Abstract
HSCCC technique in a semi-preparative-scale was successfully applied for the first time to isolation and purification of nootkatone from the essential oil of fruits of Alpinia oxyphylla Miquel. Twelve pairs of two-phase solvent systems, consisting of seven non-aqueous and five organic-aqueous solvent systems, were evaluated by HSCCC. It revealed that the separation was mainly influenced by the partition coefficient (K) of nootkatone and the separation factor (α) between nootkatone and valencene while the organic-aqueous solvent systems were more efficient than the non-aqueous systems. With the optimal two-phase solvent system composed of n-hexane-methanol-water (5:4:1, v/v) by eluting the lower phase in a head-to-tail mode, 3.1 mg of nootkatone was obtained at a purity of 92.30 % by GC-MS in one step operation from 80 mg of crude essential oil in less than 4 h. The chemical structure of nootkatone fraction was confirmed by EI-MS and 1H NMR.
Keywords: Counter-current chromatography, Alpinia oxyphylla Miquel, Sesquiterpenoid, Nootkatone, Flavor, Bioactive component
1. Introduction
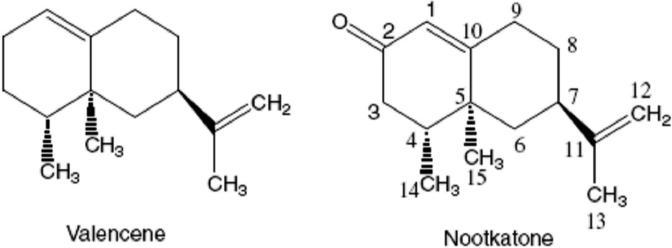
Alpinia oxyphylla Miquel (Zingiberaceae), “Yizhi” in Chinese, cultivated widely in South China, has been used as a traditional medicine for intestinal disorders and urosis in Chinese pharmacopeia. The essential oil from fruits of Alpinia oxyphylla Miq. mainly consists of various sesquiterpenoids [1-3], among which the two eremophilanes, i.d., valencene and nootkatone (shown in Fig.1), are often present in a considerable amount. Nootkatone is a flavorer (FEMA 3166) used for flavoring the food and tobacco [4]. More importantly, it is an antiulcer agent [5] and has an insecticidal activity against D. melanogastr [6]. However, due to its chiral sterostructure, the preparation of nootkatone via organic synthesis is difficult.
Fig. 1.
Chemical structures of valencene and the target compound nootkatone.
High-speed counter-current chromatography (HSCCC) has been widely applied for purification of functional components from traditional Chinese herbs and other natural products [7-10]. It is a support-free liquid-liquid partition chromatography technique so that the irreversible adsorption onto the solid stationary phase and denaturation of the compounds can be eliminated. What is more, HSCCC offers many other advantages such as choice of a wide range of the solvent systems, short separation time, high-purity of fractions, quantitative sample recovery and ease of scaling up.
In this paper, HSCCC is applied for the first time to isolation and purification of nootkatone from the essential oil of Alpinia oxyphylla fruits. The composition of the two-phase solvent system is optimized in terms of partition coefficients (K). nootkatone and proper separation factors (α) between nootkatone and valencene by testing seven non-aqueous and five organic-aqueous solvent systems. Finally, the two-phase solvent system composed of n-hexane-methanol-water (5:4:1, v/v) was selected and utilized for the isolation and purification of nootkatone from the essential oil.
2. Experimental
2.1. Apparatus
The present study employed a model TBE 300A high-speed counter-current chromatograph (Shanghai Tauto Biotech, Shanghai, China) with three ploytetrafluoroethylene coils (tubing I.D. 2.6 mm, total volume 300 ml) and a 20 ml manual injection sample loop. The evolution radius (R) is 5 cm, and the β values of the multilayer coil vary from 0.5 at the internal terminal to 0.8 at the external terminal (β= r/R, where r is the distance between the coil and the holder shaft). The rotary speed of the apparatus can be regulated at 700~1000 rpm with a speed controller. The elution of the solvent and the UV detection were performed by one ÄKTA prime system (Amersham Pharmacia Biotechnique Group, Sweden). The column temperature was controlled by an HX 1050 water-circulating constant temperature implement (Beijing Tianyou Science Development Co. Ltd., Beijing, China). The chromatogram was recorded by an N2010 workstation (Zhejiang University, Hangzhou, China).
An Agilent 6890N/5973i gas chromatograph and mass spectrometer (GC-MS) and an Agilent 6890 gas chromatograph coupled with a flame ionization detector (GC-FID) (Agilent Technologies, USA) were used for analysis. 1H NMR was performed on a Brucker Avance 400 MHz nuclear magnetic resonance (NMR) spectrometer.
2.2. Materials and reagents
The fruits of Alpinia oxyphylla Miq. were purchased from Tongrentang drugstore (Qianmen, Beijing). Before use, they were ground into powder. The organic solvents, methanol, acetonitrile, ethyl acetate, ethanol, light petroleum (boiling point ), n-hexane, chloroform and dichloromethane, all in analytical grade, were from Beijing Chemical Reagent Company. The C6-C23 n-alkanes used to analyze retention indices (RI) of the components in the essential oil, in chromatographic grade, were from Dikma Technologies in Beijing.
2.3. Preparation of essential oil by distillation and solvent extraction (SDE)
Attached to a modified Likens-Nickerson apparatus were a 2000 ml round bottom flask and a 500 ml round bottom flask. In the 2000 ml round bottom flask, 500 g of pulverized sample was suspended in 1300 ml of water. In the 500 ml round bottom flask, 300 ml dichloromethane (purified in advance) was added. The sample and the solvent were heated by one oil bath and one water bath separately. After boiled and refluxed for 12 h, the dichloromethane fractions in both the solvent flask and the solvent loop were combined, dehydrated over anhydrous Na2SO4 and concentrated mildly by N2 blowing. The essential oil obtained was pale yellow at a yield of 1.61%.
2.4. Determination of partition coefficients (K)
The partition coefficients (K) were determined as follows: a small amount of essential oil was dropped into a 10 ml test tube to which 2.0 ml of each phase of the equilibrated two-phase solvent system was added. The tube was shaken vigorously for 2 min to thoroughly equilibrate the sample between the two phases. Then an aliquot of each phase was analyzed by GC. The K values were calculated by the peak areas in GC chromatograms.
2.5. Preparation of two phase solvent systems and sample solutions
The solvents were mixed in a separatory funnel according to the selected volume ratios and thoroughly equilibrated by vigorous shaking at room temperature (ca 22 °C). Prior to use, the upper phase and the lower phase were separated and degassed by sonication for 25 min.
For selection of two-phase solvent system the sample solution was prepared by dissolving 150 mg of essential oil into 18 ml two-phase solvent system, while for the isolation and purification of nootkatone the sample solution was prepared by dissolving 80 mg of essential oil into 15 ml two-phase solvent system.
2.6. HSCCC separation
Both head to tail and tail to head elution modes were carried out. When tail to head elution was used, the ploytetrafluoroethylene tubes pertaining to entrance and exit on the injection loop were reversed. In each separation the multilayer-coiled column was first entirely filled with the stationary phase at a flow rate of 30 ml.min−1. Then the mobile phase was pumped through the column at a flow rate of 1.5 ml.min−1 while the HSCCC apparatus was rotated at a speed of 850 rpm. After hydrodynamic equilibrium was established throughout the coil, the sample solution was injected into the separation column. During the separation the column temperature was controlled at 22 °C. The UV detector was set at 254 nm. The fractions were manually collected according to the chromatogram, and concentrated by N2 blowing. The residue liquids were analyzed by GC-MS. The purity of nootkatone fraction was expressed as the percentage of its peak area relative to the total peak area in GC-MS.
2.7. GC-MS and GC analysis
The DB-5 ms 30 m × 0.25 mm × 0.25 μm capillary column (Agilent Technologies, USA) was used in GC-MS analysis. The carrier gas was helium at 1 ml.min−1. The initial oven temperature was 100 °C held for 2 min; then raised to 165 °C at 10 °C.min−1; further raised to 170 °C at 1.5 °C.min−1 kept for 2 min; and again raised to 183 °C at 1.5 °C.min−1. Finally it was raised to 280 °C at 30 °C.min−1 and kept for 2 min. The sample of 2.0 μl was injected at 300 °C in a split mode (20:1). The mass detector was operated at 150 °C in an electron impact mode at 70 eV. The ion source temperature was kept at 230 °C while the transfer line temperature was at 250 °C. The chromatograms were recorded by monitoring the total ion currents in 40-450 mass range.
The HP-5 30 m × 0.32 mm × 0.25 μm capillary column (Agilent Technologies, USA) was used in GC analysis. The carrier gas was nitrogen at 1 ml.min−1. Other chromatographic conditions utilized were identical to those in GC-MS analysis above.
2.8. NMR analysis
In addition to GC-MS, the structure of nootkatone fraction was further confirmed by 1H NMR. 1H NMR spectra were recorded on a Brucker Avance 400 MHz spectrometer with TMS (tetramethylsilane) as internal standard and CDCl3 as the solvent.
3. Results and discussion
3.1. Analysis of the essential oil
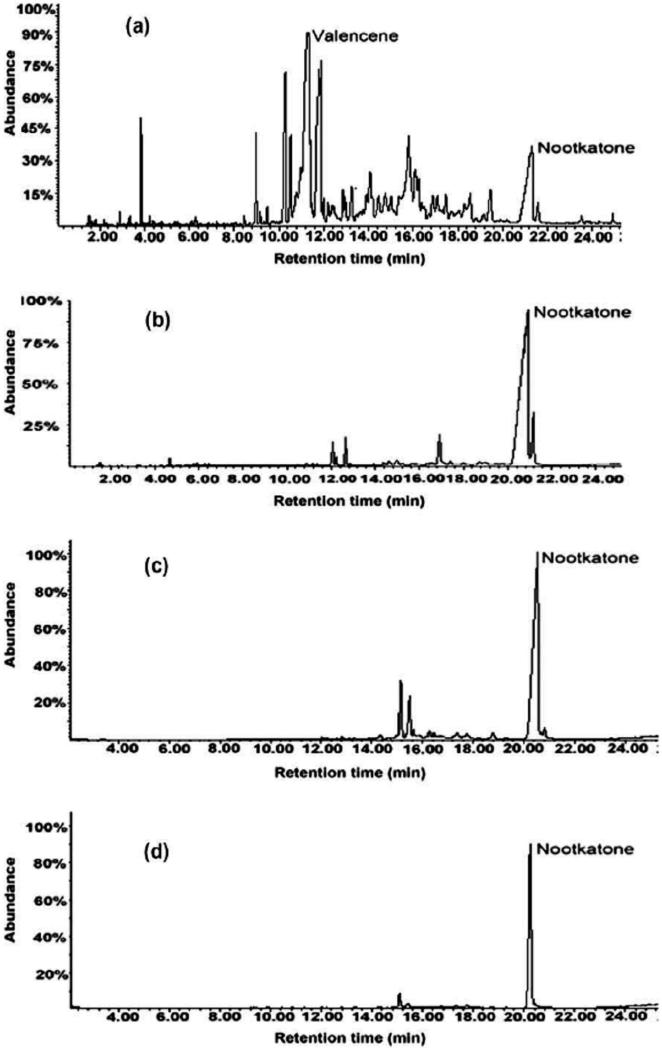
The total ion current chromatogram of the essential oil in GC-MS was shown in Fig.2a. It could be seen that most of the peaks were distributed in the retention region over 8 min. According to NIST 02 mass spectra library as well as retention indices (RI), they mainly belonged to sesquiterpenes (9.00~12.00 min) and oxygenous sesquiterpenes (12.31~24.00 min). Valencene (RI 1477) was the most abundant in the essential oil, representing 33.68% of the total peak areas, whereas nootkatone (RI 1834) was 7.64%.
Fig. 2.
Total ion current chromatograms in GC–MS analysis of the crude essential oil from fruits of Alpinia oxyphylla Miquel and the nootkatone fractions collected in HSCCC experiments. (a) The crude essential oil, valencene (RI 1477) in 33.68% and nootkatone (RI 1834) in 7.64%. (b) The nootkatone fraction corresponding to HSCCC separation in Fig. 3a, nootkatone in 80.55 %. (c) The nootkatone fraction corresponding to HSCCC separation in Fig. 3b, nootkatone in 77.39%. (d) The nootkatone fraction corresponding to HSCCC separation in Fig. 3c, nootakone in 92.30%. Operation conditions of GC–MS: DB-5 ms 30 m _ 0.25 mm _ 0.25 lm capillary column; carries gas, helium in 1 ml min_1; sample, 2.0 ll injected at 300 _C in a split mode (20:1); oven temperature: initial 100 _C held for 2 min; then raised to 165 _C at 10 _C min_1; further raised to 170 _C at 1.5 _C min_1 kept for 2 min; and again raised to 183 _C at 1.5 _C min_1; finally raised to 280 _C at 30 _C min_1 and kept for 2 min. Mass detector, 150 _C in an electron impact mode at 70 eV; ion source temperature 230 _C; transfer line temperature 250 _C; mass detection range (m/z) 40–450.
3.2 Selection and optimization of two-phase solvent systems
Successful separation by HSCCC mainly depends upon the selection of a suitable two-phase solvent system. Essentially, the non-aqueous solvent system is favorable for the isolation of non-polar target compounds like squalene [11], whereas the organic-aqueous solvent system is favorable for the polar compounds such as amygdalin [12] and inflacoumarin A [13]. Since nootkatone is an oxygenous sesquiterpene of weak polarity, both non-aqueous and organic-aqueous two-phase solvent systems were examined in the present work.
In order to find the solvent systems with suitable partition coefficients (K) (generally within the range of 0.5~2), the partition pattern of nootkatone was investigated in a series of selected two-phase solvent systems, all of which satisfy the following requirements: nootkatone was stable and soluble in the solvent system; the solvent system could form two phases with acceptable volume ratios to avoid wastage; and the settling time was less than 20 s [14]. In addition the K value of valencene was also determined, since it dominates in the essential oil and bears a chemical structure similar to nootkatone. In order to resolve nootkanone from valencene,. the ratio of their K values or the separation factor (α= K1/K2, where K1>K2) ought to be greater than 1.5 in the semi preparative multilayer separation column used in the present study [14]. It turned out that the α value between nootkanone and valencene is much greater than 1.5 in all solvent systems. Nevertheless, the value may be used as an indicator for resolving nootkanone from the rest of the impurities.
Table 1 lists the non-aqueous and organic-aqueous two-phase solvent systems examined together with various data including their K and α values for valencene and nootkatone, the purities of nootkatone fractions collected (percentage of total peak areas in GC-MS), % retention of the stationary phase, and the elution times of the two compounds in both head to tail (normal) and tail to head (reverse) elutions. For the normal elution mode in which the lower phase was used as the mobile phase, K was expressed as KU/L=CU/CL, while for the reverse elution mode the upper phase was used as the mobile phase, it was expressed as KL/U= CL/CU, where CU and CL is the solute concentration in the upper phase and the lower phase, respectively.
Table 1.
Composition of seven non-aqueous and five organic-aqueous solvent systems and summary of experimental results on separation of nootkatone in crude essential oil by HSCCC.
| Solvent system (v/v)a | KU/L (Valencene)b | KU/L (Nootkatone)b | α c | Purityd (%) |
Sf (%)e |
Elution time (min)f |
tR (min)i |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| h – tg | t – hh | h – tg | t – hh | h – tg | t – hh | h – tg | t – hh | ||||
| Hex–CHCl3–ACN (6:2:5) | 1.65 | 0.70 | 2.33 | 16.47 | 18.46 | 31.1 | 61.1 | 160–195 | 280–300 | 181 | 251 |
| Hex–CHCl3–ACN (6:1:4) | 4.46 | 0.63 | 7.22 | 53.93 | 64.98 | 82.3 | 83.0 | 105–135 | 305–350 | 139 | 298 |
| Hex–CHCl3–ACN (10:1:10) | 5.04 | 0.45 | 11.00 | 63.73 | 80.55 | 81.7 | 83.0 | 85–120 | 370–420 | 110 | 399 |
| Hex–CH2Cl2–ACN (10:3:7) | 1.97 | 0.65 | 3.00 | 53.87 | 64.82 | 75.2 | 70.0 | 230–270 | 275–315 | 147 | 274 |
| Hex–CH2Cl2–ACN (10:1:10) | 6.46 | 0.49 | 13.53 | 45.66 | 76.90 | 82.0 | 84.1 | 85–120 | 410–470 | 116 | 373 |
| Hex–CH2Cl2–EtOAc–ACN (6:1:1:4) | 2.48 | 0.72 | 3.45 | 29.02 | 70.45 | 45.2 | 66.4 | 150–180 | 255–300 | 175 | 250 |
| Hex–CH2Cl2–EtOAc–ACN (10:1:1:10) | 4.49 | 0.50 | 9.00 | 51.77 | 75.74 | 80.6 | 85.2 | 90–120 | 390–440 | 119 | 367 |
| LtPet–(Et)2O–EtOH–H2O (5:0.5:4:0.5) | 7.28 | 0.69 | 10.55 | 56.25 | 64.23 | 72.4 | 84.1 | 130–155 | 310–370 | 155 | 274 |
| LtPet–EtOH–H2O (5:4:1) | 28.64 | 0.95 | 30.17 | 75.87 | 61.85 | 79.2 | 86.9 | 160–185 | 180–230 | 192 | 209 |
| LtPet–(Et)2O–EtOH–H2O (5:0.5:4:1) | 31.50 | 1.14 | 27.63 | 66.36 | 55.44 | 76.7 | 87.6 | 300–380 | 180–240 | 221 | 177 |
| Hex– EtOAc–MeOH–H2O (5:1:4:1) | 28.68 | 1.11 | 25.84 | 75.51 | 55.26 | 82.2 | 91.2 | 185–210 | 145–170 | 218 | 182 |
| Hex–MeOH–H2O (5:4:1) | 93.56 | 1.25 | 74.85 | 77.39 | 29.55 | 81.3 | 92.6 | 190–220 | 140–200 | 241 | 163 |
Hex, n-hexane; ACN, acetonitrile; EtOAc, ethyl acetate; LtPet, light petroleum (30–60 °C); (Et)2O, diethyl ether; EtOH, ethanol; MeOH, methanol.
The partition coefficients (K): in head–tail elution using the lower phase as the mobile phase, the K value was expressed as KU/L = AU/AL, where AU and AL was the peak area of the compound in the upper phase and the lower phase by GC, respectively; whilst in tail–head elution using the upper phase as the mobile phase, the K value is the reciprocal of KU/L, that is 1/KU/L.
α is the separation factor obtained by the ratio of K values between valencene and nootkatone, e.g. α = KU/L (Valencene)/KU/L (Nootkatone).
Percentage of peak areas of nootkatone analysed by GC–MS.
Sf, retention of the stationary phase, Sf = Vs/VC, where Vs is the volume of the retained stationary phase, VC is the total column volume.
The time range of nootakone fraction being eluted out.
Head–tail elution mode, using the lower phase as the mobile phase.
Tail–head elution mode, using the upper phase as the mobile phase.
tR, the theoretical retention time of nootkatone, calculated by tR = VR/F and VR = VC[1 + (K – 1)Sf], where VR was the retention volume of nootkatone, F was the flow rate of the mobile phase and K was the partition coefficient of nootkatone.
Three types of the non-aqueous solvent systems listed in Table 1, including n-hexane-chloroform-acetonitrile, n-hexane-dichloromethane-acetonitrile and n-hexane-dichloromethane-ethyl acetate-acetonitrile, were prepared by adding chloroform, dichloromethane or a mixture of dichloromethane and ethylacetate to the solvent composed of n-hexane-acetonitrile at suitable volume ratios. It should be noted that for each system, as the amount of the modifiers (CHCl3, CH2Cl2, and EtOAc) was reduced, the KU/L values of valencene became higher whereas those of nootkatone became smaller, resulting in increase of their α values, e.g. the solvent systems n-hexane-chloroform-acetonitrile (6:2:5,v/v) versus n-hexane-chloroform-acetonitrile (6:1:4,v/v), their (KU/L) values of valencene and nootkatone were (1.65 and 0.70) versus (4.46 and 0.63), and their α values were 2.33 versus 7.22.
In most of the organic-aqueous solvent systems the KU/L values of valencene were much higher than those in the non-aqueous solvent systems due to strong hydrophobicity of valencene, whereas those of nootkatone were only slightly increased, resulting in higher α values between these two compounds. Moreover, the higher volume ratio of water in the organic-aqueous solvent system improves the α values, e.g. light petroleum-diethyl ether-ethanol-water (5:0.5:4:0.5, v/v) (α=10.55) versus light petroleum-diethyl ether-ethanol-water (5:0.5:4:1, v/v) (α=27.63). Probably due to the reduced volume ratio of water, the presence of diethyl ether or ethyl acetate in the organic-aqueous solvent systems seemed to lower the α values. For instance, light petroleum-ethanol-water (5:4:1, v/v) (α=30.17) versus light petroleum-diethyl ether-ethanol-water (5:0.5:4:1, v/v) (α =27.63), the α value was reduced even though the KU/L values of both valencene and nootkatone were increased by adding diethyl ether. So was the case of n-hexane-methanol-water (5:4:1, v/v) (α=74.85) versus n-hexane-ethyl acetate methanol-water (5:1:4:1, v/v) (α=25.84). However, it was worth mentioning that with ethyl acetate added into the solvent system composed of n-hexane-methanol-water (5:4:1, v/v), the KU/L values of valencene and nootkatone were both decreased, which was opposite to that of diethyl ether being added into the solvent system composed of light petroleum-ethanol-water (5:4:1, v/v).
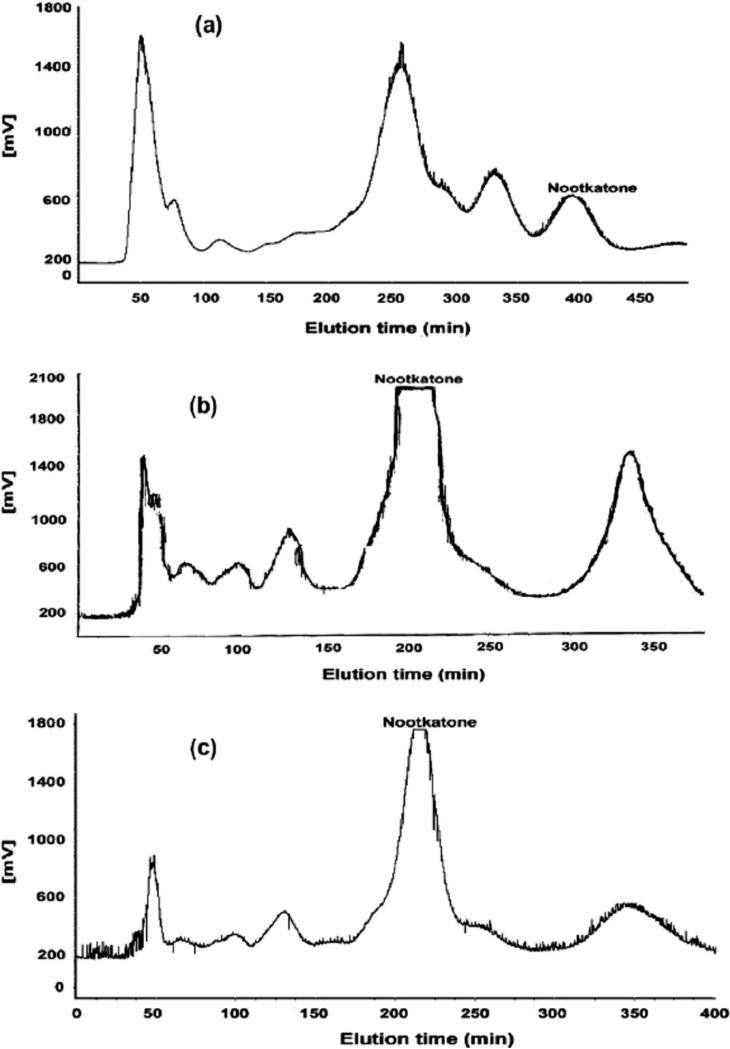
From Table 1, it may be further noted that regardless in the non-aqueous or the organic-aqueous two-phase solvent systems, separation of nootkatone from the crude essential oil was mainly influenced by the K value of nootkatone and the α value between nootkatone and valencene. In fact higher purities of nootkatone fractions shown in Table 1 were produced from the two-phase solvent systems by increasing both K and α values. For each non-aqueous two-phase solvent system listed in Table 1, the (KL/U) value of nootkatone was uniformly larger than its (KU/L) value; thus the purity of nootkatone fraction obtained by HSCCC by the reverse elution mode was higher than that obtained by the normal elution mode. Also, for the organic-aqueous two-phase solvent system, if the (KU/L) value was larger than its (KL/U) value, the purity of nootkatone fraction obtained in the normal elution mode is higher than that obtained by the reverse elution mode. In Table 1, the highest purity (80.55%) of nootkatone fraction among the non-aqueous solvent systems and that (77.39%) among the organic-aqueous solvent systems were separately achieved by the solvent systems composed of n-hexane-chloroform-acetonitrile (10:1:10, v/v) (reverse elution mode) and n-hexane-methanol-water (5:4:1, v/v) (normal elution mode). The HSCCC chromatograms in these solvent systems were shown in Fig.3a and Fig.3b, while the resulting GC-MS chromatograms on nootkatone fractions obtained by HSCCC were shown in Fig. 2b and Fig. 2c.
Fig. 3.
HSCCC chromatograms on the separation of nootkatone from the crude essential oil of fruits of Alpinia oxyphylla Miquel. Revolution speed: 850 rpm; separation temperature: 22 _C, flow rate: 1.5 ml min_1; and UV detection wavelength 254 nm. (a) Two-phase solvent system: hexane–chloroform–acetonitrile (10:1:10, v/v); mobile phase: the upper phase; sample size: 150 mg of essential oil dissolved into 18 ml two-phase solvent system. (b) Two-phase solvent system: n-hexane–methanol–water (5:4:1, v/v); mobile phase: the lower phase; sample size: 150 mg of essential oil dissolved into 18 ml two-phase solvent system. (c) Two-phase solvent system: n-hexane–methanol–water (5:4:1, v/v); mobile phase: the lower phase; sample size: 80 mg of essential oil dissolved into 15 ml two-phase solvent system.
At any rate in comparing the purity of nootkatone fraction obtained in n-hexane-dichloromethane-acetonitrile (10:1:10, v/v) (reverse elution mode) with that in n-hexane-chloroform-acetonitrile (10:1:10, v/v) (reverse elution mode), it is supposed that the separation would be more influenced by the K value of nootkatone if the α value had been large enough for the resolution of nootkatone from valencene.
This was also the case in the organic-aqueous solvent systems, e.g. n-hexane-ethyl acetate-methanol-water (5:1:4:1,v/v) (KU/L=1.11, α=25.84) versus n-hexane-methanol-water (5:4:1,v/v) (KU/L=1.25, α=74.85), where the α value in the former had already reached the level to resolve nootkatone from valencene and its analogs. Though the α value in the latter was much larger, the purity obained in the normal elution mode was just slightly improved since its KU/L value was only a little higher than the former.
It is important to observe that in comparing the purity of nootkatone fraction with the elution time, HSCCC separation using the organic-aqueous solvent systems (normal elution mode) is more efficient than that with non-aqueous solvent systems (reverse elution mode). It can be seen from Table 1 that due to higher α values or greater selectivity attained by most of the organic-aqueous solvent systems, the high purities of nootkatone fractions (over 70%) were often achieved in shorter elution times when these organic-aqueous solvent systems were applied on HSCCC in the normal elution mode. Besides, separation in the non-aqueous solvent systems (reverse elution mode) was unfavorable for the collection and recovery of nootkatone, since longer elution time led to a broader and more dilute peak. As shown in Fig.3b, the HSCCC peak corresponding to nootkatone fraction given by the organic-aqueous solvent system composed of n-hexane-methanol-water (5:4:1, v/v) (normal elution mode) was acceptable. However, the nootkatone peak resulting from the non-aqueous solvent system composed of n-hexane-chloroform-acetonitrile (10:1:10, v/v) (reverse elution) in HSCCC was quite lower and relatively broader, as shown in Fig.3a. From Table 1 the organic-aqueous solvent composed of n-hexane-methanol-water (5:4:1,v/v) is considered to be the most suitable system to isolate nootkatone from the essential oil.
3.3. Isolation of nootkatone from the essential oil by HSCCC
In fact, the crude essential oil may be more complex than those samples reported on preparative separation by HSCCC [10-12,15], since various sesquiterpenoids having similar polarities to nootkatone are likely present in the essential oil and even the presence of the target nootkatone is not so prominent as shown in Fig.2a. Thus, after the two-phase solvent system had been selected and optimized for the above work, the purity of nootkatone obtained was still unsatisfactory, as shown in Table 1, Fig.2 and Fig.3. It is well known that sample size often affects the separation resolution [13]. Therefore, in order to improve the purity of nootkatone in one step HSCCC separation, the separation was similarly carried out with the solvent system composed of n-hexane-methanol-water (5:4:1, v/v) in the normal elution mode by reducing the sample load (shown in Fig.3c). It turned out that 3.1mg of nootkatone, with a purity at 92.30 % by GC-MS (shown in Fig.2d), was obtained from 80 mg of crude essential oil.
However, when less sample loaded, separation on the non-aqueous solvent composed of hexane-chloroform-acetonitrile (10:1:10,v/v) (reverse elution mode) was still not improved, and the HSCCC peak corresponding to nootkatone fraction often became too flat to be identified.
3.4 The structural identification
The nootkatone fraction obtained in purity of 92.30 % was an pale oil with sweet, woody and orange-like odors. Its chemical structure was identified by EI-MS (in GC-MS) and 1H NMR as follows:
MS (70 eV, m/z): 218 [M+] (23); 147(100); 121(72); 91(69); 41(64). 1H NMR (CDCl3) δ(ppm): 5.80 (1H, s, H-1); 4.78 (2H, d, H-12); 1.77 (3H, s, H-13); 1.15 (3H, s, H-15); 0.99 (3H, d, H-14). The MS and 1H NMR spectra data were in agreement with those reported [6, 16].
4. Conclusions
The HSCCC method in a semi-preparative scale was established and applied successfully in the isolation and purification of nootkatone from the essential oil of fruits of Alpinia oxyphylla Miq. Five organic-aqueous and seven non-aqueous two-phase solvent systems were evaluated their performance by HSCCC in terms of the partition coefficients of nootkatone and valencene and their α values using both normal (lower phase mobile) and reverse (upper phase mobile) elution modes. The results indicated that due to larger α values between valencene and nootkatone, the organic-aqueous solvent systems demonstrated more efficient separation in the separation of nootkatone from various sesquiterpenoids in the essential oil. Therefore, the organic-aqueous solvent system composed of n-hexane-methanol-water (5:4:1, v/v) was selected for the solvent system for preparative separation of nootkatone. By eluting the lower phase at a flow rate of 1.5 ml.min−1 under 850 rpm of revolution speed, 80 mg of crude essential oil was separated yielding 3.1 mg of nootkatone at a purity of 92.3% in less than 4 h. The chemical structure of nootkatone fraction obtained was confirmed by EI-MS and 1H NMR.
Acknowledgements
The present work was supported by Beijing Municipal Talent Development Organization (No.20051D0500310) and National Natural Science Foundation of China (NSFC20676003).
References
- 1.Liu H, Guo S-Y, Xiao K-J, Cai M-Y, Han C-R. Journal of South China University of Technology. (Natural Science Edition) 2006;34:54. [Google Scholar]
- 2.Luo X-Z, Yu J-G, Xu L-Z, Yang S-L, Feng J-D, Ou S-L. Chinese Journal of Chinese Materia Medica. 2001;26:262. [PubMed] [Google Scholar]
- 3.Yi M-H, Xiao H, Liang Z-Y. China Tropical Medicine. 2004;4:339. [Google Scholar]
- 4.Chen Y-K, Xie B, Liu X-Y, Kong N-C, Li C. Fine Chemicals. 2006;23:980. [Google Scholar]
- 5.Yamahara J, Yu H, Tamai Y. Chem. Pharm. Bull. 1990;38:3053. doi: 10.1248/cpb.38.3053. [DOI] [PubMed] [Google Scholar]
- 6.Miyazawa M, Nakamura Y, Ishikawa Y. J Agric. Food Chem. 2000;48:3639. doi: 10.1021/jf000325z. [DOI] [PubMed] [Google Scholar]
- 7.Frighetto RTS, Welendorf RM, Nigro EN, Frighetto N, Siani AC. Food Chem. 2008;106:767. [Google Scholar]
- 8.Ma C-J, Li G-S, Zhang D-L, Liu K, Fan X. J Chromatogr. A. 2005;1078:188. doi: 10.1016/j.chroma.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Shi S, Zhang Y, Huang K, Liu S, Zhao Y. Food Chem. 2008;108:402. [Google Scholar]
- 10.Yan J, Chen G, Tong S, Feng Y, Sheng L, Lou J. J Chromatogr. A. 2005;1070:207. doi: 10.1016/j.chroma.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 11.Lu H-T, Jiang Y, Chen F. J Chromatogr. A. 2003;994:37. doi: 10.1016/s0021-9673(03)00454-0. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Tong S, Li J. Journal of liquid Chromatography & Related Technologies. 2006;29:1271. [Google Scholar]
- 13.Wang Q-E, Lee FS-C, Wang X. J Chromatogr. A. 2004;1048:51. doi: 10.1016/j.chroma.2004.06.135. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y. J Chromatogr. A. 2005;1065:145. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 15.Cao X-L, Xu Y-T, Zhang G-M, Xie S-M, Dong Y-M, Ito Y. J Chromatogr. A. 2006;1127:92. doi: 10.1016/j.chroma.2006.05.083. [DOI] [PubMed] [Google Scholar]
- 16.Luo X-Z, Yu J-G, Xu L-Z, Li K-L, Tan P, Feng J-D. Acta Pharmaceutia Sinica. 2000;35:204. [Google Scholar]