteria from blood samples from patients suspected of bacteremia. SPS prevents the killing of bacteria by innate cellular and humoral factors. We analyzed the effect of SPS on the three complement activation pathways: the classical, alternative, and lectin pathways, respectively. Inhibition of complement activity by SPS is caused by a blocking of complement activation and is not a result of complement consumption. The classical pathway is inhibited at SPS concentrations greater than 0.1 mg/ml, and complete inhibition is seen at 0.4 mg/ml. An SPS concentration of 0.5 mg/ml completely inhibits the binding of C1q and subsequent incorporation of C3, C4, and C9. The same was observed for the alternative pathway with an inhibition at SPS concentrations from 0.1 mg/ml and a complete inhibition from 0.4 mg/ml. Here, properdin binding was completely absent, and no incorporation of C3 and C9 was observed. In contrast, the lectin complement pathway remains unaffected at these SPS concentrations, and inhibition is first observed from 0.7 mg/ml. A complete inhibition required concentrations greater than 1 mg/ml. SPS is used in growth media (e.g., BACTEC and BacT/Alert) at concentrations from 0.3 to 0.5 mg/ml. The well-known finding that certain bacteria are growth inhibited by blood factors could therefore be a consequence of the lectin pathway, which is not inhibited at these concentrations. In addition, our findings also open up the possibility of a new assay for the assessment of the functional capacity of the lectin complement pathway.
Sodium polyanethole sulfonate (SPS) is the most common anticoagulant used in commercial blood culture bottles. Blood from patients with symptoms of bacteremia has been drawn under sterile conditions into bottles containing growth medium containing SPS for culture of bacteria (3, 11, 19). SPS has been shown to function as an anticoagulant (4) and as an inhibitor of humoral and cellular elements that might interfere with bacterial growth. SPS has also been shown to influence the complement system and to inhibit phagocytosis and leukocyte functions (2). Under the trade name Liquoid, SPS is used in concentrations of 0.25 to 0.5 mg/ml, most often 0.35 mg/ml in growth media. The exact function of SPS on the complement system is, however, not clear; some studies have demonstrated that SPS functions through an activation of the complement system via activation of complement component C1 (6), while other studies have indicated an inhibition of complement activation (7).
The complement (C) system involves a large number of distinct plasma proteins that react with one another in sequence to opsonize or directly kill invading microorganisms and to induce a series of inflammatory responses. There are three distinct pathways through which the C system can be activated. These pathways—the classical pathway (CP), the alternative pathway (AP), and the lectin pathway (LP)—depend on different molecules for their initiation. They all converge to generate the same central effector molecule, C3b, through the activity of C3-activating enzyme complexes, the C3-convertases (17, 18). The CP is initiated as a result of the formation of antibody-antigen complexes and involves the C1 complex [C1q-(C1s)2-(C1r)2]. Binding of the C1 complex to an antibody-antigen complex leads to cleavage of C4 and C2 and subsequent downstream complement activation. The AP is initiated by the presence of foreign surfaces, e.g., complex carbohydrates and the absence of cellular inhibitors, leading to the formation of the C3-convertase C3bBb, stabilized by properdin. The LP is activated when mannan-binding lectin (MBL) or ficolins bind to restricted patterns of carbohydrate structures on the surface of various pathogens, which triggers the MBL-associated serine proteases (MASPs) to cleave C4 and C2, leading to further downstream complement activation (16). With a prevalence of 5 to 7% in the general Caucasian population, MBL deficiency is the most common known immunodeficiency (14). Several studies have shown that MBL deficiency is associated with increased susceptibility to several infectious diseases, including extracellular pathogens and particularly bacteria, which can cause acute respiratory tract infections during early childhood (5, 12).
By setting up assays for the three individual complement pathways, we analyzed the effect of SPS on each individual pathway. We could not confirm an effect of SPS as an activator of complement, but our results show a dose-dependent inhibition of the CP and the AP, whereas the LP remains unaffected in a concentration window where SPS completely inhibits the two other pathways. The experiments also indicate that currently used bacterial growth media may not contain enough SPS for efficient inhibition of the LP and thus the killing of certain bacteria. These findings also open up possibilities for designing assays for the LP with no interference from the other pathways.
(Preliminary data from this study were presented at the XIth European Meeting on Complement in Human Disease in Cardiff, Wales, in 2007.)
MATERIALS AND METHODS
Serum sources.
A pool serum of normal human sera (pNHS) from 12 healthy volunteers was used as a complement source. Blood was collected in dry glass tubes (Venoject) and allowed to clot at room temperature for 3 h. The clotted blood was centrifuged at 970 × g for 15 min at 4°C, followed by collection of serum. The sera were immediately pooled and frozen in aliquots at −80°C. Complement-deficient sera (C2 deficient and MBL deficient, B/B genotype [12]) were from individual donors and were collected as described above.
Reagents and buffers.
The following monoclonal antibodies (MAbs), previously developed in our laboratories, were used: Hyb 131-10 and 131-11 against MBL, all IgG1κ and reacting with different epitopes on the MBL molecule. Hyb 162-02 against the β-chain of C4b, HAV3-4 against the β-chain of C3b, Hyb 04-22 against C9, Hyb 39-04 against properdin, and the MAb HuC3c F1-4, which is a C3c-specific antibody reacting against an epitope only exposed on the C3c fragment (15). All of the MAbs, except for HuC3c F1-4, are available from Bioporto A/S (Gentofte, Denmark).
The following polyclonal antibodies were used: biotin-labeled goat anti-human C1q (Lot 68-001-1; Miles Laboratories). Rabbit anti-human serum albumin (A001), horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (PO260), rabbit anti-human C3c (A0062), and HRP-conjugated streptavidin (P0397) were all from Dako, Glostrup, Denmark.
The buffers used for enzyme-linked immunosorbent assays (ELISAs) included coating buffer (35 mM NaHCO3, 15 mM Na2CO3; pH 9.6), developing buffer (35 mM C6H8O7, 65 mM Na3HPO4; pH 5), and dilution-washing TBS buffer (10 mM Tris, 140 mM NaCl; pH 7.4) containing 0.05% Tween 20. SPS (Sigma catalog no. P2008-5G) was dissolved in TBS to a final concentration of 10 mg/ml. The following media were used: BACTEC (Plus Aerobic/F, lot 8177750; Becton Dickinson) and BacT/Alert (Aerobic, lot 1020692; bioMérieux).
Activation of complement.
Inulin (Sigma catalog no. 13754-25G), zymosan (Sigma catalog no. Z4250-25G), cobra venom factor (CVF; Sigma catalog no. V-9125), and heat-aggregated human IgG (HAG; prepared from human IgG, for intravenous use; Statens Serum Institut, Denmark) were used as activators of C.
Aggregated human IgG was prepared by incubating human IgG (10 mg/ml) at 56°C for 60 min. Large aggregates were removed by centrifugation at 1,400 × g for 5 min. Serum was incubated with 1 mg of inulin, zymosan, CVF, HAG, or SPS/ml or no additives at 37°C for 60 min, centrifuged at 1,400 × g for 5 min, and frozen at −80°C until further analysis.
Assessment of functional capacity of the CP.
The assay to measure the capacity of the CP was performed essentially as described by Zimmermann-Nielsen et al. (20) and Baatrup et al. (1), with modifications including the use of human serum albumin (HSA) and rabbit anti-HSA IgG as the activation matrix and the use of 1 μg of the following detection antibodies/ml: C1q (goat anti-C1q), C4 (Hyb 162-02), C3 (HAV3-4), and C9 (Hyb 04-22). Serum samples were diluted 1:1 in dilution buffer containing 4 mM Ca2+ and 2 mM Mg2+, with or without 0.5 mg of SPS/ml (final concentration), and then preincubated for 10 min on ice and added in serial dilutions to rabbit anti-HSA IgG-coated plates, followed by incubation for 1 h at 37°C.
Assessment of functional capacity of the AP.
The protocol for the functional capacity of the AP was similar to the method used for the CP assay, with the following modification: lipopolysaccharide (LPS) W Salmonella enterica serovar Typhimurium (lot 51974; Difco Laboratories) was immobilized on microtiter plates (Nunc, Roskilde, Denmark) at 10 μg/ml and used as an activating surface for AP activation. The serum samples were diluted 1:1 in dilution buffer containing 4 mM Mg2+ and 10 mM EGTA, with or without 0.5 mg of SPS/ml (final concentration), and then preincubated for 10 min on ice and added in serial dilutions to LPS-coated plates, followed by incubation for 60 min at 37°C. Detection of C3 (HAV3-4), properdin (Hyb 39-04), and C9 (Hyb 04-22) deposition was conducted as described above.
Assessment of the functional MBL pathway capacity.
To measure the MBL pathway, mannan (Sigma catalog no. 7204) was used as a ligand. Mannan was dissolved in phosphate-buffered saline (PBS; 10 mg/ml) and stored at 4°C until use. Microtiter plates (Nunc) were coated overnight at 4°C with 10 μg of mannan/ml in coating buffer. The plates were emptied and then washed in washing buffer containing 4 mM Ca2+, and the residual binding sites were blocked in washing buffer for 30 min at room temperature. Serum samples were diluted 1:1 in dilution buffer containing 4 mM Ca2+ and 2 mM Mg2+, with or without 0.5 mg of SPS/ml (final concentration), and preincubated for 10 min on ice and added in serial dilutions to the mannan-coated plates, followed by incubation for 2 h at 37°C. After a washing step, detection of MBL, C4, and C3 was performed as described for the CP assay with the addition of anti-MBL MAbs (a pool of Hyb 131-10 and 131-11).
For the three ELISA settings described above (CP, AP, and LP), the binding or deposition of the components was reduced to background levels when 10 mM EDTA was present in the dilution buffers (data not shown).
Quantification of C3c level.
Sandwich ELISA was used to measure the C3c level. Microtiter plates were coated with the 6 μg of anti-human C3c-specific antibody (HuC3c, F1-4)/ml in coating buffer overnight at 4°C. After incubation with appropriate activators, sera were made 10 mM with respect to EDTA (to quench additional in vitro activation), diluted 1:320, and incubated for 1 h. The amount of bound C3c was measured by sequential incubation with the biotin-labeled polyclonal C3c antibody (A0062) and HRP-streptavidin (PO397).
General procedure for ELISA development.
To prepare the microtiter plates, 100 μl of secondary reagent, either HRP-conjugated rabbit anti-mouse (diluted 1:1000) or HRP-conjugated streptavidin (diluted 1:2,000), was added to the each plate. The plates were incubated 1 h at room temperature and washed three times. The plates were developed with 100 μl/well of ortho-phenylenediamine (0.4 mg/ml; Kem-En-Tec Diagnostics, Denmark) dissolved in citrate buffer (35 mM citric acid, 65 mM Na2PO4 [pH 5]) containing 0.12‰ (vol/vol) H2O2 for 20 min in the dark at room temperature. The color reaction was stopped by the addition of 100 μl of 1 M H2SO4 to each well. Optical density (OD) levels were measured at 490 to 650 nm by using a V-Max kinetic reader (Molecular Devices).
SDS-PAGE and Western blotting.
Electrophoresis was performed on 4 to 12% (wt/vol) polyacrylamide gradient gels using the NuPAGE system (Invitrogen) according to the manufacturer's recommendations. Proteins were electroblotted onto polyvinylidene difluoride membranes (PVDF-HyBond; Amersham Bioscience). After blocking in PBS, 0.05% Tween 20 membranes were incubated with anti-C3 MAb (HAV3-4) at 1 μg/ml for 2 h in PBS-0.05% Tween 20, followed by incubation with HRP-conjugated rabbit anti-mouse immunoglobulin (P0260) diluted 1:1,000 in PBS-0.05% Tween 20 at room temperature for 1 h. The membranes were washed and developed with 0.04% 3-amino-9-ethylcarbazole (Sigma) and 0.015% H2O2 in 50 mM sodium acetate buffer (pH 5.0).
RESULTS
Analysis for complement activation after incubation of serum with SPS.
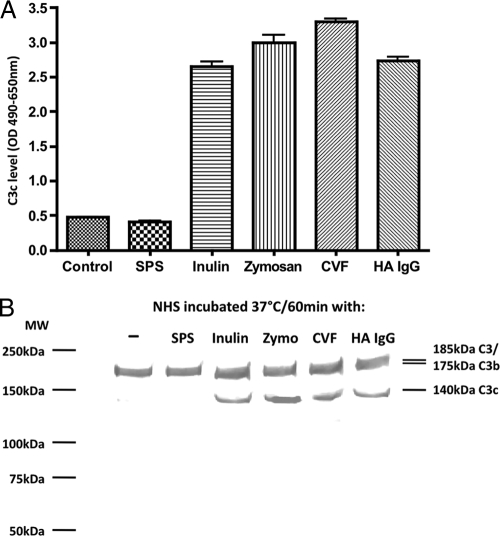
Complement activity can be abolished either through activation, followed by the consumption of complement factors, or through inhibition of the activity of essential components. The exact function of SPS on the complement system is not clear, but adding SPS to serum would result in increased levels of complement split products such as C3c if SPS acts as an activator of complement. To study this, serum was incubated with either SPS or known activators of complement. The levels of generated serum C3c were measured by a sandwich ELISA. Incubation with inulin, zymosan, heat-aggregated IgG, or CVF all resulted in increased levels of C3c (Fig. 1A). Serum incubated with SPS resulted in C3c levels similar to the control serum without addition of an activator.
FIG. 1.
(A) C3c levels in serum samples measured by a C3c-specific sandwich ELISA. Serum was incubated with inulin, zymosan, aggregated IgG (HA IgG), CVF, or SPS and control serum without application. After incubation, sera were made 10 mM with respect to EDTA, diluted 1:320. The C3c levels were assessed with a C3c-specific MAb (F1-4) as catching antibody and levels were measured as OD490-650 units. Error bars indicate two times the standard deviation of double determinations. (B) Western blot of human serum activated with inulin, zymosan (Zymo), aggregated IgG (HA IgG), CVF, or SPS and control serum without application. Nonreducing SDS-PAGE of serum samples diluted 1:40. C3 bands were visualized with the anti-human C3 monoclonal antibody (HAV3-4). We observed three bands with apparent molecular sizes of 180, 175, and 140 kDa corresponding to native C3, C3b/iC3b, and C3c, respectively. The MW marker was Precision Plus Protein Prestained (Bio-Rad).
The presence of C3 split products after incubation with SPS was also evaluated by Western blot analysis. We observed in all serum samples bands with apparent molecular sizes 180 and 175 kDa corresponding to intact C3 and C3b/iC3b, respectively. In addition, we observed clear bands of ∼140 kDa under nonreducing conditions corresponding to the C3c fragment in the lanes with sera activated with inulin, zymosan, aggregated IgG, or CVF. This band was very weak or absent in the control or the serum incubated with SPS, demonstrating that SPS does not activate complement (Fig. 1B).
Influence of SPS on the pathway-specific deposition of the complement factor C3 on activating surfaces.
To study the influence of SPS on the three complement pathways, we measured the pathway-specific C3 deposition during solid-phase activation. pNHS at 10% was incubated together with various SPS concentrations on different activating surfaces. C3 deposition was subsequently monitored. Figure 2 shows the effect of various SPS concentrations on the pathway-specific C3 depositions. Incubation of 10% pNHS without SPS induced a deposition of C3 after activation of all of the three pathways. This deposition could be dose dependently inhibited by SPS. Inhibition of the CP on an immune complex-coated surface was observed with SPS concentration from 0.1 mg/ml and complete inhibition at 0.4 mg/ml or higher. Likewise, C3 deposition on an LPS surface was affected by SPS concentrations from 0.1 mg/ml and completely inhibited at 0.4 mg of SPS/ml or higher. In contrast to these findings, C3 deposition via the LP on a mannan-coated surface remained unaffected by SPS until the SPS reached a concentration of 0.7 mg/ml. Complete inhibition of the LP was accomplished only at SPS concentrations higher than 1 mg/ml.
FIG. 2.
Incorporation of C3 on surface-bound activators of the three described complement activation pathways: CP, AP, and LP. pNHS was diluted 1:1 in dilution buffer in the presence of final SPS concentrations from 0 to 1 mg/ml. Afterward, the samples were further diluted 1:5 equivalent to a final serum concentration of 10%, and assays were carried out as described in Materials and Methods. Incorporation of C3 was determined as OD490-650 units. Error bars indicate two times the standard deviation of double determinations.
Thus, the C3 deposition via CP and AP was completely inhibited at 0.4 mg of SPS/ml, whereas the C3 deposition via LP at this concentration of SPS remained unaffected. Subsequent experiments to evaluate the suppressive effects of SPS on complement function utilized a final SPS concentration of 0.5 mg/ml, which slightly exceeds that required to completely inhibit complement activation by the classical or the alternative pathway.
Influence of SPS on the deposition or binding of complement factors C1q, C4, C3, and C9 as a result of activation of the classical complement pathway.
The effect of SPS on the functional capacity of CP was evaluated by using pNHS and MBL-deficient (B/B) serum on an immune complex-coated surface in the presence or absence of SPS. The incorporation of C1q, C4, C3, and C9 was measured by using specific MAbs. Incubation with various concentrations of SPS showed inhibition of the incorporation of all four components and complete inhibition at a concentration of 0.4 mg of SPS/ml (data not shown).
In the absence of SPS the CP activation resulted in a concentration-dependent deposition or binding of C1q, C4, C3, and C9 in both pNHS and MBL-deficient serum (twofold diluted from 1/10 to 1:5,120). In contrast, application of an SPS concentration of 0.5 mg/ml to the sera resulted in a complete inhibition of the deposition of all four components (Fig. 3A).
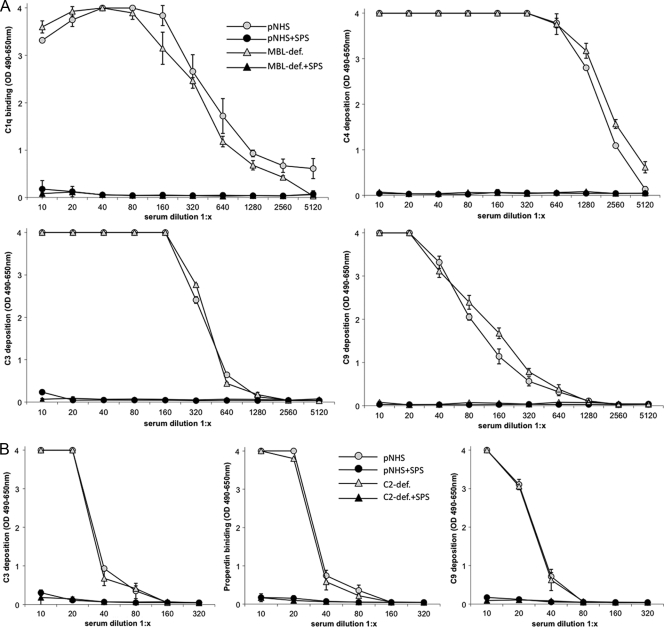
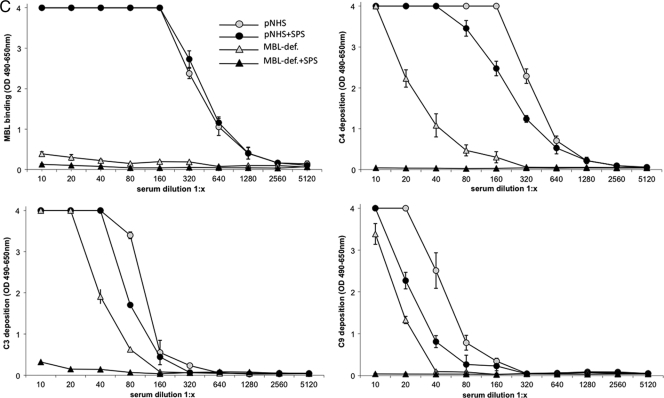
FIG. 3.
(A) Classical pathway. Twofold dilutions of pNHS or MBL-deficient serum in the presence or absence of SPS (final concentration of 0.5 mg/ml) incubated on an immune complex surface. The binding, activation, and deposition of C components C1q, C4, C3, and C9 were determined as OD490-650 units. Error bars indicate two times the standard deviation of double determinations. (B) Alternative pathway. Twofold dilutions of pNHS or C2-deficient serum in the presence or absence of SPS (final concentration, 0.5 mg/ml) incubated on an LPS surface with Mg2+-EGTA binding, activation, and the deposition of C3, properdin, and C9 was assessed by using specific MAbs and determined as OD490-650 units. Error bars indicate two times the standard deviation of double determinations. (C) Lectin pathway. Twofold dilutions of pNHS or MBL-deficient serum in the presence or absence of SPS (final concentration, 0.5 mg/ml) incubated on a mannan surface. Binding, activation, and deposition of MBL, C3, C4, and C9 were assessed by using specific MAbs and determined as OD490-650 units. Error bars indicate two times the standard deviation of double determinations.
Influence of SPS on the deposition or binding of complement factors C3, properdin and C9 as a result of activation of the alternative complement pathway.
To assess the influence of SPS on complement activation via the AP, we used pNHS and C2-deficient serum applied to an LPS-coated surface and diluted in buffer containing Mg2+-EGTA in the presence or absence of 0.5 mg of SPS/ml. Incubation of pNHS and C2-deficient serum in the absence of SPS resulted in a concentration-dependent deposition or binding of C3, properdin, and C9 (Fig. 3B). Incubation of serum samples in the presence of 0.5 mg of SPS/ml resulted in a complete inhibition of the deposition or binding of C3, properdin, and C9. Thus, as observed for the CP, the AP activation of complement is also abolished in the presence of an SPS concentration of 0.5 mg/ml.
Influence of SPS on deposition or binding of complement factors MBL, C4, C3, and C9 as a result of activation of the lectin complement pathway.
The effect of SPS on the lectin pathway was analyzed on a mannan-coated surface. Dilutions of pNHS and MBL-deficient serum were incubated with or without 0.5 mg of SPS/ml (final concentration). MBL-deficient serum (from a donor with B/B genotype) showed no binding of MBL to mannan, but some deposition of C3, C4, and C9 (Fig. 3C). This deposition of C3, C4, and C9 probably represents residual activity associated with the classical or the alternative pathway, since MBL-deficient serum cannot activate the lectin pathway. When MBL-deficient serum was incubated with 0.5 mg of SPS/ml, the deposition of C3, C4, and C9 was completely abolished, further demonstrating the capacity of SPS to inhibit the classical and the alternative pathway. Incubation of pNHS on the mannan-coated surface resulted in a concentration-dependent deposition of MBL, C3, C4, and C9. When pNHS was incubated with SPS, the deposition of MBL was unaltered, whereas the deposition of C3, C4, and C9 was slightly reduced. The failure of SPS to alter deposition of MBL strongly suggests that SPS does not inhibit the lectin pathway. The slight reductions in C3, C4, and C9 deposition probably represent the ability of SPS to inhibit residual complement activation by the classical and the alternative pathway.
Effect of Ca2+ and Mg2+ on the CP and AP inhibition mediated by SPS.
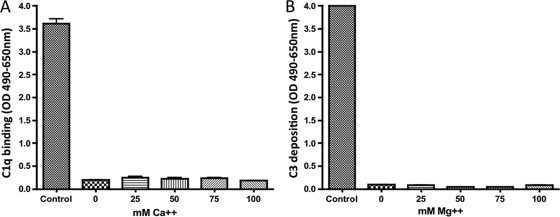
To examine whether the effect of SPS on the CP and the AP was due to a chelation of the divalent ions Mg2+ and Ca2+, pNHS with an concentration of 0.5 mg of SPS/ml was analyzed at different concentrations of Ca2+ or Mg2+, and the C1q binding and C3 deposition was subsequently measured (Fig. 4). We found that increasing the concentration (up to 100 mM) of Ca2+ did not restore the C1q binding to immune complexes in pNHS with 0.5 mg of SPS/ml (Fig. 4A). Similarly, no effect was observed when the Mg2+ concentrations were increased (up to 100 mM) on the C3 deposition to LPS (Fig. 4B). Accordingly, the SPS inhibition of the CP and AP is not due to a chelation of Mg2+ or Ca2+.
FIG. 4.
(A) Effect of increasing concentration of Ca2+ on the C1q binding in pNHS. (B) Effect of increasing concentration of Mg2+ on the C3 deposition in pNHS. In both experiments, pNHS was diluted 1:1 in the presence of a final SPS concentration of 0.5 mg/liter. The samples were assayed at 10% pNHS and carried out as described in Materials and Methods. Controls were pNHS without SPS application. The C3 deposition was determined as OD490-650 units. Error bars indicate two times the standard deviation of double determinations.
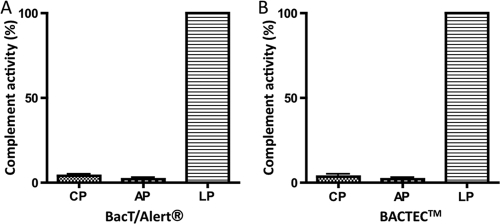
Effect of SPS in bacterial growth medium on complement activity.
We analyzed the effect of SPS in two commonly used bacterial growth media, BACTEC and BacT/Alert, that both contained 0.35 mg of SPS/ml. Figure 5 shows the complement activity measured as C3 incorporation when pNHS was incubated with growth medium (1 volume of pNHS plus 2 volumes of growth medium). This corresponds to an efficient SPS concentration of ∼0.35 mg/ml compared to Fig. 2. C3 incorporation was completely inhibited when assayed as CP or AP activity, whereas there was still deposition of C3 when the lectin pathway was activated. Altogether, these experiments show that the amount of SPS in the blood culture medium used in clinical microbiology laboratories only inhibits classical and alternative complement activity, whereas the activity through the lectin pathway is still functional.
FIG. 5.
Complement activity after incubation of pNHS with the growth media BacT/Alert (A) and BACTEC (B). One volume of pNHS was added to two volumes of growth medium, followed by assessment of the complement activity through the three complement pathway assays as described in Materials and Methods. The efficient concentration of SPS corresponds to ∼0.35 mg/ml in the final dilution.
DISCUSSION
Bacteremia is a serious and often life-threatening clinical condition. An important diagnostic tool for this condition is to analyze a blood specimen for the growth of bacteria on selected growth media. Such media often contain SPS as an anticoagulant and as an inhibitor of the bacteriostatic and bactericidal effects of blood cells and plasma factors. The effect of SPS is largely unknown, although the effect on plasma factors in the SPS has been ascribed to complement consumption or blocking of individual complement factors. We found that incubation of normal human serum with SPS did not lead to the activation (and consumption) of complement using split products of C3 as the readout. The effect of SPS on the individual complement activation pathways was subsequently investigated. For the classical and alternative pathways, inhibition of the incorporation of C3 started at a SPS concentration of 0.1 mg/ml with a complete inhibition at SPS concentrations greater than 0.3 mg/ml. In contrast to this, inhibition of C3 in the lectin pathway started at SPS concentrations above 0.7 mg/ml, and more than 1 mg/ml was required to obtain a complete inhibition. We also analyzed the effect of SPS on the incorporation and binding of other key components of the complement system. Using a fixed SPS concentration of 0.5 mg/ml (within the concentration window, where the LP remains unaffected) we analyzed the incorporation or binding of C1q, C4, C3, and C9 for the classical pathway; properdin, C3, and C9 for the alternative pathway; and MBL, C4, and C3 for the lectin pathway as a function of the serial serum-SPS dilutions. Various complement-deficient sera (C2-deficient serum with blocked activation of CP and LP and MBL-deficient serum that will not activate the LP) were also used for these analyses. The presence of SPS totally eliminated the binding of C1q and incorporation of C4, C3, and C9 in the classical pathway. This could well be explained by the inhibition of C1q binding to immune complexes, giving an overall inhibition of the classical pathway. Whether other components of the classical pathway are also inhibited remains unclear. For the alternative pathway, properdin binding, and incorporation of C3, C4 was also eliminated at this SPS concentration, but whether this inhibition is due to inhibition of the serine protease activity of factor D and/or factor Bb or whether SPS interferes with the interaction of C3b with factor B is not known. The lectin pathway, on the other hand, gave only a slight inhibition of the incorporation of C3, C4, and C9, whereas the MBL binding was unaffected by SPS. To look closer into this inhibition, we included serum samples from individuals with MBL deficiencies. These samples showed incorporation of C3, C4, and C9 when incubated on a mannan surface. However, addition of SPS at 0.5 mg/ml resulted in a complete inhibition of C3, C4, and C9 incorporation. We therefore conclude that the partial reduction of C4 incorporation seen with normal serum without SPS after activation on a mannan surface is due to interference from the classical pathway (due to the presence of anti-mannan antibodies in most normal sera [9]). This was also the case for the partial reduction of C3 and C9 incorporation involving interference from both classical and alternative pathway. Incorporation of C3 and C9 measured in the presence of SPS is therefore due to lectin pathway activation only, without any influence from the CP and AP. The commonly used culture media for culturing bacteria in blood contain between 0.035 and 0.05% (wt/vol) SPS and, based on our data, we conclude that this concentration of SPS will efficiently abolish complement activation via the classical or alternative pathway, whereas the lectin complement pathway still shows activity and may kill bacteria susceptible to the action of this pathway. Since the LP remains functionally active, it could be one of the reasons why some bacteria grow slow in those growth media.
Additionally, the effect of SPS on the three complement pathways outlines an easy platform for the assessment of the functional capacity of the lectin complement pathway in serum samples. The existing ELISA-based methods do not quench the interference from the AP (10, 13), or they rely on nonphysiologic conditions with exogenously added complement sources (8). Thus, using SPS to measure complement capacity of the lectin pathway will offer novel possibilities for patient diagnostics in individuals suspected of an immunodeficiency, as well as for the study of disease association linked to the lectin complement pathway.
Acknowledgments
The use of SPS as a tool for complement pathway-specific assay has been patented (PCT/DK2008/050221) by Immunobond ApS in cooperation with Yaseelan Palarasah, Mikkel-Ole Skjoedt, Lars Vitved, and Claus Koch.
Footnotes
Published ahead of print on 30 December 2009.
REFERENCES
- 1.Baatrup, G., H. Jonsson, A. Sjoholm, G. Sturfelt, and S. E. Svehag. 1988. Complement fixation by solid phase immune complexes; reduced capacity in SLE sera. J. Clin. Lab. Immunol. 26:73-79. [PubMed] [Google Scholar]
- 2.Belding, M. E., and S. J. Klebanoff. 1972. Effect of sodium polyanethole sulfonate on antimicrobial systems in blood. Appl. Microbiol. 24:691-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eng, J. 1975. Effect of sodium polyanethol sulfonate in blood cultures. J. Clin. Microbiol. 1:119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kindness, G., F. B. Williamson, and W. F. Long. 1979. Effect of polyanetholesulfonic acid and xylan sulfate on antithrombin III activity. Biochem. Biophys. Res. Commun. 88:1062-1068. [DOI] [PubMed] [Google Scholar]
- 5.Koch, A., M. Melbye, P. Sorensen, P. Homoe, H. O. Madsen, K. Molbak, C. H. Hansen, L. H. Andersen, G. W. Hahn, and P. Garred. 2001. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA 285:1316-1321. [DOI] [PubMed] [Google Scholar]
- 6.Loos, M., and D. Bitter-Suermann. 1976. Mode of interaction of different polyanions with the first (C1,C1) the second (C2) and the fourth (C4) component of complement. IV. Activation of C1 in serum by polyanions. Immunology 31:931-934. [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami, Y., I. H., E. Kitano, H. Kitamura, and Y. Ikada. 2001. Interaction of poly(2-acrylamido 2-methylpropane sulfonate)-grafted polystyrene beads with cationic complement proteins. J. Biomater. Sci. Polym. 12:451-465. [DOI] [PubMed] [Google Scholar]
- 8.Petersen, S. V., S. Thiel, L. Jensen, R. Steffensen, and J. C. Jensenius. 2001. An assay for the mannan-binding lectin pathway of complement activation. J. Immunol. Methods 257:107-116. [DOI] [PubMed] [Google Scholar]
- 9.Roos, A., P. Garred, M. E. Wildenberg, N. J. Lynch, J. R. Munoz, T. C. Zuiverloon, L. H. Bouwman, N. Schlagwein, F. C. Fallaux van den Houten, M. C. Faber-Krol, H. O. Madsen, W. J. Schwaeble, M. Matsushita, T. Fujita, and M. R. Daha. 2004. Antibody-mediated activation of the classical pathway of complement may compensate for mannose-binding lectin deficiency. Eur. J. Immunol. 34:2589-2598. [DOI] [PubMed] [Google Scholar]
- 10.Roos, A., B. L. H., Munoz, J., Zuiverloon, T., Faber-Krol, M. C., F. C. Fllaux-van den Houten, N. Klar-Mohamad, C. E. Hack, M. G. Tilanus, and M. R. Daha. 2003. Functional characterization of the lectin pathway of complement in human serum. Mol. Immunol. 39:655-688. [DOI] [PubMed] [Google Scholar]
- 11.Rotter, M. 1977. Microbiological diagnosis of septicemia. Infection 5:55-59. [DOI] [PubMed] [Google Scholar]
- 12.Roy, S., K. Knox, S. Segal, D. Griffiths, C. E. Moore, K. I. Welsh, A. Smarason, N. P. Day, W. L. McPheat, D. W. Crook, and A. V. Hill. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 359:1569-1573. [DOI] [PubMed] [Google Scholar]
- 13.Seelen, M. A., R. A., J. Wieslander, T. E. Mollnes, A. G. Sjöholm, R. Wurzner, M. Loos, F. Tedesco, R. B. Sim, P. Garred, E. Alexopoulos, M. W. Turner, and M. R. Daha. 2005. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. Immunol. Methods 296:187-198. [DOI] [PubMed] [Google Scholar]
- 14.Sjoholm, A. G., G. Jonsson, J. H. Braconier, G. Sturfelt, and L. Truedsson. 2006. Complement deficiency and disease: an update. Mol. Immunol. 43:78-85. [DOI] [PubMed] [Google Scholar]
- 15.Skjødt, M. O., J. Brandt, and L. Vitved. 2007. C3c as a fluid phase marker for inflammation: generation and characterization of monoclonal antibodies reacting against neoepitopes on split products of C3. Mol. Immunol. 44:3917. [Google Scholar]
- 16.Thiel, S., T. Vorup-Jensen, C. M. Stover, W. Schwaeble, S. B. Laursen, K. Poulsen, A. C. Willis, P. Eggleton, S. Hansen, U. Holmskov, K. B. Reid, and J. C. Jensenius. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386:506-510. [DOI] [PubMed] [Google Scholar]
- 17.Walport, M. J. 2001. Complement: part I. N. Engl. J. Med. 344:1058-1066. [DOI] [PubMed] [Google Scholar]
- 18.Walport, M. J. 2001. Complement: part II. N. Engl. J. Med. 344:1140-1144. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins, T. D., and S. E. West. 1976. Medium-dependent inhibition of Peptostreptococcus anaerobius by sodium polyanetholsulfonate in blood culture media. J. Clin. Microbiol. 3:393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann-Nielsen, E., L. H. Iversen, S. E. Svehag, O. Thorlacius-Ussing, and G. Baatrup. 2002. Activation capacity of the alternative and classic complement pathways in patients operated on for colorectal cancer. Dis. Colon Rectum 45:544-553. [DOI] [PubMed] [Google Scholar]