Abstract
Understanding the form of clinical change can help guide the development of more effective treatment strategies for opiate dependence. This study investigated the process of change by modeling transitions among four clinical states encountered among 64 detoxified opiate-dependent individuals treated with daily oral naltrexone: no opiate use, blocked opiate use (i.e. opiate use while adhering to oral naltrexone), unblocked opiate use (i.e. opiate use after having discontinued oral naltrexone), and treatment drop-out. The effects of baseline characteristics and two psychosocial interventions of differing intensity, Behavioral Naltrexone Therapy (BNT) and Compliance Enhancement (CE), on these transitions were investigated. Heavier opiate users were more likely to be retained in a comprehensive psychosocial intervention (i.e. BNT). Markov modeling indicated a transition from abstinence to the discontinuation of treatment was approximately 3.56 times greater among participants in CE relative to BNT indicating the more comprehensive psychosocial intervention kept participants engaged in treatment longer. Transitions to stopping treatment were more likely to occur following unblocked opiate use in both treatments. Continued opiate use while being blocked (i.e. extinction) accounted for a relatively low proportion of transitions to abstinence and may have more deleterious effects later in the treatment episode.
Keywords: Oral Naltrexone, Markov Modeling, Behavior Therapy
1.0 Introduction
What does change look like during treatment? Who is more likely to demonstrate it? And how can the process of change guide our clinical decisions? The answers to these questions are relevant for understanding the effects of specific treatment procedures, identifying factors that can moderate specific intervening efforts, and for developing therapy programs that are more responsive to the ongoing process of change. However, understanding therapeutic change and developing more effective interventions is a fluid process that offers a wide range of conceptual, clinical, and empirical challenges and numerous avenues for possible investigation. Thus, employing conceptual frameworks that help constrain the wide range of methodological possibilities may yield a more directed and fruitful therapy development process.
The stage model of behavioral therapies research provides a programmatic blueprint for the development and testing of new interventions (Rounsaville et al, 2001a). The model emphasizes an iterative strategy for developing therapies that is guided by the results of preliminary investigations (e.g. Stage Ia or Ib). Strengths of the model include guidance on the operationalization and assessment of therapy procedures, direction for moving from pilot work to a stronger test of a therapy’s efficacy, and the allowance for varying degrees of conceptual specificity during the development process (Rounsaville et. al., 2001b). However, other developmental frameworks place more directed tests of a treatment’s theoretical and conceptual foundations as the central guiding mechanism during program development and assessment (Kazdin, 2001). While each model stipulates different necessary conditions for successful therapy development, the common goals of developing more efficacious interventions and ultimately gaining a deeper understanding of how therapies promote change suggests that aspects of each perspective may provide a useful strategy for improving our intervention efforts. The goal of the present investigation is to provide a more direct test of the conceptual underpinnings and obtain a better understanding of the process of change as part of an iterative strategy to refine a behaviorally based naltrexone intervention for opiate dependence.
Behavior change is a dynamic process that may be instantiated by specific clinical states. Thus, identifying theoretically relevant behaviors can provide important landmarks for assessing how specific interventions affect transitions along a continuum of change and promote feedback that can guide improvements to our treatments. This requires a conceptual model of change and an analytical method that allows for the empirical evaluation of the change process. However, evaluations of therapeutic procedures have traditionally employed analytical methods that assess average change over wide intervals of time (Kazdin, 2001). While providing information on the outcome of change, this methodological approach can also obscure the process of change. Modifications to therapy programs based on the process of change may ultimately yield therapeutic procedures that are more sensitive to the various manifestations of change and provide a better blueprint for clinical decision making. Markov models provide an alternative method for investigating the process of change. They provide a means for modeling the probability of transitioning between important clinical states during the course of treatment and offer a useful framework for delineating the effects of different interventions on the transition process (Gallop, Ten Have, & Crits-Christoph, 2006; Xiaowei et al., 2007). This analytical technique may provide a more fruitful method for addressing how interventions work by empirically assessing the direction of change at conceptually designated points and provide a platform for developing intervention strategies that can respond to different manifestations of the change process.
Naltrexone, an opiate antagonist, has held significant promise as a pharmacotherapy for opiate dependence and adherence is central to its overall efficacy (Johansson, Berglund, and Lindgren, 2006). Numerous studies have investigated techniques for increasing naltrexone adherence (Carroll, et al., 2001; Fals-Stewart et al., 2003; Preston et al., 1999; Rothenberg et al., 2002). However, little is known about the process of change during naltrexone based interventions for opiate dependence. The aggregation of opiate use over time and across participants (Caroll et al., 2001; Nunes et al., 2006; Preston et al., 1999) has limited our understanding of the relationship between opiate use, naltrexone compliance, and treatment retention. This is an important limitation since the process of extinction - a transition from a state of opiate use to a state of opiate abstinence - is the conceptual underpinning of a naltrexone based intervention. Because it is a powerful opioid receptor antagonist, naltrexone blocks the reinforcing effects of opioids, producing an essential condition necessary for extinction. However, opiate use during treatment can be either a transitional state towards future naltrexone adherence and abstinence (Hulse & Basso, 2000) or a predictor of treatment failure (Sullivan et al., 2007). Thus, gaining a better understanding of the trajectory of treatment engagement and transitions from opiate use to abstinence during naltrexone maintenance has important implications for decisions concerning the appropriateness of naltrexone maintenance in the presence of continued opiate use.
Behavioral Naltrexone Therapy (BNT: Rothenberg et al., 2002) is an individually-based treatment program that incorporates elements from Network Therapy (Galanter, 1993), Relapse Prevention Therapy (Caroll, 2002), and voucher based Contingency Management (Higgins et al., 1991) to increase adherence to oral naltrxone in order to promote abstinence among opiate dependent individuals. BNT was demonstrated to be feasible in a Stage Ia development trial, although it was less effective among those with greater baseline depression, heroin use, or methadone use (Sullivan et al., 2006). These preliminary results led to several treatment modifications (i.e. excluding heavy methadone use, extending the length of the initial inpatient detoxification for low level methadone users or heavy opiate users to reduce precipitated withdrawal symptoms, and aggressively treating depression) prior to a Stage 1b randomized trial. The 1b trial demonstrated BNT was superior to medication management for increasing participant retention (Nunes et al., 2006).
This study used data from the Stage 1b clinical trial (Nunes et al., 2006) to investigate two important issues. First, in the spirit of the stage model of therapy development, we sought to investigate if treating depressive symptoms during the first month of outpatient counseling sessions, excluding methadone users who reported using greater than 30mg per week, and extending the length (from 7 days to 9 days) of the initial inpatient detoxification for low level methadone users or heavy opiate users would improve retention and reduce the negative impact of these factors on treatment retention. We also sought to identify patient characteristics that may continue to influence the efficacy of different psychosocial interventions employing a naltrexone maintenance component. Second, we investigated the conceptual underpinning of naltrexone based interventions by modeling transitions among treatment engagement, medication adherence, and opiate use over time using a Markov model framework. The effects that treatment and patient characteristics have on these transitions were also tested. This combined analytical approach incorporates the strengths of an iterative approach to therapy development as well as a more direct test of the conceptual underpinnings of naltrexone treatment with an eye towards providing useful feedback that can guide the clinical decision making process.
2.0 Methods
A more detailed account of the screening, detoxification, and treatment procedures employed in the study were presented elsewhere (Nunes et al., 2006). A description of the procedures relevant to the present study is presented below.
2.1. Participants and Procedures
This study was approved by the New York State Psychiatric Institute Institutional Review Board and all participants signed informed consent prior to enrolling in the treatment study. All participants were heroin users seeking treatment at a university-affiliated research clinic who completed screening and met predetermined eligibility criteria that included: a DSM-IV diagnosis of opiate dependence, psychiatric and medical stability, and having a non-substance dependent support person who was willing to participate in the treatment program. Potential participants were excluded if they had a diagnosis of schizophrenia or bipolar disorder, presented with unstable hypertension, diabetes, or hepatitis (liver enzymes 2-3 times the normal limit), were physiologically dependent on alcohol or sedatives, or presented with a significant risk of suicide. Participants acting as the support person were excluded if they were currently using heroin or cocaine, presented with an active psychiatric disorder (e.g. psychosis), or reported a history of violence with the opiate dependent participant. All potential support persons were interviewed by a member of the treatment staff and provided a urine sample that was assessed for the presence of illicit substances.
Of the 403 individuals screened for participation, 80 were eligible, consented, and began the detoxification procedures on the inpatient unit. Three hundred and twenty-three potential participants attended at least one screening session but did not enroll in the study. A majority of these individuals did not return for a second appointment. Not wanting an immediate detoxification and not wanting to include a support person in the treatment process were primary reasons for not participating after the first screening session. Sixty-nine of the 80 participants enrolled, completed the detoxification and were randomized to a treatment condition. Of the 11 participants who were not randomized: 6 voluntarily withdrew, 3 were administratively discharged due to violating unit rules, and 2 completed the detoxification but left prior to being assigned to a treatment condition. Of the 69 participants randomize, 64 attended at least one post-detoxification clinic appointment (BNT n=34; CE n=30).
A majority of the participants were male (81.3%) with an average age of 35.3 years (SD=9.1). Participants were using an average of 6.4 bags of heroin per day (SD=3.6) prior to entering treatment and had been using heroin regularly for 7.8 years (SD=7.0). About half of the participants (51%) reported a previous anxiety or depressive disorder. Depression severity for those reporting symptoms prior to entering treatment was in the mild to moderate range (Mean HAMD = 13.7; SD = 7.2). Demographic characteristics, baseline drug use, methadone use, and psychiatric severity at baseline did not differ between treatment conditions and are presented in Table 1. The groups did differ in average years of regular drug use. Participants in BNT reported more years of regular heroin use.
Table 1.
Demographic, Baseline Substance Use, and Retention variables by Treatment Group
| Variable | BNT (n=34) | CE (n=30) | Test Statistic |
|---|---|---|---|
| Males | 79% | 83% | χ2(1) = 0.27, p=.60 |
| Age (yrs.) | 36. (10.3) | 35 (7.7) | t (62) = 0.72, p= 0.47 |
| Single | 79% | 84% | χ2(1) = 0.27, p=.60 |
|
Race Caucasian African-American Hispanic |
52.9% (n=18) 17.6% (n=6) 29.4% (n=10) |
53.3% (n=16) 13.3% (n=4) 33.3% (n=10) |
χ2(2) = 0.30, p=.87 |
| Psychiatric Diagnoses | |||
| Antisocial Personality Disorder | 15% | 6% | χ2(2) = 0.51, p=.47 |
| Any Depressive or Anxiety Disorder | 58% | 45% | χ2(1) = 0.99, p=.32 |
| Major Depressive Disorder | 21.2% | 12.9% | Exact p=.51 |
| Baseline Depression Severity (HAMD) | 14.7 (7.1) | 12.6 (7.3) | t (62) = 1.14, p= 0.26 |
| Substance Use | |||
| Methadone use at baseline | 9% | 3% | Exact p=.61 |
| Years of Heroin Use | 9.5 (8.2) | 6.0 (5.0) | t (53.5) = 2.11, p= .04* |
| Avg bags of heroin per day | 6.6 (3.5) | 6.1 (3.8) | t (62) = 0.57, p= 0.57 |
|
Route of Administration IV Intranasal Smoke |
36% 61% 3% |
39% 61% 0% |
Exact p=1.0 |
| Treatment Outcome | |||
| Weeks in treatment | 12.9 (9.9) | 7.7 (8.5) | t (62) = 2.25, p= 0.03* |
| Percentage of Participants completing 6 months | 24.2% | 9.7% | χ2(1) = 1.47, p=.15 |
| Percentage of Participants completing only 1 week of treatment | 6.1% | 22.6% | Exact p=.08 |
| Percentage of Participants retained to week 4 | 75.8% | 51.6% | χ2(1) = 3.04, p=.08 |
| Proportion of weeks heroin was used in treatment | 0.38 (0.37) | 0.28 (0.38) | t (60) = 1.13, p= 0.26 |
Means and Standard deviations are presented for continuous variables.
The inpatient buprenorphine-assisted detoxification procedure was conducted over a 6- to 9 day period with a gradual transition to naltrexone during this time. Other adjunctive, as needed, medications (e.g. clonidine, and clonazepam) were used to reduce some of the physical discomfort experienced during the detoxification process. Following the detoxification, all participants continued with oral naltrexone and were randomly assigned to one of two psychosocial interventions, Behavioral Naltrexone Therapy (BNT) or Compliance Enhancement (CE), and began a 26-week outpatient counseling program.
Behavioral Naltrexone Therapy (BNT) is a manual-guided intervention that combines elements from Motivational Interviewing, Relapse Prevention Therapy, Network Therapy, and contingency management with voucher incentives to increase adherence to naltrexone and reduce the probability of relapse to opiates (Rothenberg et al., 2002). Participants attended two therapy sessions per week that consisted of one individually based counseling session and one session that included the participant’s support person. The support person was a family member or another non-relative (e.g. friend) from the participant’s social network that the participant nominated to serve as a medication monitor and resource during treatment. Individuals serving as the support person received information on the function of naltrexone in the treatment of opiate dependence and were orientated to their primary role as a monitor of naltrexone adherence. The first treatment session included a negotiated medication maintenance schedule outlining the times and days that naltrexone would be administered. This was developed with the participant, support person, and therapist. Treatment sessions including the support person focused on monitoring naltrexone adherence and implementing relapse prevention strategies. The contingency management program included voucher points worth $2.00 earned for each opiate- negative urine and documented naltrexone adherence. All points could be exchanged for goods and services consistent with the treatment plan. Support participants also earned vouchers worth $1.00 for each naltrexone pill monitored (recorded by the monitor on a daily basis) with an additional $300 bonus for attending at least 75% of the scheduled network treatment sessions.
Compliance Enhancement (CE) is a manual-guided intervention designed as a control condition for medication trials (Carroll et al., 1999). It is intended to model standard medical care that consists of a supportive helping relationship (e.g. non-specific therapeutic factors) with an emphasis on medication adherence. CE encourages 12 Step-involvement and the use of personal resources to help promote change. It does not incorporate training in specific coping skills, the use of voucher incentives, or the direct involvement of a participant’s social network. CE was administered in two weekly visits over the 26-week treatment program, one session to meet with the physician and the other to meet with nursing staff for clinical monitoring. CE sessions included the discussion of naltrexone compliance, supportive problem-solving, and encouragement of 12-step participation. Therapists for both conditions received individual training from either an experienced doctoral level psychologist (BNT) or board-certified addiction psychiatrist (CE). Fidelity measures (checklists and audiotaped session ratings) indicated that both treatments were administered in an equally skillful and consistent manner (Nunes et al., 2006).
Participants in both counseling conditions attended the clinic three times per week during the first two weeks of outpatient treatment. The thrice weekly visits included the administration of naltrexone under the direct observation of clinic staff and urine toxicology. Urine was collected under the observation of a staff member and tested on site for opioids with the Accuttest method (Roche Diagnostics, Indianapolis, IN) to provide immediate feedback to participants and clinical staff. Naltrexone 50 mg pills were packaged in gelatin capsules together with riboflavin. All collected urines were tested with ultraviolet light for fluorescence, demonstrating compliance with the riboflavin-labeled naltrexone. Participants who demonstrated opiate-negative urines after the first two weeks of outpatient care began home-based administration of naltrexone and attended the treatment clinic twice per week for the duration of the program. Opiate use during the outpatient portion of the program resulted in a return to clinic-based naltrexone administration. Clinic-based administration continued until three consecutive opiate-free urines were demonstrated, at which point home-based administration was resumed. Urine was tested for the presence of opiates and riboflavin at every clinic visit throughout the duration of the study.
2.3 Measures
Diagnostic Evaluation
The Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 2002) was used to determine participant diagnostic eligibility. The SCID-IV assessed DSM-IV Axis I disorders including mood disorders, psychotic symptoms, drug and alcohol use disorders, and anxiety disorders. It was administered during the initial evaluation period prior to study entry. Inter-rater reliability has been good in field trials with trained raters with the Standard SCID (Williams et al., 1992).
Depression
The Hamilton Rating Scale for Depression (HAM-D; Bagby et al., 2004; Hamilton, 1967) is a widely used structured clinician-rated instrument for current depression. The present study used a 25-item structured interview version (SIGH-D-DES; Williams 1988) that incorporates the reverse vegetative symptoms of atypical depression but permits calculation of the standard 17 and 21 item scores that are imbedded in the scale. The HAM-D was administered at baseline, weekly during the first month of treatment, and monthly thereafter.
Addiction Severity
The Addiction Severity Index (McLellan et al., 1992) was administered at baseline to assess substance related problem severity in seven life areas: alcohol use, drug use, psychological, legal, medical, employment, and family.
Substance Use
The Substance Use Inventory, a modification of the Time-Line Follow-back method (Sobell et al., 1980) was administered weekly to record the number of days and the amount of opiates, cocaine, marijuana, alcohol and other drug use that occurred during the period between each treatment session. In addition, an observed urine sample was collected at baseline and at all outpatient visits. All samples were tested immediately with Acccutest for opioids and sent for a full panel screen for opiates, cocaine, benzodiazepines, cannabinoids, alcohol, barbiturates, amphetamines, methadone, and Phencyclidine (PCP).
2.4 Outcomes
Treatment Retention
Treatment retention was defined as the number of days to dropout. Dropout was delineated as the day on which the patient relapsed and was removed from the trial or the 14th day of treatment absence for participants who had no treatment contact for two consecutive weeks. The time to dropout for participants who completed the trial (the 182 days) was treated as being right censored (i.e. the drop-out event did not occur for completers by the 26 week observation period). There were no missing data for the time to drop out outcome (i.e. it was defined for every patient).
Opiate Use and Naltrexone Compliance
Heroin use and naltrexone compliance were conceptualized as three behavioral states operationalized from a participant’s urine samples (see above). Each clinic visit entailed a urine toxicology. The results of the urine toxicologies for a particular week were combined to yield a weekly opiate use-naltrexone compliance measure. The opiate use-naltrexone compliance measure was a categorical variable defined by three levels: 1) no use of opiates (i.e. All urine samples for a given week were opiate negative), 2) blocked opiate use (i.e. all urine samples were riboflavin-positive and at least one urine was opiate-positive for a given week), and 3) unblocked opiate use (i.e. at least one urine had a concurrent opiate-positive and riboflavin-negative result during a given week).
2.5 Data Analyses
Differences among the treatment groups on baseline demographics and baseline drug use were tested with chi-square and F-tests for categorical and continuous variables, respectively. The specific analyses used to test each research question are presented below.
2.5.1 Testing Stage 1a Modifications: The effect of participant characteristics on treatment retention
Baseline participant characteristics were tested as moderators of the effect of treatment condition (BNT vs CE) on treatment retention by including them as covariates in Cox proportional hazard models. The moderators tested were demographic information (gender, age, race, marital status), the severity of heroin use prior to entering treatment (average bags of heroin used per day in the month prior to treatment), the presence of methadone before beginning detoxification (yes/no), and the number of years that heroin was used regularly. The presence of a psychiatric disorder and antisocial personality disorder diagnosis were demonstrated to influence the efficacy of BNT in a pilot trial (Sullivan et al 2006), and these were included in the model. The presence of a psychiatric disorder was included in the model in 2 ways: having a current anxiety or depressive disorder (yes/no) and the severity of depression symptoms at baseline (The Hamilton Depression Rating Scale: HAM-D score). Antisocial personality disorder was entered as a dichotomous variable (present vs. absent). For the analyses, each characteristic was entered as a main effect in addition to treatment group assignment (BNT vs. CE). The interactions between baseline characteristics and treatment condition were tested with the inclusion of all two-way interaction terms (baseline characteristics by treatment condition). Non-significant interaction terms were removed from the analyses and the main effects were re-estimated for the final model.
2.5.2 Modeling Change: The effect of heroin use and naltrexone compliance on treatment retention
The analytical strategy for investigating the effect of treatment on heroin use and naltrexone compliance was to fit a continuous Markov model on the transitional probabilities among the participants’ four defined behavior states 1. no opiate use (abstinence), 2. blocked opiate use, 3. unblocked opiate use, and 4. the cessation of treatment, (or dropout)). Let X(t) be the state of an patient at week t with state space Ω={1,2,3,4}. The transition rate from state 1 to state 2 in the model, λ12 (t) is defined by
The transition intensity matrix Q for the model is
Then, the transit probability matrix P(t) can be calculated by taking the exponential of the scaled transition intensity matrix, P(t) = exp(tQ). The effect of the covariates (e.g. treatment) on a particular transit intensity can be investigated using a proportional hazard model (Marshall and Jones, 1995) as follows
| (1) |
where λrs0 is an unspecified baseline transit intensity, and Z is an indicate variable for treatment and a time-dependent variable, X(t). The time-dependent variable was entered as a dichotomous variable in which the first three weeks of treatment were contrasted with weeks 4 to 26. It was entered in this manner since prior research has demonstrated that treatment attrition is greater within the first several weeks following detoxification from opiates (Rothenberg et al., 2002; Nunes et al., 2006). If the interaction terms (e.g. time by treatment) were not significant, a model without that term was tested. Together, these analyses tested if the transition rate among different opiate use and naltrexone adherence states varied across the psychosocial interventions and if the treatment effects varied across time. The goodness-of-fit of the model was assessed using a likelihood ratio test (Kalbfleisch and Lawless, 1985).
3.0 Results
3.2. Predictors of treatment outcome
Approximately 17% of the sample completed the 6-month treatment program with the average number of treatment weeks completed being approximately 10 (SD=9.6). Fourteen percent completed only one week of treatment and 64% percent of the sample completed at least three weeks of treatment. Participant characteristics (gender, age, race, marital status, having a history of a comorbid psychiatric disorder (anxiety or depressive disorder), an Antisocial Personality Disorder diagnosis, or baseline depression severity (HAM-D)) did not predict treatment retention nor did they moderate the effect of treatment condition on time to dropout. However, baseline heroin use interacted with treatment condition to predict time to dropout (χ2(1) = 3.2 p = 0.07). Participants using relatively more bags of heroin per day prior to entering treatment demonstrated greater retention in BNT relative to heavier baseline heroin users in the CE treatment condition.
3.2. The effect of heroin use and naltrexone compliance on treatment retention
Five-hundred and thirty-one transition events were modeled. Of the transitions to one of the four defined clinical states (i.e. no opiate use (abstinence), blocked opiate use, unblocked opiate use, and or dropout), 436 were from no opiate use, 43 were from blocked opiate use, and 52 were from unblocked opiate use. Fifty-three transition events were to drop-out (26 from abstinence, 4 from blocked opiate use, and 23 from unblocked use). Table 2 presents the estimated proportions of transition events among the four clinical states modeled across all participants (no opiate use (abstinence), blocked opiate use, unblocked opiate use, treatment dropout) according to treatment group and time period (weeks 1 to 3 and weeks 4 to 26). The first column denotes the clinical state at a particular week and columns 2 to 5 represent the clinical state the next week.
Table 2.
Estimated proportions of transition eventsa by treatment condition (BNT and CE) and time since detoxification (Weeks 1 to 3 versus Weeks 4 to 24)
| Weeks (1-3 Post Detox) | ||||
|---|---|---|---|---|
| Transitions From: | Transitions to: | |||
| BNT | ||||
| No Opiate Use | Blocked Use | Unblocked Use | Drop Out | |
| No Opiate Use | .886 | .031 | .043 | .039* |
| Blocked Use | .497 | .283 | .162 | .057 |
| Unblocked Use | .067 | .102 | .517 | .314 |
| CE | ||||
| No Opiate Use | .823 | .025 | .026 | .127* |
| Blocked Use | .604 | .252 | .062 | .082 |
| Unblocked Use | .126 | .132 | .317 | .426 |
| Weeks (4-26 Post Detox) | ||||
|---|---|---|---|---|
| Transitions From: | Transitions to: | |||
| BNT | ||||
| No Opiate Use | Blocked Use | Unblocked Use | Drop Out | |
| No Opiate Use | .925 | .023 | .029 | .022* |
| Blocked Use | .387 | .448 | .139 | .026 |
| Unblocked Use | .032 | .091 | .661 | .216 |
| CE | ||||
| No Opiate Use | .883 | .019 | .019 | .078* |
| Blocked Use | .487 | .418 | .057 | .038 |
| Unblocked Use | .067 | .133 | .489 | .312 |
= 531 transitions were modeled. The transitional events occurred among a total sample size of 64 participants.
Pairwise comparison (No opiate use to dropout: CE vs BNT) significant at p < .05
Descriptively, several transitional patterns in the table deserve comment. First, regardless of treatment condition, a week of abstinence was more likely to be followed by another week of abstinence than any other clinical state (i.e. blocked opiate use, unblocked opiate use, or treatment drop out). This pattern was more likely after the first three weeks of treatment (BNT: .925 vs. .886; CE: .883 vs. .823, Table 2). Second, during the first three weeks of treatment, the use of opiates while blocked (i.e. “testing”), was more likely to be followed by a week of no opiate use (i.e. extinction process), than a week in which blocked use, continued unblocked use, or dropout occurred. This transitional pattern was more pronounced in the CE condition (BNT .497, CE .604, Table 2). Third, if unblocked opiate use occurred, it was more likely to be followed by another week in which unblocked opiate use occurred. However, unblocked opiate use in the first three weeks of treatment was more likely to be followed by dropout in CE compared to BNT. Fourth, a return to blocked use was less likely to occur after unblocked opiate use in both treatments. Fifth, transitions to drop-out from either no opiate use (abstinence) or opiate use (blocked or unblocked) were more likely to occur in the first three weeks of treatment following detoxification.
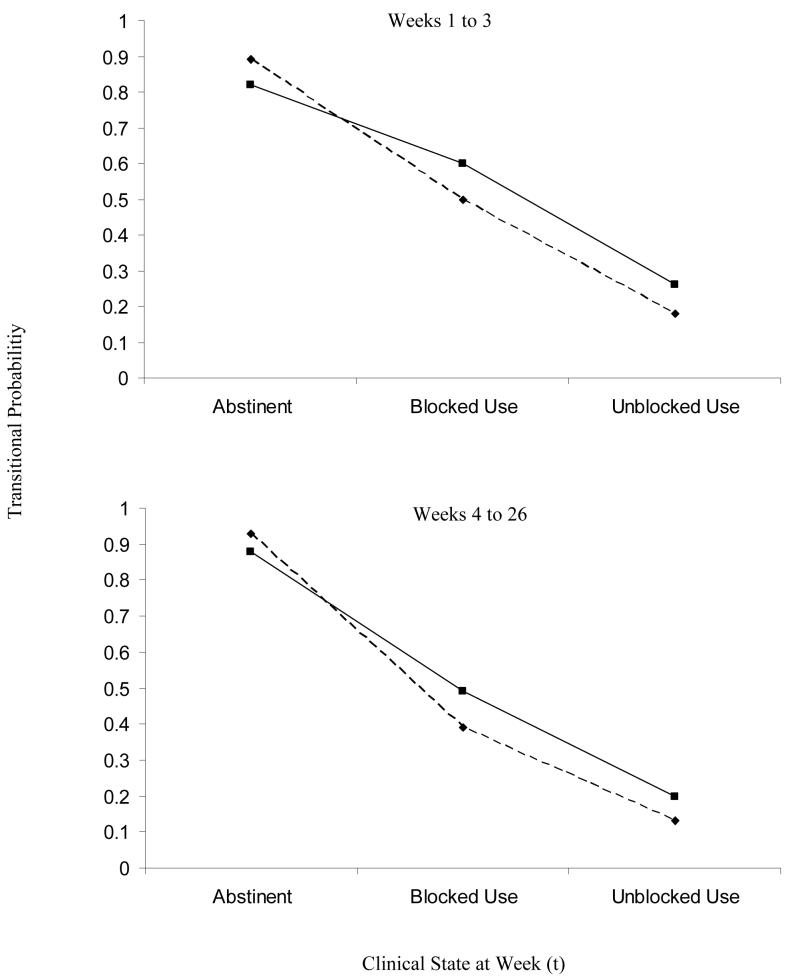
Figure 1 presents the probability of transitioning to an improved clinical state in the next week of treatment both early in the treatment process and after the first three weeks of treatment. Both panels indicate that the chances of no opiate use (being abstinent) during the next week of treatment are relatively high if the urine analysis indicated current abstinence (no opiate use). This held for both treatment conditions, although was slightly higher in BNT. However, as substance use increased (Blocked Use) and medication adherence decreased (Unblocked Use), the probability of transitioning to an improved clinical state (i.e. abstinence for those with blocked use and abstinence or blocked use for those with unblocked use) in the next week of treatment decreased. CE also demonstrated a somewhat greater proportion of transitions to an improved clinical state when blocked or unblocked use occurred.
Figure 1.
Estimated probability of transitioning to an improved clinical state at week (t+1) given a clinical state at week (t) by treatment group (BNT (---◆---) and CE (──■──)) and time in treatment.
Inferential tests of treatment effects in the Markov model (equation 1) yielded a non-significant interaction between treatment and time (χ2(6) = 9.57, p=0.14). A re-estimation of the model without the treatment by time interaction term indicated a significant independent effect for treatment condition on the transition rates (χ2(6) = 14.06, p < 0.03; Hazard Ratio 3.56 (95% CI: 1.03; 12.30). The transition rate from abstinence to treatment dropout was approximately 3.56 times (i.e. exp(1.27)) times greater among those in the CE condition relative to those in BNT, independent of the treatment time period. The treatment effects on other transitions were not statistically significant (see Table 3). The significant effect for time segment (χ2(1) = 4.03, p=0.04) indicated, on average, transitions to treatment dropout were greater in the first three weeks of treatment. The overall goodness-of-fit test indicated that the main effect only model provided an adequate fit to the data (χ2=218.7, df=199; p=0.16).
Table 3.
Parameter estimates and (95 % CIs) for the effect of treatment group (BNT versus CE) on the transition among four clinical states
| Transition to: | ||||
|---|---|---|---|---|
| No Opiate Use | Blocked Use | Unblocked Use | Dropout | |
| From: | ||||
| No Opiate Use | 0 | 0.34 (-1.18,1.87) |
-0.16 (-1.59,1.27) |
1.27* (0.03, 2.51) |
| Blocked Use | 0.37 (-0.62,1.36) |
0 | -2.78 (-9.35,3.78) |
4.57 (-8.13,17.27) |
| Unblocked Use | -1.57 (-9.96,6.81) |
1.24 (-0.48,2.95) |
0 | 0.25 (-1.18,1.68) |
p<0.05
Note: parameters with a value of 0 were held constant and were not estimated.
4.0 Discussion
The present study sought to investigate the effect of prior modifications on a behavioral naltrexone treatment program and model the process of clinical change to help guide both the refinement of treatment and clinical decisions during an oral naltrexone based treatment for opiate dependence. Most participant characteristics at baseline did not predict or modify the effect of different behavioral regimens for naltrexone maintenance. Further, several participant characteristics previously associated with a poorer response to Behavioral Naltrexone Therapy (Rothenberg et al., 2002; Sullivan et al., 2006) were not predictive of treatment retention nor did they interact with either treatment condition. This finding suggests that the more aggressive treatment of depression in the first month following detoxification, excluding methadone users who were receiving greater than 30mg per week, and extending the detoxification and naltrexone initiation phase for several days for low level methadone users, reduced the deleterious effects of methadone and depression on retention that was demonstrated in initial pilot results (Sullivan 2006).
Participants using a greater amount of heroin per day prior to entering treatment were more likely to be retained in BNT. The relative benefit of including a member of an individual’s social network in treatment and employing a contingency management program, both elements of BNT, is consistent with other treatment studies employing these techniques in naltrexone-based interventions (Carroll et al., 2001; Fals-Stewart et al., 2003; Nunes et al., 2006; Preston et al., 1999). These results extend previous findings by suggesting that the benefit of a broader therapeutic strategy is more specific to heavier opiate users. In contrast, lower level opiate users may benefit less intensive interventions that use a supportive treatment environment that emphasizes naltrexone adherence and contacting other supportive resources. The relative benefit of less intensive interventions that focus on naltrexone adherence and accessing other supportive services has been demonstrated in naltrexone-based trials for alcohol dependence (Anton et al., 2006; Volpicelli, et al., 2001). The present results suggest the severity of opiate use is an important indicator for deciding the type of psychosocial intervention to employ as part of an oral naltrexone based intervention strategy.
Investigating transitions during treatment offered a more detailed perspective on the mechanisms of change and provided some preliminary suggestions for clinical decision making and continued treatment development. A week of abstinence was very likely to be followed by another week of abstinence in both treatments. However, once opiate use was initiated, transitions back to abstinence were somewhat more likely to occur in the first several weeks of treatment and were more probable in CE. Similarly, unblocked opiate use, was more likely to be followed by improvement (blocked use or abstinence) among those in the CE treatment in the first several weeks. These patterns suggest while BNT is more likely to retain participants, CE’s more direct message of medication adherence, risk of using opiates, and connecting with support groups may offer a more effective strategy for moving individuals from unblocked or blocked opiate use to a more favorable clinical state during treatment.
Is extinction the primary behavioral mechanism of change in a naltrexone based treatment? A steady state was the most probable event on the continuum of change. A week of abstinence at one point in time was most likely followed by another week of abstinence. This pattern does not support behavioral extinction as the primary trajectory of change and suggests that other therapeutic or patient factors may account for naltrexone’s effect on preventing opiate use. BNT incorporated a wide range of therapeutic strategies including a contingency management program for reinforcing opiate-negative urine specimens and a medication monitoring program both of which reduce substance use in other drug dependent populations (Caroll et al., 2001; Prendergast et al., 2006; Preston et al., 1999). However, the CE treatment condition employed neither a contingency management nor a monitoring component (alternative reinforcers) but demonstrated a similar steady abstinent state pattern. Evidence suggests that social contingencies operating in the therapeutic milieu can play an important role in the therapeutic change process (Hayes & Wolf, 1984; Rosenfarb & Hayes, 1984), which may have contributed to the overall effect in both treatment conditions. In addition, independent lines of evidence suggest that rule governed behavior is an important factor that may significantly affect how people respond to the contingencies operating in their environment (Hayes et al., 1986). Future studies focusing on how verbal and nonverbal factors influence the change process may help facilitate the development of more effective treatment strategies by delineating the learning processes most likely to yield higher rates of treatment adherence and subsequent decreases in substance use.
A subgroup of transitional events was consistent with behavioral extinction. If blocked use occurred during a treatment week it was more likely to be followed by a week of abstinence than unblocked use or treatment dropout. This effect was more likely to occur in the first three weeks of outpatient treatment and was more pronounced in the CE condition. In contrast, blocked use was more predictive of continued blocked use in BNT at later points in treatment. These findings suggest that blocked opiate use maybe an important part of the change process and that its occurrence within the first three weeks following opiate detoxification is not necessarily indicative of treatment failure or relapse. The directive aspects of CE therapy (e.g. “stay on naltrexone and do not use”) may be more helpful in promoting this transition back to abstinence in the first month of treatment. In contrast, the relatively greater persistence of blocked use in the later stages of treatment suggests that different factors may be involved in the initiation or maintenance of opiate use at time points more distant from the completion of detoxification and may be a warning signal for clinical deterioration and a greater likelihood of relapse (Sullivan et al., 2007).
Unblocked opiate use increased the probability of another unblocked use episode or treatment dropout. The fact that only a small percentage of unblocked use episodes were followed by a transition back to naltrexone use or abstinence is consistent with other studies demonstrating that unblocked use is a strong predictor of treatment dropout (Sullivan et al, 2007). This pattern also reinforces the notion that continued naltrexone adherence is an important factor in promoting treatment retention and better outcome (Weiss, 2004). The transition from unblocked use to treatment dropout, although low, was more pronounced in the first three weeks of outpatient treatment for both treatment groups. The pattern of early discontinuation reinforces the importance of naltrexone adherence in the first several weeks following detoxification for promoting better long term outcome (Hulse and Basso, 2000). These findings also highlight the potential utility of newly developed depot formulations of naltrexone for reducing the chances of an unblocked use episode during this critical time period. An investigation of the utility of a depot formulation during the first month of outpatient counseling is currently underway and may help answer this important question.
A proportion of transition events were abstinence to treatment cessation. This transitional pattern was significantly more pronounced in the CE condition, suggesting that the contingency management program and the involvement of a participant’s social network may have decreased the rate of this treatment exiting pattern in BNT. It also is reasonable to assume that a notable percentage of participants demonstrating this pattern initiated opiate use after which they never returned to the clinic so that blocked or unblocked use was not measured. Since most baseline characteristics did not predict the termination of treatment in the present study, identifying those participants more likely to leave treatment prior to opiate use remains an important empirical and clinical issue. However, these speculations are constrained by the duration of observation. A significant proportion of participants leaving treatment were lost to follow-up. Thus, their status at six months was unknown. Hulse and Basso (2000) found that approximately one third of the participants beginning oral naltrexone discontinued its use and used heroin prior to reinitiating its use at a 6-month follow-up. In their study those more likely to be naltrexone-adherent at six months received more intensive medication supervision in the first six weeks of treatment. The similar monitoring procedures employed in BNT during this trial suggest that a proportion of those transitioning from abstinence to treatment cessation may have been abstinent at six months. Longer observation periods over the course of treatment trials may help identify the parameters associated with different treatment trajectories and delineate those more likely to succeed in stopping opiate use over time.
The overall sample size was a limitation of the present study. Estimations and direct tests of all transitional probabilities are based on the frequencies of prior clinical states and the study sample size. Although transitional patterns can be informative, the modest sample size (n=69) in this study restricted the power to statistically detect group differences on many of the transition probabilities and may have increased the likelihood of a Type II error. Further, the sample size reduced the precision with which estimates of effect were obtained as demonstrated by the wide confidence intervals in Table 2. Future studies employing larger samples may provide a more reliable estimate of all the transitions demonstrated during a naltrexone based intervention and a more refined analysis of the change process. Further, although participants were randomly assigned to a treatment condition, participants in BNT reported a longer history of heroin use. This may have influenced the study results. However, the relationship between length of heroin use and retention was non-significant when adjusting for severity of daily heroin use at the time of treatment entry (e.g. bags of heroin used per day). Bags per day was demonstrated to have more predictive power in the multivariate analyses and interact with treatment condition such that heavier opiate users were retained longer in BNT. The groups did not differ in terms of quantity of heroin used per day.
In sum, modeling predictors of outcome as well as transitions among predefined clinical states offered a more detailed view of how clinical change occurred during a naltrexone based treatment and provided useful information for guiding treatment recommendations for a behavioral and pharmacological strategy for treating opiate dependence. Treating depression in the first month of therapy and restricting naltrexone use for recently detoxified methadone dependent patients mitigated the deleterious effects these factors had on treatment retention and improved the overall success of BNT. Employing contingency management and medication monitoring procedures may be particularly useful for heavy opiate users. While behavioral extinction played a relatively minor role during the process of change, opiate use in the first several weeks of treatment may be more responsive to a direct message about medication adherence. If blocked opiate occurs later in treatment it is more likely to be indicative of clinical worsening and considering other treatment options may be useful at this juncture.
Acknowledgements
This work was supported by grants R01 DA-10746, K02 00288 (Edward Nunes), and K23 021850 (Kenneth M. Carpenter) from the National Institute on Drug Abuse. The authors thank Allison Dorlen-Pastor, Greg Bornstein, and Laura Curtiss for their assistance in conducting the study.
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence: The COMBINE Study: A Randomized Controlled Trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bagby MR, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: Has the Gold Standard Become a Lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Caroll KM, Ball SA, Nich C, O’Connor PG, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence. Archives of General Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroll KM, O’Malley SS, Nuro KF. Compliance Enhancement: A manual for the pharmacotherapy of drug abuse and dependence. Yale University Psychotherapy Development Center; West Haven, CT: 1999. [Google Scholar]
- Fals-Stewart W, O’Farrell TJ. Behavioral family counseling and naltrexone for male opiod-dependent patients. Journal of Consulting and Clinical Psychology. 2003;71:432–442. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID- I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2002. [Google Scholar]
- Gallop RJ, Ten Have TR, Crits-Christoph P. A mixed effects Markov model for repeated binary outcomes with non-ignorable dropout. Statistics in Medicine. 2006;25:2398–2426. doi: 10.1002/sim.2333. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:276–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Brownstein AJ, Zettle RD, Rosnefarb I, Korn Z. Rule- governed behavior and sensitivity to changing consequences of responding. Journal of Experimental Analysis of Behavior. 1986;45:237–256. doi: 10.1901/jeab.1986.45-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Wolf MR. Cues, consequences and therapeutic talk: Effects of social context and coping statements on pain. Behaviour Research and Therapy. 1984;22:385–392. doi: 10.1016/0005-7967(84)90081-0. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Basso MR. The association between naltrexone compliance and daily supervision. Drug and Alcohol Review. 2000;19:41–48. [Google Scholar]
- Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opiod dependence: a meta-analytical review. Addiction. 2006;101:491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch JD, J.F. Lawless JF. The analysis of panel data under a Markov assumption. Journal of the American Statistical Association. 1985;80:863–871. [Google Scholar]
- Kazdin AE. Progression of therapy research and clinical application of treatment require better understanding of the change process. Clinical Psychology: Science and Practice. 2001;8:143–151. [Google Scholar]
- Marshall G, Jones RH. Multi-state Markov models and diabetic retinopathy. Statistics in Medicine. 1995;14:1975–1984. doi: 10.1002/sim.4780141804. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: A ceiling on effectiveness? The American Jounral of Drug and Alcohol Abuse. 2006;32:503–517. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug and Alcohol Dependence. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rosenfarb I, Hayes SC. Social standard setting: The Achilles heel of informational accounts of therapeutic change. Behavior Therapy. 1984;15:515–528. [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, et al. Behavioral Naltrexone therapy: an integrated therapy of opiate dependence. Journal of Substance Abuse Treatment. 2002;23:351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice. 2001a:133–142. [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. Methodological diversity and theory in the stage model: Reply to Kazdin. Clinical Psychology: Science and Practice. 2001b;8:152–154. [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Sanders B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MD, Ward E, editors. Evaluating Alcohol and Drug abuse Treatment Effectiveness: Recent Advances. Pergamon Press; New York: 1980. pp. 129–150. [Google Scholar]
- Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, Anen SJ, Brooks AC, Jiang H, Akerele E, Nunes EV. Management of relapse in naltrexone maintenance for heroin dependence. Drug and Alcohol Dependence. 2007;91:289–292. doi: 10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Rothenberg JL, Vosburg SK, Church SH, Feldman SJ, Epstein EM, Kleber HD, Nunes EV. Predictors of retention in naltrexone maintenance for opioid dependence: Analysis of a stage I trial. The American Journal on Addictions. 2006;15:150–159. doi: 10.1080/10550490500528464. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Pettinati HM, McLellan AT, O’Brien CP. Combining medication and psychosocial treatment for addictions: The Brenda approach. Guilford Press; New York: 2001. [Google Scholar]
- Xiaowei Y, Shoptaw S, Kun N, Juanmei L, Belin TR. Markov transition models for binary repeated measures with ignorable and nonignorable missing values. Statistical Methods in Medical Research. 2007;16:347–364. doi: 10.1177/0962280206071843. [DOI] [PubMed] [Google Scholar]
- Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99:1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]