Abstract
RNA interference (RNAi), an effective technique for regulating/silencing specific genes, can be applied to treat various diseases. Multiple clinical trials using RNAi are ongoing and molecular imaging can serve as a powerful tool in RNAi-based therapies. This brief review will highlight the current progress on in vivo imaging of RNAi delivery and silencing effects. Incorporation of suitable molecular imaging techniques into future RNAi-based clinical trials will provide more pieces of the puzzle, thus facilitating the transformation of RNAi into a powerful therapeutic modality in the clinic.
Keywords: RNA interference (RNAi), molecular imaging, small interfering RNA (siRNA), small hairpin RNA (shRNA), positron emission tomography (PET)
Since the discovery in 1998 (1), RNA interference (RNAi) has emerged as a powerful tool for therapeutic gene silencing because of its unique specificity, broad applicability, and high efficiency. Small RNAs regulate gene expression by transcriptional and post-transcriptional gene silencing mechanisms (2). The effector RNA molecules of RNAi consist of 20~30 nucleotides, which are complexed with the RNA-induced silencing complex (RISC) to generate a cascade effect, causing sequence-specific messenger RNA (mRNA) cleavage or translation repression (3). Using small interfering RNA (siRNA) or small hairpin RNA (shRNA), clinical applications of RNAi focus on the treatment of several diseases: age-related macular degeneration (AMD), viral infections, cancer, and neurological disorders (2). In 2004, the first siRNA-based therapeutics (Bevasiranib) entered a clinical trial for the treatment of AMD (2, 4). Currently, there are many multi-center clinical trials of RNAi ongoing around the world.
However, the clinical utility of RNAi faces a number of challenges. RNAi is an important endogenous regulatory mechanism in cells and introduction of foreign siRNAs can cause undesired side effects. Certain siRNA molecules show off-target effects and synthetic siRNAs can induce type I interferon responses and stimulate the production of inflammatory cytokines. Furthermore, efficient and specific delivery of siRNA/shRNA to target cells or organs is highly challenging (5). The ultimate goal of RNAi-based therapies will be hard to achieve without improving the biodistribution, safety, effectiveness, and reliability of gene delivery systems.
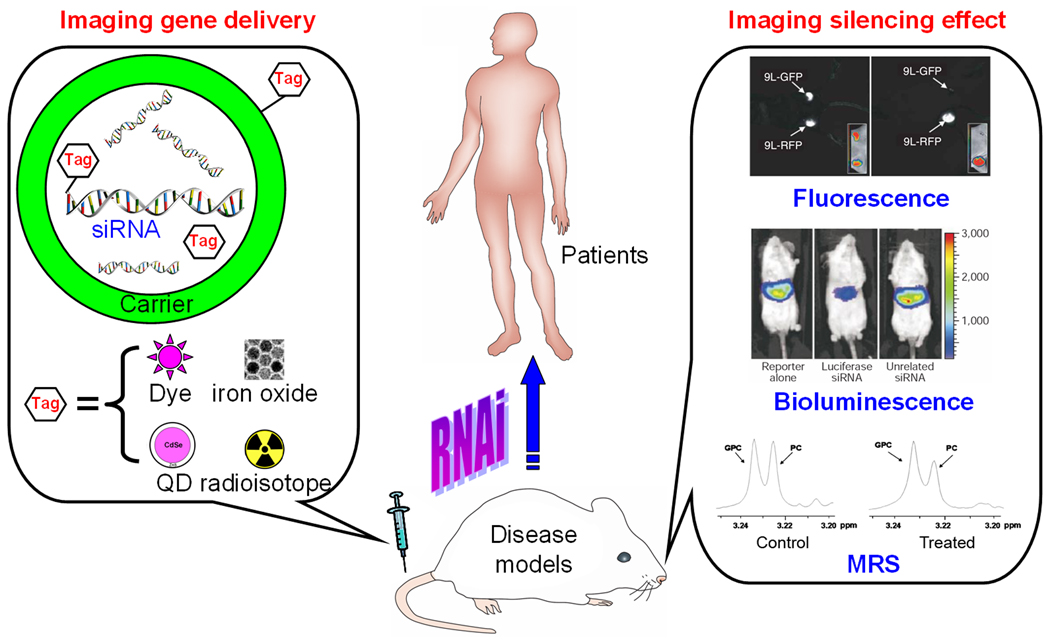
Molecular imaging techniques can serve as powerful tools for tracking siRNA/shRNA delivery in vivo, as well as for assessing the silencing effects (6, 7). To date, multiple molecular imaging modalities have been applied in RNAi-related research, including optical imaging (fluorescence and bioluminescence), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), single photon emission computed tomography (SPECT), and positron emission tomography (PET) (Figure 1).
Figure 1.
Molecular imaging techniques can be used to monitor both gene delivery efficiency and the silencing effect of RNAi. In some cases the carriers can also be the image tags. Upon further optimization and rigorous validation, many of these techniques may be translated into the clinic in the future.
IMAGING OF SIRNA DELIVERY
The development/optimization of targeted delivery strategies (viral or non-viral) to enable efficient, systemic delivery of siRNA will be a big step forward for RNAi-based therapies. Optical imaging, radionuclide-based imaging (i.e. SPECT and PET), and MRI have been employed for the assessment of various siRNA delivery techniques.
Fluorescence Imaging
Optical imaging of siRNA delivery generally adopts two strategies: labeling the siRNAs or labeling their carriers. An siRNA-based molecular beacon was developed for the detection and knockdown of telomerase expression in human breast cancer cells (8). The molecular beacon incorporates a fluorescence resonance energy transfer (FRET) fluorophore pair (Cy3/Cy5), and is activated upon binding to the telomerase mRNA in cells. Aside from excellent gene silencing efficiency (~80%), the activation of the molecular beacon in cancer cells could also be visualized with fluorescence imaging. Thus, this strategy could be useful for both imaging and therapeutic applications.
Quantum dots (QDs) have been used to label siRNA carriers (mostly nanoparticles) in cell-culture studies. For example, cationic liposomes were employed to deliver both QDs and siRNA to murine fibroblasts (9). The gene silencing effect correlated directly with intracellular fluorescence (i.e. the QD signal), and > 90% gene knockdown was achieved in the highly fluorescent cells. Subsequently, a new system was reported which incorporated a polyethylene glycol-modified QD core with siRNA and tumor-homing peptides attached (10). However, only modest gene knockdown (< 30% based on fluorescence) was achieved with this system. In another study, QDs were encapsulated into antibody-conjugated chitosan nanoparticles to monitor the delivery of HER2 siRNA to cancer cells (11).
While fluorescent QDs remain attractive tools for cell- and animal-based studies, many barriers prevent their immediate clinical translation (12). In general, due to poor tissue penetration and lack of quantitation capability, optical imaging has very limited clinical potential.
Dual-modality MRI and Fluorescence Imaging
In 2007, a multifunctional agent was described for in vivo transfer of siRNA and simultaneous imaging of tumor targeting by MRI and near-infrared fluorescence (NIRF) imaging (13). The NIRF dye-labeled magnetic nanoparticle, covalently linked to siRNA molecules specific for either model or therapeutic targets, was further modified with a membrane translocation peptide for intracellular delivery. In vivo tracking of this multifunctional agent in two tumor models was demonstrated by MRI and NIRF imaging. Further, in vivo optical imaging in the GFP channel confirmed that efficient gene silencing was achieved in 9L rat gliosarcoma tumors stably transfected with GFP. This study was the first example of combining non-invasive multimodality imaging and RNAi using a nanoparticle.
SPECT Imaging
The inherent characteristics of radionuclide-based imaging (e.g. sensitive, quantitative, and tomographic) make SPECT and PET more suitable for in vivo imaging of siRNA delivery than MRI and optical imaging. 99mTc and 111In have been used to determine the cellular delivery and biodistribution of siRNAs (14, 15). In addition, the stability of 111In-labeled siRNA-poly(ethyleneimine) complexes has been investigated with SPECT (16). Although these studies provided encouraging results regarding the absolute accumulation of radiolabeled siRNAs in tumors, it was not clear whether the delivered siRNA was functional and if any therapeutic effect was achieved.
PET Imaging
Due to its high sensitivity, PET has the greatest clinical potential among all molecular imaging modalities. PET and bioluminescence imaging (BLI) have been used to evaluate the biodistribution and function of siRNA-containing nanoparticles in mice inoculated with luciferase-transfected tumor cells (17). Conjugation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to the 5' end of the siRNA allowed for 64Cu-labeling. PET data showed that non-targeted and transferrin-targeted siRNA-containing nanoparticles exhibited similar biodistribution and tumor accumulation (17). However, BLI revealed that transferrin-targeted nanoparticles resulted in lower tumor luciferase activity than the non-targeted nanoparticles. This strategy enabled direct and non-invasive evaluation of siRNA delivery, which can be combined with BLI to further characterize the therapeutic potential of RNAi. In future studies, certain issues such as in vivo stability of the 64Cu-DOTA complex, and whether DOTA-conjugated siRNAs have comparable RNAi efficiency as unlabeled siRNAs, deserve further investigation.
Utilizing the most widely used positron-emitter, 18F, a recent study examined the biodistribution of radiolabeled siRNAs (18). Replacement of the 2′-hydroxyl group of certain nucleotides in a siRNA sequence with a fluorine atom or a methoxy group was found to be compatible with RNAi. PET imaging, performed after intravenous injection of 18F-labeled siRNAs (fluorine/methoxy substituted or unmodified) in rodents, showed rapid kidney and liver clearance of radioactivity. Although tissue distribution profiles of these siRNAs were similar, fluorine-modified siRNAs exhibited better blood persistence and stability than both the methoxy-substituted and unmodified siRNAs.
The two strategies for imaging siRNA delivery are: labeling the siRNA itself (which may affect its function/specificity and RNAi efficiency) or labeling the siRNA carrier. The latter will not affect the efficacy of siRNA, but the imaging data represent localization of the carrier rather than the siRNA. In future studies, labeling the siRNA and the carrier with different imaging tags should be investigated. Comparison of the biodistribution of labeled siRNA and labeled carrier will provide a more complete picture and lead to better understanding of siRNA delivery. One study described above concluded that fluorine substitution did not affect the activity of siRNAs (18), thus optimizing 18F chemistry for siRNA labeling may provide another means for accurate evaluation of siRNA delivery.
In conclusion, molecular imaging has the potential to play a key role in selecting siRNA delivery systems with optimal biodistribution, pharmacokinetics, and targeting efficacy for future RNAi-based therapies. An alternative approach to confirm the efficiency of RNAi is to measure the silencing effect and/or therapeutic response. Currently, the majority of such studies use optical imaging techniques (both bioluminescence and fluorescence), which allow semi-quantitative or quantitative measurement of gene inhibition.
IMAGING THE THERAPEUTIC/SILENCING EFFECTS
Molecular imaging of the therapeutic/silencing effects of RNAi is still at the preclinical stage, mainly through visualizing the levels of marker gene expression in living animals. These imaging markers are mostly bioluminescent enzymes, whose activity can be accurately measured based on the light output since there is minimal background signal in animals. The use of fluorescent proteins (e.g. EGFP or RFP) is also feasible, however tissue autofluorescence may interfere with the EGFP/RFP signal.
MRS is also useful in evaluating the efficacy of RNAi-based therapies because of its potential to directly detect the biological outcomes. By measuring the chemical shifts of certain compounds (e.g. choline whose concentration in tissues/tumors can be altered by certain therapeutic intervention), MRS may have better clinical utility than optical imaging.
Bioluminescence Imaging
Many proof-of-principle studies on in vivo RNAi adopted the strategy of silencing luciferase expression as a measurement of siRNA/shRNA activity (19, 20). In several recent stuides, BLI has been employed to investigate the dose/time-dependence of vector-based RNAi (21) and to optimize various siRNA delivery methods (22, 23).
Multi-drug resistance (MDR), a major obstacle for successful chemotherapy of cancer, can be caused by overexpression of P-glycoprotein, a product of the MDR1 gene. In an elegant study, BLI was used for non-invasive assessment of P-glycoprotein silencing (24). Taking advantage of the fact that the substrate of Renilla luciferase (i.e. coelenterazine) is effluxed out of the cells by P-glycoprotein, BLI was used to evaluate the shRNA-mediated down-regulation of P-glycoprotein activity in both cultured cells and tumor implants in living animals (24). Further, a similar strategy was also shown to be applicable to firefly luciferase (24).
In another report, the role of hypoxia-inducible factor-1alpha (HIF-1α) in glioma growth was investigated in vivo with RNAi (25). BLI revealed that RNAi therapy could significantly attenuate glioma growth by reducing HIF-1α levels constitutively (using shRNA) or transiently (using siRNA).
Despite the success of BLI in evaluating gene silencing efficiency of RNAi, it should be interpreted with caution because firefly luciferase has a relatively short half-life in living cells (a few hours). In contrast, fluorescent proteins have much longer half-lives (up to 26 h). Therefore, fluorescence imaging with EGFP or other fluorescent proteins can be more advantageous than BLI in evaluating the long-term effects of RNAi.
Fluorescence Imaging
EGFP and its variants have been primarily used for ex vivo imaging studies to monitor the efficiency of RNAi. Many of these studies provided answers to important questions such as the subcellular distribution and bioavailability of siRNAs delivered by various formulations, including tumor-targeted carriers. In several of the studies mentioned above, EGFP was used to evaluate the silencing effect of siRNAs and confirm their delivery to the target organs (10, 13). In another report, the effect of intratumoral injection of siRNA (against EGFP) into EGFP-expressing B16F10 melanoma tumors, followed by application of an external electric field, was evaluated with fluorescence imaging (26). A significant decrease in tumor EGFP fluorescence was observed by in vivo imaging within 2 days after treatment.
MRS
MRS, with unique specificity but low sensitivity, can be used for imaging the metabolic changes which accompany many diseases such as cancer. Elevated levels of phosphocholine (PC) and total choline (tCho) metabolites are well-established characteristics of many cancer cells, thus monitoring them with MRS can be very effective in assessing the therapeutic effect of RNAi. Recently, a breast cancer model was used to investigate lentiviral vector-mediated shRNA down-regulation of choline kinase (chk), the enzyme that converts choline to PC (27). After intravenous injection of lentiviruses (which express the shRNA against chk) into MDA-MB-231 tumor-bearing mice, non-invasive 31P MRS revealed that PC and phosphomonoester levels in the tumor significantly decreased. More importantly, chk silencing resulted in reduced tumor growth and proliferation. This study demonstrated the proof-of-principle that non-invasive MRS could be used to monitor RNAi-based therapies with high accuracy.
CONCLUSION AND FUTURE PERSPECTIVES
A variety of molecular imaging techniques have been explored for in vivo imaging of RNAi (Table 1). Clearly, continued development of non-invasive imaging strategies for monitoring both siRNA/shRNA delivery and their gene silencing effect will provide more insights into RNAi-based therapies. Optical techniques used to be the only tools for non-invasively evaluating the effects of RNAi. Although useful in preclinical settings, the clinical potential of these techniques is limited. MRS, recent applied to RNAi-based therapies, has certain clinical potential but may be limited by its sensitivity. Imaging siRNA delivery with SPECT/PET is clinically relevant, however the labeling chemistry needs to be chosen carefully. Much future effort will be required to optimize various imaging techniques for use in RNAi-based therapies.
Table 1.
A brief summary of in vivo imaging of RNAi. (N/A: not applicable).
| Modality | Imaging delivery or effect? |
Image tag | Labeling gene or carrier? |
Clinical potential |
Ref |
|---|---|---|---|---|---|
| Fluorescence | both | fluorescent dye, QD, GFP, etc. |
both | + | (8–11, 13, 26) |
| MRI | delivery | iron oxide | carrier | ++ | (13) |
| SPECT | delivery | 99mTc and 111In | both | +++ | (14–16) |
| PET | delivery | 18F and 64Cu | gene | +++ | (17, 18) |
| BLI | effect | luciferases | N/A | None | (17, 20–25) |
| MRS | effect | chemical shift | N/A | ++ | (27) |
The most significant barrier to the widespread use of RNAi in the clinic is delivery. Solving this problem will require the development of clinically suitable, safe, and effective gene delivery systems. Incorporation of molecular imaging techniques to monitor the gene delivery efficiency and/or the silencing effect, which is missing from most of the currently ongoing RNAi-based clinical trials, may dramatically facilitate the transformation of RNAi into a powerful therapeutic modality in the clinic.
Over the last decade, molecular imaging with PET/SPECT has advanced dramatically and many PET/SPECT probes are already in clinical trials for a wide variety of targets (28, 29). Some of these probes may be directly used to monitor the therapeutic effect of RNAi in the future, if the imaging target is (related to) the target of RNAi. Lastly, monitoring RNAi-based therapies (both gene delivery and the silencing effect) with a single imaging modality may not be sufficient in many cases. Rational design and use of dual-modality or multimodality imaging in future RNAi-related studies will allow researchers to acquire more pieces of the puzzle, thereby fulfilling the enormous potential of RNAi.
ACKNOWLEDGMENTS
The authors are grateful to the financial support from Wisconsin Partnership Program, UW Carbone Cancer Center, NCRR 1UL1RR025011, and Susan G. Komen for the Cure.
REFERENCES
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm D. Small silencing RNAs: state-of-the-art. Adv Drug Deliv Rev. 2009;61:672–703. doi: 10.1016/j.addr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Samul R, Silva RL, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 5.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdanov AA., Jr Merging molecular imaging and RNA interference: early experience in live animals. J Cell Biochem. 2008;104:1113–1123. doi: 10.1002/jcb.21689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore A, Medarova Z. Imaging of siRNA delivery and silencing. Methods Mol Biol. 2009;487:93–110. doi: 10.1007/978-1-60327-547-7_5. [DOI] [PubMed] [Google Scholar]
- 8.Chang E, Zhu MQ, Drezek R. Novel siRNA-based molecular beacons for dual imaging and therapy. Biotechnol J. 2007;2:422–425. doi: 10.1002/biot.200600257. [DOI] [PubMed] [Google Scholar]
- 9.Chen AA, Derfus AM, Khetani SR, Bhatia SN. Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Res. 2005;33:e190. doi: 10.1093/nar/gni188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derfus AM, Chen AA, Min DH, Ruoslahti E, Bhatia SN. Targeted quantum dot conjugates for siRNA delivery. Bioconjug Chem. 2007;18:1391–1396. doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 11.Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials. 2007;28:1565–1571. doi: 10.1016/j.biomaterials.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Hsu AR, Li ZB, Chen X. Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Res Lett. 2007;2:265–281. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 14.Liu N, Ding H, Vanderheyden JL, Zhu Z, Zhang Y. Radiolabeling small RNA with technetium-99m for visualizing cellular delivery and mouse biodistribution. Nucl Med Biol. 2007;34:399–404. doi: 10.1016/j.nucmedbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Behe M, Kissel T. In vivo SPECT and real-time gamma camera imaging of biodistribution and pharmacokinetics of siRNA delivery using an optimized radiolabeling and purification procedure. Bioconjug Chem. 2009;20:174–182. doi: 10.1021/bc800408g. [DOI] [PubMed] [Google Scholar]
- 16.Merkel OM, Librizzi D, Pfestroff A, et al. Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: Effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. J Control Release. 2009;138:148–159. doi: 10.1016/j.jconrel.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viel T, Boisgard R, Kuhnast B, et al. Molecular imaging study on in vivo distribution and pharmacokinetics of modified small interfering RNAs (siRNAs) Oligonucleotides. 2008;18:201–212. doi: 10.1089/oli.2008.0133. [DOI] [PubMed] [Google Scholar]
- 19.Kurreck J. RNA interference: from basic research to therapeutic applications. Angew Chem Int Ed Engl. 2009;48:1378–1398. doi: 10.1002/anie.200802092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi N, Matsui Y, Kawase A, et al. Vector-based in vivo RNA interference: dose- and time-dependent suppression of transgene expression. J Pharmacol Exp Ther. 2004;308:688–693. doi: 10.1124/jpet.103.059931. [DOI] [PubMed] [Google Scholar]
- 22.Hassani Z, Lemkine GF, Erbacher P, et al. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J Gene Med. 2005;7:198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- 23.Dassie JP, Liu XY, Thomas GS, et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27:839–846. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pichler A, Zelcer N, Prior JL, Kuil AJ, Piwnica-Worms D. In vivo RNA interference-mediated ablation of MDR1 P-glycoprotein. Clin Cancer Res. 2005;11:4487–4494. doi: 10.1158/1078-0432.CCR-05-0038. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie DL, Whang K, Ragel BT, Flynn JR, Kelly DA, Jensen RL. Silencing of hypoxia inducible factor-1alpha by RNA interference attenuates human glioma cell growth in vivo. Clin Cancer Res. 2007;13:2441–2448. doi: 10.1158/1078-0432.CCR-06-2692. [DOI] [PubMed] [Google Scholar]
- 26.Golzio M, Mazzolini L, Ledoux A, et al. In vivo gene silencing in solid tumors by targeted electrically mediated siRNA delivery. Gene Ther. 2007;14:752–759. doi: 10.1038/sj.gt.3302920. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamachary B, Glunde K, Wildes F, et al. Noninvasive detection of lentiviral-mediated choline kinase targeting in a human breast cancer xenograft. Cancer Res. 2009;69:3464–3471. doi: 10.1158/0008-5472.CAN-08-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49 Suppl 2:113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 29.Cai W, Niu G, Chen X. Multimodality imaging of the HER-kinase axis in cancer. Eur J Nucl Med Mol Imaging. 2008;35:186–208. doi: 10.1007/s00259-007-0560-9. [DOI] [PubMed] [Google Scholar]