Abstract
Activation of the innate immune system typically precedes engagement of adaptive immunity. Cells at the interface between these two arms of the immune response are thus critical to provide full engagement of host defense. Among the innate T cells at this interface are γδ T cells. γδ T cells contribute to the defense from a variety of infectious organisms, yet little is understood regarding how they are activated. We have previously observed that human γδ T cells of the Vδ1 subset accumulate in inflamed joints in Lyme arthritis and proliferate in response to stimulation with the causative spirochete, Borrelia burgdorferi. We now observe that murine γδ T cells are also activated by B. burgdorferi and that in both cases the activation is indirect via Toll-like Receptor (TLR) stimulation on dendritic cells or monocytes. Furthermore, B. burgdorferi stimulation of monocytes via TLR, and secondary activation of γδ T cells, are both caspase-dependent.
Introduction
Lyme disease is the most common vector-borne disease in the United States (1, 2), which is caused by the spirochete Borrelia burgdorferi and transmitted to humans by Ixodes ticks (3, 4). Infection often produces a distinct rash and flu-like illness in its initial stage, but can later progress to more chronic cardiac, neurologic, and rheumatic manifestations (1, 5–7). Several lines of evidence point to a role of T lymphocytes in Lyme arthritis. These include the ability of adoptively transferred B. burgdorferi-specific T cells to confer arthritis (8), the presence of activated T cells in Lyme arthritis synovial tissue that proliferate to B. burgdorferi (7, 9), an association of HLA-DR4 with chronic antibiotic-resistant Lyme arthritis (10), similar to rheumatoid arthritis (11), and effective therapeutic treatments that partially inhibit T cell function (1, 2).
γδ T cells are activated in a variety of infectious and inflammatory disorders. They are often anatomically sequestered at epithelial barriers or sites of inflammation (12), and can manifest cytotoxicity toward a wide array of targets (13). γδ T cells are moderately protective in infections due to Listeria (14), Leishmania (15), Mycobacterium (16), Plasmodium (17), and Salmonella (18). γδ T cells also accumulate at inflamed sites in autoimmune disorders such as rheumatoid arthritis (19), Lyme arthritis (20), celiac disease (21), and sarcoidosis (22). Evidence suggests that they may be beneficial in certain autoimmune models. Both collagen-induced arthritis in mice (23) and adjuvant arthritis in rats (24) are worse after depletion of γδ T cells, as are murine lupus (25) and a model of orchitis (26).
The predominant human γδ T cell subset in peripheral blood is Vγ2Vδ2, which reacts to nonpeptide antigens from Mycobacterium. These include isoprenyl pyrophosphates (27–30), as well as alkylamine antigens (31). These are products of microbes as well as self-antigens. By contrast, the Vδ1 subset accumulates in inflamed synovium in rheumatoid arthritis (19, 32) and Lyme arthritis (20). Very little is known regarding the mechanism of activation of this γδ T cell subset or their function. Our earlier studies on synovial Vδ1 cells revealed that they can potently activate myeloid dendritic cells (DC) in a Fas/Fas-ligand-dependent manner (33). In this capacity, Vδ1 cells may be important at initiating the adaptive immune response. We have also previously observed that Lyme synovial Vδ1 cells proliferate in response to a sonicate of Borrelia burgdorferi plus DC, but it was uncertain whether this process was a direct or indirect activation by Borrelia components. We now observe that activation of Vδ1 cells by B. burgdorferi is predominantly an indirect process via Toll-like receptor (TLR) signaling and requires cell contact with metabolically active DC or monocytes. Studies with both human Vδ1 T cells and murine γδ T cells show that Borrelia activates γδ T cells primarily via TLR2, but stimulation by other TLR ligands can also indirectly activate γδ T cells. These new findings serve to form a stronger connection by γδ T cells between innate and adaptive immune responses.
Material and Methods
Mice
C57BL/6 mice, either wild-type, TLR2−/−, TLR9−/−, or MyD88−/−, were housed and bred in the University of Vermont animal facility and used at 2–6 months of age. The facility is AALAC-approved, and protocols were approved by IACUC. Original breeding pairs were obtained from Jackson Laboratories (Bar Harbor, ME).
Derivation of synovial fluid γδ T cell clones and monocyte-derived DC
Lymphocytes were purified from synovial fluid of Lyme arthritis patients by Ficoll-Hypaque centrifugation (Sigma Chemical Company, St. Louis, MO), and cultured in AIM-V medium (GIBCO BRL, Gaithersburg, MD) containing 5% fetal bovine serum (FBS) (HyClone, Logan, UT) and 50 U/ml recombinant human IL-2 (Cetus, Emeryville, CA). Cells were stimulated with 10 µg/ml of a sonicate of B. burgdorferi, strain N40, grown in BSK II medium (Sigma) as previously described (20, 34). From these bulk cultures, responding cells were cloned at 0.3 cells/well in AIM-V with 10% FBS in the presence of irradiated peripheral blood lymphocytes (3 × 105/well), human recombinant IL-2 (100 U/ml), and 10 µg/ml of B. burgdorferi. After 14–21 days, cells from positive wells were phenotyped and those containing γδ T cells were expanded by restimulation with either B. burgdorferi (10 µg/ml) or PHA (1 µg/ml) (Murex Biotech, Dartford, Kent, England), at approximately 14-day intervals. All synovial γδ clones were Vδ1 by antibody screening and DNA sequencing and proliferated in response to Borrelia stimulation (35).
Human monocytes were purified as CD14+ cells from peripheral blood of healthy volunteers (Miltenyi Biotech, Auburn, CA). Myeloid DC were prepared by culture of monocytes with 800 U/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF), (BioSource International, Camarillo, CA) and 500 U/ml IL-4 (BioSource).
Preparation of murine γδ T cells and bone marrow-derived dendritic cells
Spleen cells were depleted of erythrocytes by hypotonic lysis followed by negative selection to enrich for γδ T cells using rat monoclonal antibodies to CD4 (GK1.5), CD8 (Tib105), B220 (RA3-6B2), MHC class II (3F12), and CD11b (M1/70) for 30 min. The samples were washed and then incubated with goat anti-rat IgG-labeled magnetic beads (Qiagen, Inc.) for 45 min followed by magnetic field separation. The purified cells were cultured in 48-well plates coated with 5µg/ml of anti-TCR-γδ antibody (GL3) in complete culture medium [RPMI 1640 supplemented with 25 mM HEPES, 2.5 mg/ml glucose (Sigma Chemical Corp., St. Louis, MO), 10 µg/ml folate (Invitrogen, Carlsbad, CA), 110 µg/ml pyruvate (Invitrogen), 5×10−5 M 2-ME (Sigma), 292.3 µg/ml glutamine (Invitrogen), 100 U/ml penicillin-streptomycin (Gibco Lifesciences), and 10% FBS] containing 100 U/ml recombinant human IL-2. After two days, cells were moved to uncoated wells for further expansion with complete medium plus IL-2. On day 7, cells were used for experiments with γδ T cell purity >95%.
The preparation of bone marrow-derived dendritic cells (BMDC) was done according to the method of Lutz, et al. (36) and used on day 10.
T cell-monocyte/DC co-cultures
Cultures of monocytes or DC (5 × 105/ml) with Vδ1 clone cells (1 × 106/ml) were made in AIM-V medium with IL-2 (100 U/ml) and 10% FBS in the absence or presence of B. burgdorferi sonicate (10 µg/ml), purified native lipidated or delipidated OspA from B. burgdorferi as previously described (35) (10 µg/ml), or in some experiments other TLR ligands, including Pam3Cys (InvivoGen, San Diego, CA), E. Coli 0111:B4LPS (Sigma), CpG (Coley Pharmaceutical, Wellesley, MA), or Poly I:C (Sigma) (each at 1 µg/ml). After 24 h cells supernatants were removed for cytokine analysis by ELISA, and cells were stained for expression of TCR-γδ and CD25 by flow cytometry using an LSR II (BD Biosciences, San Jose, CA). Blocking studies were performed using either control IgG, anti-TLR2 (a kind gift of Dr. Robert Finberg, University of Massachusetts), anti-IL-1β (clone 8516, R&D Systems), anti-IL-6 (cat. #AF206NA, R&D Systems), anti-TNFα (clone J1D9, Ancell) (each at 20 µg/ml).
Transwell cultures were performed using 1 × 106 Vδ1 cells in 1 ml AIM-V/FBS/IL-2 medium placed in the lower chamber, with 5 × 105 DC in 100 µl placed on top of the membrane of the upper chamber with B. burgdorferi sonicate at 10 µg/ml. After 24 h the Vδ1 cells were assessed for expression of CD25.
Chemical fixation of DC was performed by incubating the cells in the absence or presence of B. burgdorferi at 10 µg/ml at 37°C overnight, then washed in 5% FBS in RPMI, and fixed by the addition of ice-cold 1-ethyl-3(3’-dimethyl-aminopropyl)-carbodiimide (EDCI, Sigma) at 75 mM in PBS for 60 min on ice. Following fixation the DC were extensively washed with 5% FBS/RPMI and then incubated with Vδ1 cells in the absence of additional B. burgdorferi. Expression of CD25 by the Vδ1 cells was examined after an additional 24 h.
Antibodies and flow cytometry
Antibodies used were to the following determinants: TCR-γδ (5A6.E9, Invitrogen/Caltag), CD25 (CD25-3G10, Invitrogen/Caltag), CD1a (HI149, Invitrogen/Caltag), CD1b (M-T101, BD-Pharmingen), CD1c (M241 Ancell), and CD1d (CD1d42, BD-Pharmingen). Samples were analyzed on an LSR II flow cytometer (BD Biosciences).
Biotin-VAD-fmk active caspase precipitation assay
Cells were lysed in lysis buffer containing 20 µM biotin-VAD-fmk (MP Biomedicals). 600 µg of lysate were precleared by rocking with 40 µl Sepharose 6B agarose beads (Sigma) at 4°C for 2 h. Supernatants were then rocked with 60 µl streptavidin-sepharose beads (Zymed, Invitrogen) at 4°C overnight. Beads were washed 5 times in lysis buffer, then boiled in loading buffer. Beads were removed by centrifugation and immunoblot analysis was then performed on supernatants.
Immunoblot analysis
T cells were lysed in buffer containing 0.2% NP40, 20 mM Tris HCl (pH 7.4, American Bioanalytical, Natick, MA), 2 mM sodium orthovanadate (Sigma), 10% glycerol (Fisher Scientific, Pittsburgh, PA), 150 mM NaCl (Sigma), complete protease inhibitor (Roche Diagnostics, Indianapolis, IN), and 20 µM z-VAD-fmk (MP Biomedicals). Protein concentration was determined by Bradford assay (BioRad Laboratories, Hercules, CA). Protein lysates were boiled for 5 min in loading buffer containing 2-ME and separated using SDS-PAGE on 10%, or 12.5% gels. Proteins were transferred onto PVDF membranes (BioRad) and blocked using 4% milk in Tris-buffered saline plus 0.1% Tween-20 (American Bioanalytical) at room temperature for 1 h. Membranes were incubated at 4°C overnight in milk containing anti-human caspase-8 (BD Biosciences). Immunoreactive proteins were visualized using HRP-labeled conjugates (Santa Cruz Biotechnology, Santa Cruz, CA, Southern Biotech, Birmingham, AL, Biomeda, Foster City, CA) and developed using LumiGLO (KPL, Gaithersburg, MD).
Caspase activity assay
Relative caspase activities were determined using the Apo-ONE Caspase Assay (Promega, Madison, WI). Monocytes, either freshly isolated or following two days culture with B. burgdorferi (10 µg/ml), or cultured DC, were resuspended in culture medium at 10 × 106/ml. 100 µl of cells were serially diluted in 100 µl culture medium and then mixed with 100 µl of caspase reagent (DEVD-rhodamine) according to the manufacturer’s protocol. Spectrophotometric readings were taken over a range of times using a Fluorescence reader (Bioteck Instruments, Winooski, VT).
Statistical analysis
Unpaired t tests were used to assess the significance of differences in production of cytokinies by monocytes and DC assessed by ELISA.
Results
Borrelia burgdorferi activates γδ T cells indirectly via TLR pathways
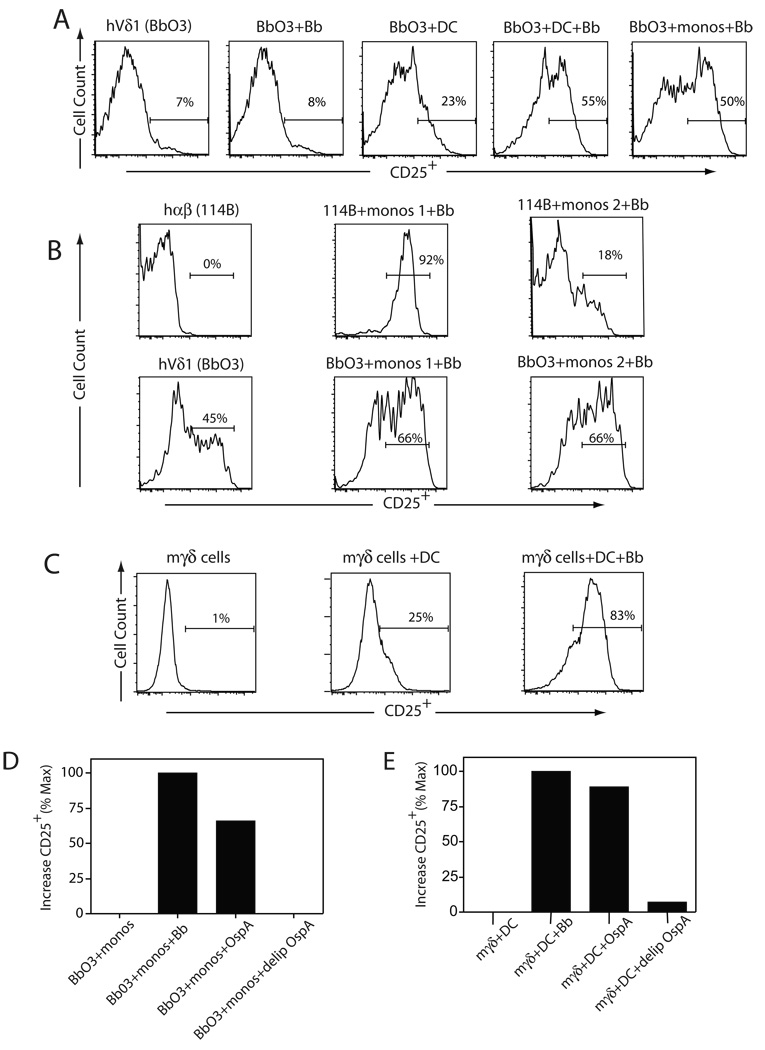
To examine the pathway by which B. burgdorferi might activate γδ T cells, we considered whether this would be a direct or indirect process. As shown in Figure 1A, Lyme arthritis synovial Vδ1 clones (20, 35), as represented by clone Bb03, were stimulated to express CD25 by the addition of B. burgdorferi in the presence of myeloid DC. Borrelia alone did not induce CD25 expression, and myeloid DC without Borrelia induced only a modest upregulation of CD25 expression by the Vδ1 clones. Borrelia stimulation of the Vδ1 clones was also possible using fresh CD14+ monocytes from which the DC were derived, using HLA mismatched donors (Fig. 1B lower panels). By contrast, activation of a Borrelia-specific CD4+ αβ T cell clone, 114B, was achieved only by use of autologous monocytes (Fig. 1B upper panels).
Figure 1. Borrelia burgdorferi activates both human and murine γδ T cells.
(A) Induction of CD25 expression by human Lyme arthritis synovial Vδ1 T cell clone Bb03 following 24 h of stimulation with medium alone (Bb03), a sonicate of B. burgdorferi alone (Bb03+Bb), dendritic cells alone (Bb03+DC), DC plus B. burgdorferi (Bb03+DC+Bb), or fresh monocytes plus B. burgdorferi (Bb03+monos+Bb). The findings were consistent in three separate experiments using two Vδ1 clones from two patients. (B) Human CD4+ TCR-αβ (hαβ) Borrelia-specific T cell clone 114B (upper panel) was stimulated with monocytes and B. burgdorferi using either autologous monocytes (114B+monos 1+Bb) or HLA-mismatched monocytes (114B+monos 2+Bb). CD25 expression was measured after 24 h. A human Vδ1 (hVδ1) clone Bb03 was used under identical stimulation conditions (lower panel)._(C) Murine splenic γδ T cells were purified by negative selection (see Methods) and then stimulated with anti-γδ plus IL-2 and propagated for 8 days until surface CD25 levels had downregulated. The γδ T cells were then restimulated with syngeneic bone marrow-derived DC (BMDC) in the absence or presence of B. burgdorferi, and CD25 levels measured after 24 h. The findings were consistent in two experiments. (D) Human Vδ1 T cell clone Bb03 was stimulated with fresh CD14+ monocytes (monos) in the presence of either no additives, B. burgdorferi sonicate, lipidated OspA (OspA), or delipidated OspA (delip OspA). CD25 expression was measured after 24 h by flow cytometry and results expressed as the percent increase compared to minimum defined by γδ T cells with monos or DC alone, and maximum CD25 induction defined by stimulation with Borrelia sonicate plus DC. (E) The same conditions as in (C) were used to activate murine γδ T cells with BMDC.
To further test the generality of the activation of γδ T cells by Borrelia, we also examined the activation of murine γδ T cells in this system. Splenic γδ T cells were enriched by negative selection and then activated with anti-γδ antibody plus IL-2 and expanded over 7 days. Cultures at this time contained >95% γδ T cells bearing low levels of surface CD25. As such they resembled the activation state of the human synovial Vδ1 T cell clones. The murine γδ T cells were then restimulated using syngeneic bone marrow-derived dendritic cells (BMDC) with or without B. burgdorferi. Similar to the human synovial Vδ1 cells, murine splenic γδ T cells were also strongly stimulated to upregulate CD25 in the presence of BMDC plus Borrelia, but to a much lesser extent with BMDC alone (Fig. 1C). Further dissection of the components of B. burgdorferi that were responsible for the stimulation of CD25 expression by the γδ T cells revealed that a single surface lipoprotein, OspA, was capable of stimulating both human Vδ1 T cells (Fig. 1D) and murine γδ T cells (Fig. 1E) to nearly the same degree as the Borrelia sonicate. However, when the tripalmitic acid lipid component of OspA was absent, the ability to stimulate human and murine γδ T cells was lost.
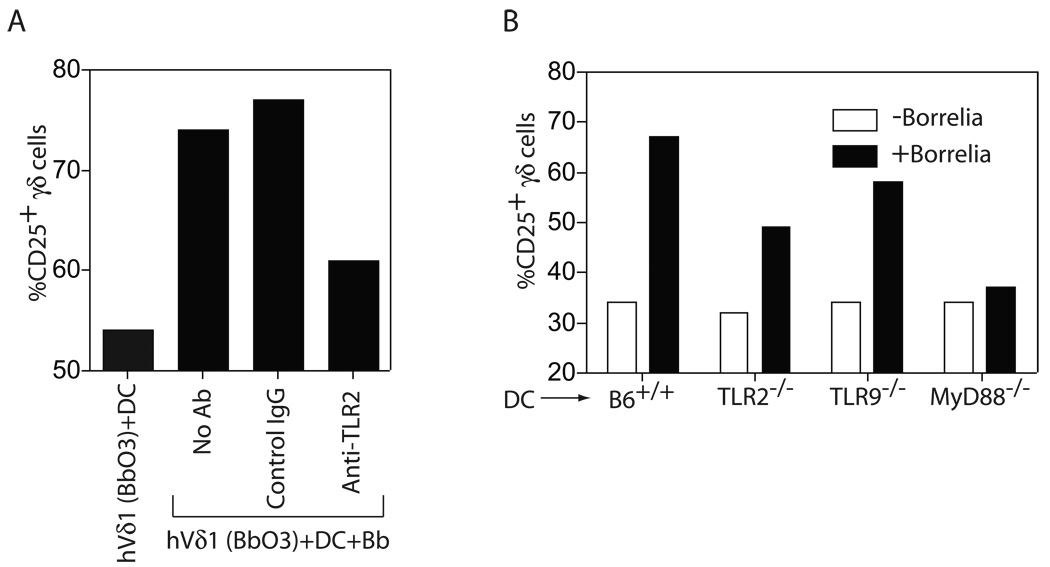
Lipidated OspA has been shown to be a TLR2 ligand, and B. burgdorferi contains no LPS, a TLR4 ligand (37). We thus examined the requirement of TLR2 signaling in the activation of the human and murine γδ T cells. A blocking anti-human TLR2 antibody inhibited the induction of CD25 expression by the human Vδ1 cells by about 50%, which was consistent in three experiments (Fig. 2A). A similar decline in CD25 induction was observed in the murine γδ T cells when BMDC were used from TLR2−/− mice (Fig. 2B). Less decline of CD25 expression was observed in the absence of TLR9 (a receptor for bacterial DNA), but in the absence of MyD88, a signaling intermediate for most TLR, there was essentially complete inhibition of CD25 induction (Fig. 2B). The murine γδ T cell findings were consistent in three experiments.
Figure 2. Activation of human and murine γδ T cells by B. burgdorferi requires TLR signals.
(A) Human Vδ1 T cell clone Bb03 was either not stimulated, or activated by DC plus Bb in the presence of no antibody, control IgG, or anti-TLR2, both at 20 µg/ml. CD25 expression was measured by flow cytometry after 24 h. (B) Murine γδ T cells were stimulated in the absence (white bars) or presence (black bars) of B. burgdorferi sonicate using DC from wild-type C57BL/6 mice (B6+/+), or B6 mice deficient for TLR2, TLR9, or MyD88. CD25 expression was measured by flow cytometry after 24 h. The findings were consistent in two separate experiments.
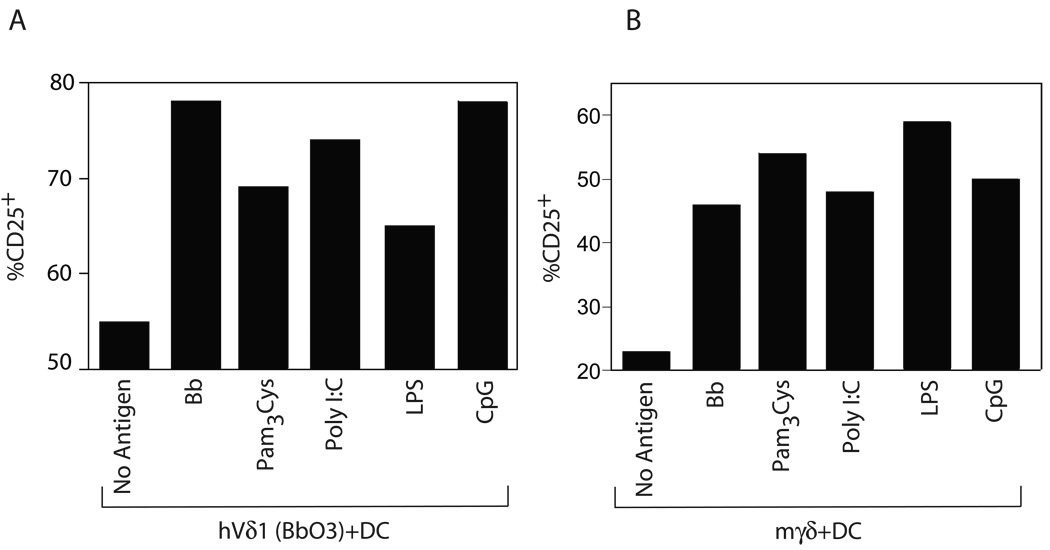
The ability of Borrelia lipopeptides to activate both human and murine γδ T cells raised the question whether this phenomenon might be a property of other TLR ligands. We therefore examined the ability of several TLR ligands to activate human and murine γδ T cells. As shown in Figure 3, in the presence of DC, Pam3Cys (TLR2 ligand), poly I:C (TLR3 ligand), LPS (TLR4 ligand), or CpG (TLR9 ligand) were each able to activate human (Fig. 3A) and murine (Fig. 3B) γδ T cells. As with Borrelia stimulation, no induction of CD25 expression by the Vδ1 clones was observed in the absence of DC, and we have not observed expression of these TLR by the γδ T cells (data not shown). Collectively these findings suggested that activation of γδ T cells by B. burgdorferi and other TLR ligands is an indirect process.
Figure 3. Ligands for various TLR can activate γδ T cells.
(A) Human Vδ1 T cell clone Bb03 (A) or (B) murine γδ T cells were either not activated, or activated in the presence of DC with B. burgdorferi (Bb) or TLR ligands Pam3Cys (TLR2), Poly I:C (TLR 3), LPS (TLR4), or CpG (TLR9), and surface CD25 expression measured after 24 h. Results were consistent in three separate experiments.
Activation of human Vδ1 cells by B. burgdorferi requires cell contact with DC
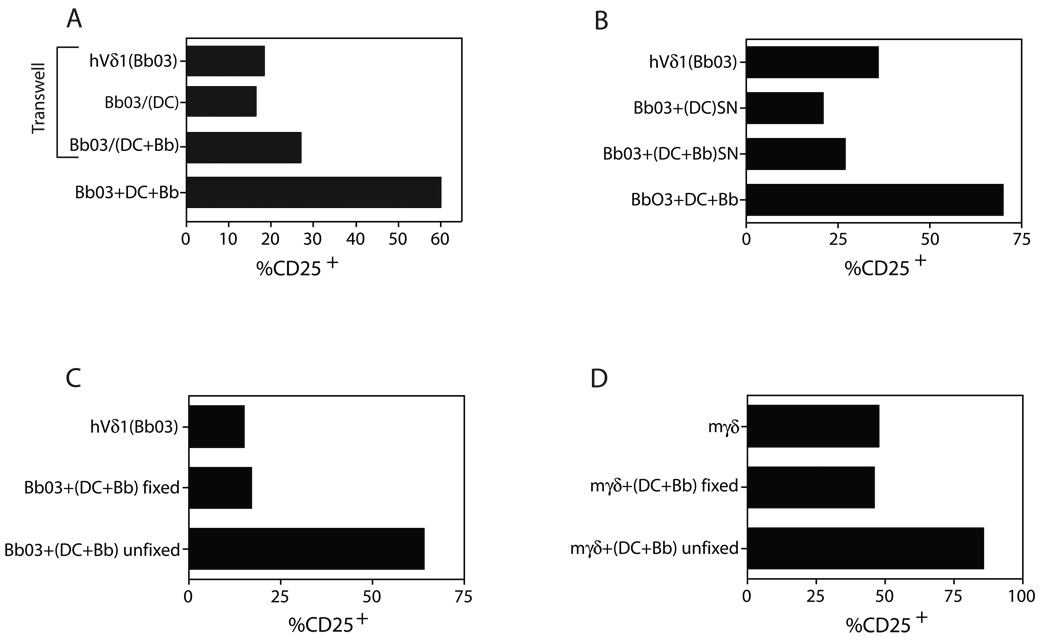
To determine whether cell contact between γδ T cells and DC was required for activation of γδ T cells, a transwell system was used in which Vδ1 T cells were placed in the lower chamber (106/ml) and DC (5 × 105 in 100 µl) placed on the membrane of the upper chamber with or without B.burgdorferi. Cell densities and Borrelia sonicate concentration (10 µg/ml) were the same as used in conventional contact cultures, which were used as a positive reference control for induction of CD25 on γδ T cells. Figure 4A shows that no induction of CD25 expression on human γδ T cells was observed in the transwell system using DC alone, and this was not significantly increased with the addition of Borrelia to the transwell cultures (Fig. 4A). To examine this further, supernatants from various DC culture conditions were used to activate human Vδ1 cells. Neither supernatants from cultures of DC alone, nor DC plus Borrelia for 24 h could activate the γδ T cells (Fig. 4B). We further studied this question following fixation with EDCI of human or mouse DC after 24 h incubation with Borrelia sonicate. Fixation resulted in the complete loss of the ability of DC to activate either human (Fig. 4C) or mouse (Fig. 4D) γδ T cells. However, DC pretreated with Borrelia in a similar manner, washed thoroughly after 24 h, but not fixed, were able to strongly activate human and mouse γδ T cells (Fig. 4C, D). These collective findings supported the view that full activation of γδ T cells by B. burgdorferi required direct cell contact with metabolically active DC.
Figure 4. Activation of γδ T cells requires contact with metabolically active DC.
(A) Transwell culture in which Vδ1 clone Bb03 (106/ml) was placed in the lower chamber and DC (5 × 105 in 100 µl) placed on the membrane of the upper chamber, in the absence [Bb03/(DC)] or presence of B. burgdorferi sonicate [Bb03/(DC+Bb)]. Bb03+DC+Bb served as a comparison for activation by conventional non-transwell contact stimulation, shown at the bottom. Surface CD25 was examined after 24 h by flow cytometry. (B) Vδ1 clone Bb03 was stimulated with supernatants (SN) from 24 h cultures of DC alone [(DC)SN] or DC + B. burgdorferi [(DC+Bb)SN]. CD25 expression was measured after 24 h. Comparison was made to conventional stimulation of Bb03+DC+Bb shown at the bottom. (C) Human or (D) murine DC were incubated overnight with B. burgdorferi extract and then either fixed with ECDI or unfixed before the addition of either human Vδ1 T cell clone Bb03 (C) or murine γδ T cells (D) for an additional 24 h. CD25 expression was then measured gated on the γδ T cells. Findings are representative of three separate experiments.
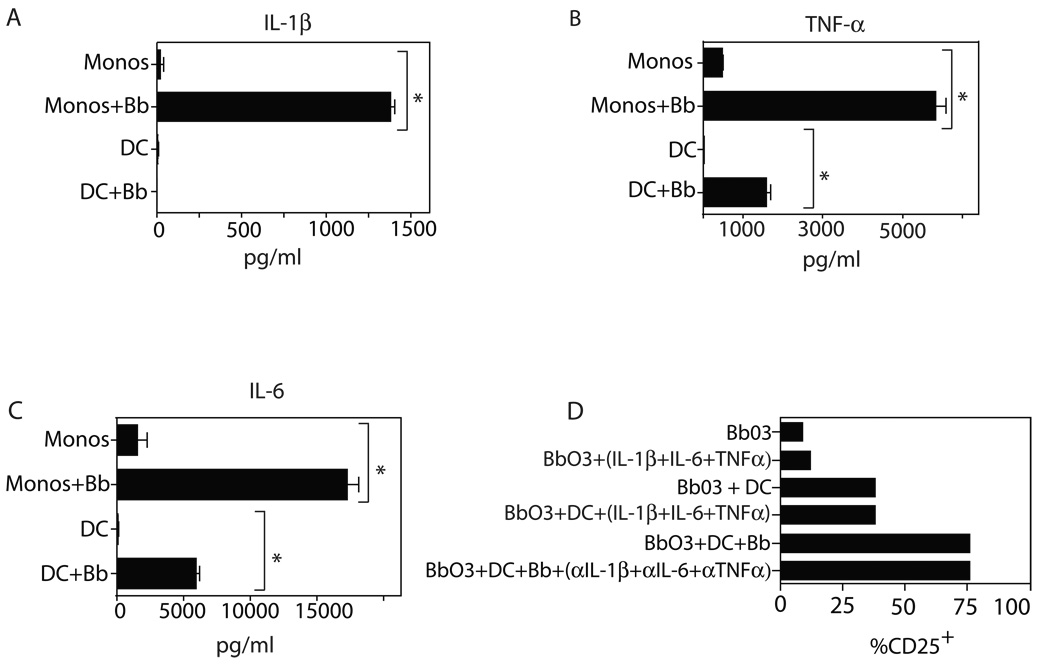
To further examine whether secreted cytokines from APC might contribute to the activation of γδ T cells, we performed bioplex analysis of supernatants of monocytes and DC following stimulation for 24 h with B. burgdorferi. Findings for the most prominently secreted cytokines, IL-1β, IL-6, and TNFα, were confirmed by ELISA assay, and are shown for monocytes in Figure 5A–C. Similar findings were observed for DC, with the exception of IL-1β (Fig. 5A–C). Using this information, we attempted to use this cytokine combination to supplant the effects of DC alone or DC plus Borrelia in activating γδ T cells. However, synovial Vδ1 clones cultured with a cocktail of IL-1β + IL-6 + TNFα, either in the absence or presence of DC, failed to upregulate CD25 expression (Fig. 5D). Conversely, a combination of blocking antibodies to these same cytokines did not diminish the induction of CD25 expression with DC plus Borrelia in conventional cultures (Fig. 5D). These results suggest that human Vδ1 T cells respond to a surface determinant on Borrelia-activated DC and monocytes.
Figure 5. Monocyte/DC-derived cytokines alone are insufficient to activate γδ T cells.
Production of (A) IL-1β, (B)TNFα, and (C) IL-6 by human monocytes (monos) or dendritic cells (DC) stimulated for 24 h with B. burgdorferi sonicate. Supernantants were analyzed by ELISA (* indicates p<0.05 by unpaired t test). (D) Vδ1 clone Bb03 was cultured without or with a combination of IL-1β + IL-6 + TNFα in the absence or presence of DC, and without B. burgdorferi. Alternatively, Bb03 was stimulated by DC plus Borrelia in the absence or presence of blocking antibodies to IL-1β, IL-6, and TNFα. CD25 expression was measured after 24 h. Findings were consistent in two experiments.
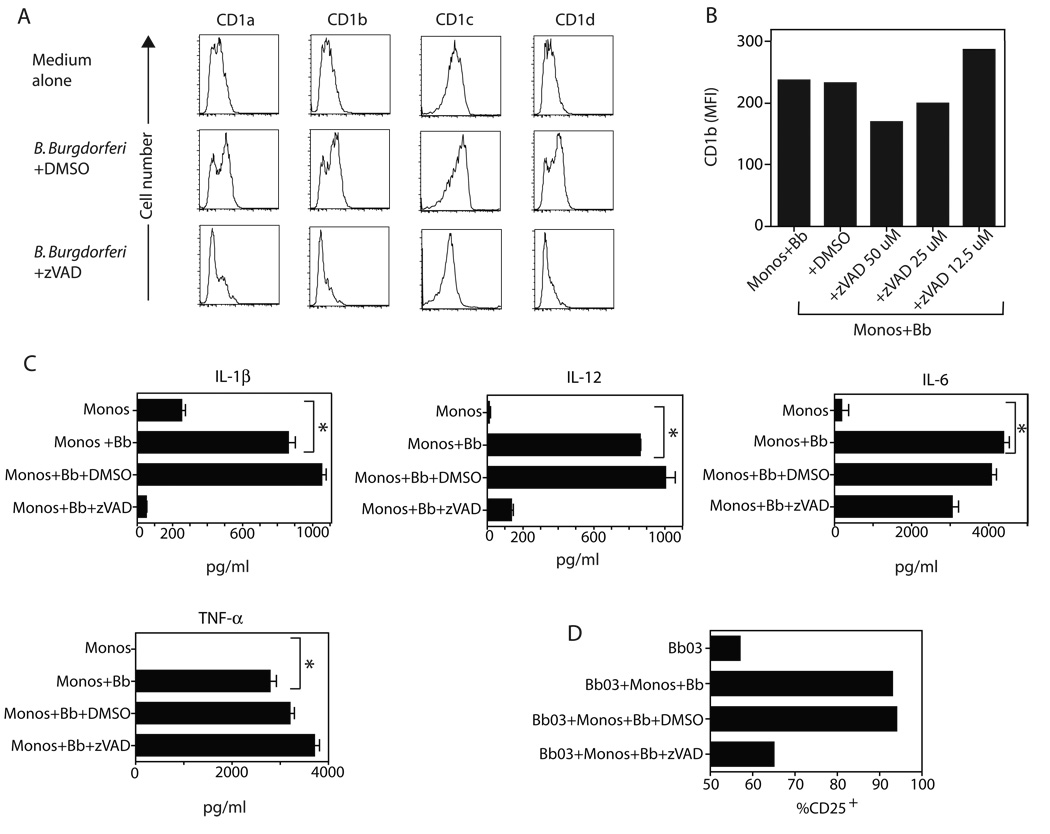
Caspase activity is required for activation of monocytes by B. burgdorferi and stimulation of CD25 by γδ T cells
The ligands for most human and murine γδ T cells are unknown, although suggestions have been made that certain human Vδ1 T cells may react to CD1c (38) and that murine Vγ4 T cells may react to CD1d (39). We thus examined the expression of surface CD1 molecules on monocytes following Borrelia stimulation. As shown in Figure 6A, surface expression by human monocytes of CD1a, CD1b, CD1c, and CD1d was upregulated by 48 h of Borrelia stimulation. It was also recently reported that TLR activation of B cells required caspase activity (40). We therefore examined the effect of caspase blockade by the pan-caspase blocker z-VAD-fmk on CD1 expression and cytokine production by monocytes, as well as its effect on activation of the Vδ1 T cells. Administration of vehicle control DMSO did not disturb the ability of B. burgdorferi to stimulate CD1 expression by monocytes. By contrast, the pan-caspase blocker z-VAD-fmk inhibited Borrelia induction of CD1 in a dose-dependent manner (Fig. 6A,B). A similar induction of CD1d expression by Borrelia was also observed for murine DC that was also blocked by z-VAD (data not shown). Furthermore, z-VAD also blocked monocyte production of IL-1β and IL-12, but not IL-6 or TNFα (Fig. 6C). This supports the view that z-VAD-fmk was not simply non-specifically toxic to monocytes, but did block the upregulation of several molecules, consistent with a role of caspases in TLR signaling by B. burgdorferi. Caspase activity was also critical for the ability of monocytes to activate synovial Vδ1 clones. Figure 6D shows that the presence of caspase blockade during the Borrelia stimulation of monocytes prevented the induction of CD25 by the γδ T cell clones.
Figure 6. Caspase-dependent induction of expression of CD1 and certain cytokines by B. burgdorferi.
(A) Freshly isolated human CD14+ monocytes were cultured for 48 h in medium alone or B. burgdorferi plus vehicle control DMSO or the pan-caspase blocker z-VAD-fmk (100 µM). Monocytes were then stained for expression of CD1a, CD1b, CD1c, and CD1d. Similar results were observed in five experiments. (B) Monocytes were stimulated with B. burgdorferi for 24 h in the absence or presence of DMSO or the indicated concentrations of z-VAD. Cells were then stained for expression of CD1b as well as with live/dead stain. Results are gated on live cells only and displayed as mean fluorescence intensity (MFI) of CD1b. (C) Supernatants from the same cultures were assessed for levels of IL-1β, IL-12, IL-6, and TNFα (* indicates p<0.05 by unpaired t test). (D) CD25 expression by Vδ1 clone Bb03 after 24 h culture with monocytes and Borrelia in the absence or presence of either DMSO or z-VAD-fmk (50 µM).
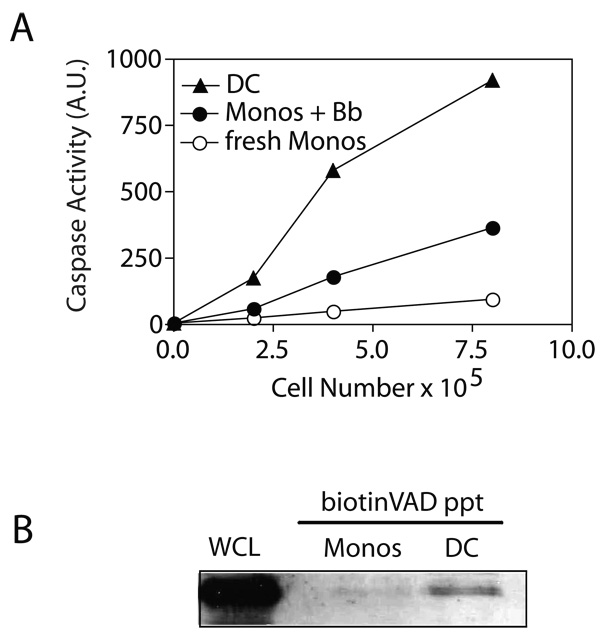
These findings suggested that Borrelia stimulation of monocytes would generate caspase activity. The presence of caspase activity in Borrelia-activated monocytes was therefore more closely defined, initially using the caspase substrate DEVD linked to rhodamine. Cleavage at the C-terminal aspartate residue releases the rhodamine to become fluorescently activated (41). Using this assay it was apparent that freshly isolated monocytes had no detectable caspase activity, whereas monocytes stimulated 48 h earlier with B. burgdorferi manifested readily detectable caspase activity (Fig. 7A). The levels of caspase activity were even more pronounced in myeloid DC that were derived from monocytes following culture in GM-CSF and IL-4. The findings using DEVD-rhodamine were further confirmed through direct labeling of only active caspases using biotin-labeled z-VAD-fmk. This allowed the precipitation with avidin-sepharose of selectively the active caspase fraction, as we have previously shown in activated T cells (41). This assay revealed that freshly isolated monocytes did not have detectable active caspase-8 (Fig. 7B). However, DC manifested easily detectable caspase-8 (Fig. 7B). Thus, the transition from resting monocyte to immature myeloid DC in the presence of GM-CSF and IL-4 results in the induction of caspase activity, which is critical for certain effector functions of these cells.
Figure 7. Caspase activity in Borrelia-activated monocytes and DC.
(A) Caspase activity was measured using the DEVD-rhodamine assay in CD14+ monocytes either freshly isolated or after 48 h stimulation with B. burgdorferi, and compared to myeloid DC derived from monocytes using GM-CSF plus IL-4. (B) Active caspase-8 in DC. Cells were lysed in buffer containing biotin-VAD-fmk and active caspases selectively precipitated using avidin-sepharose and then immunoblotted for caspase-8. Whole cell lysates (WCL) from fresh monocytes was included as a positive control for caspase-8 staining.
Discussion
The current results support a model in which synovial γδ T cells of the Vδ1 subset are activated indirectly by B. burgdorferi via TLR signals, in part by TLR2. The findings are consistent with earlier reports that Borrelia lipopeptides bind TLR2 and this required the tripalmitic acid side chains (37). We also observed that removal of the fatty acid moieties eliminated the ability of OspA to induce CD25 expression on the Vδ1 cells. This may extend to other TLR ligands and demonstrate a novel link between the innate and adaptive immune responses. The fact that the findings also apply to the activation of murine γδ T cells serves to enhance the validity of these results. We found no evidence for surface expression of TLR2 by the Vδ1 cells themselves, nor evidence for direct activation of the γδ T cells by B. burgdorferi, whereas TLR2 is abundantly expressed by monocytes and DC. Thus, the current evidence supports a model in which activation of Vδ1 cells by B. burgdorferi is not antigen-specific, but rather, indirect, in which Borrelia stimulates antigen presenting cells to upregulate a ligand(s) for the Vδ1 TCR. The ligand for the TCR-Vδ1 is currently unknown and is the subject of active investigation.
The predominant human γδ T cell subset in peripheral blood is Vγ2Vδ2, which reacts to nonpeptide antigens from Mycobacterium. These include isoprenyl pyrophosphates (27–30), as well as alkylamine antigens (31). These are products of microbes as well as self-antigens. By contrast, the Vδ1 subset is resident in the intestine (21) and accumulates at sites of inflammation, such as the synovium in rheumatoid arthritis (19) and Lyme arthritis (20). Little is known regarding the specificity of Vδ1 cells. A report has suggested that some Vδ1 cells react to CD1c (38), and that murine Vγ4+ T cells respond to CD1d during Coxsackievirus infection (39). We have not observed any suggestion of CD1c reactivity by our Lyme arthritis synovial Vδ1 cells (C. Collins, unpublished observations). There is, nonetheless, a suggestion that the synovial TCR-Vδ1 determinant may indeed be non-polymorphic, given that the Vδ1 clones can be activated by B. burgdorferi using monocytes or DC from HLA mismatched donors. Most human γδ T cells, including the synovial Vδ1 cells, express the activating NK receptor, NKG2D, which binds MICA, a non-polymorphic MHC class I-related molecule (42, 43). However, the DC used in these studies expressed no detectable surface MICA. Nonetheless, the transwell studies indicate that cell contact is required with DC to activate the Vδ1 cells.
It is also possible that DC- and monocyte-derived cytokines may potentiate activation of γδ T cells, either synergistically with, or independently of TCR signals. Similar indirect cytokine signaling has been observed in other T cell subsets such as NKT cells (44) and during homeostatic proliferation of T cells (45). In addition, the main cytokines induced by Borrelia stimulation of monocytes or DC (IL-1β, IL-6, and TNFα) have been observed to augment proliferation of CD4+ αβ T cells (46). However, we could not detect any significantly augmented CD25 expression by the addition of these cytokines alone or together, nor any reduced CD25 induction by blocking antibodies to these cytokines. We have also previously observed that the activation of the Vδ1 clones by DC and Borrelia is blocked by anti-TCR-γδ but not by blocking antibodies to MHC class I or class II, indicating that the TCR-γδ is involved with this response, but not MHC classical class I or class II molecules (35). The collective findings thus suggest that DC activate γδ T cells primarily via one or more surface determinants.
In addition to its pro-apoptotic function in death receptor pathways, caspase-8 has been noted to be critical for proliferation of T cells and a growing number of other cell types (47–49). Humans bearing a germ line point mutation of caspase-8 manifest a defect in T cells, B cells, and NK cells (50). More recently it was discovered that caspase-8 is also important for certain types of TLR signaling. B cells lacking caspase-8 were observed to have an attenuated proliferation and antibody response following signaling of TLR3 or TLR4 (40). We now observe that Borrelia stimulates caspase activity in fresh human monocytes, and this is required to produce certain cytokines, upregulate surface CD1 family members, as well as being able to activate Vδ1 T cells. The active caspase complex in DC contains caspase-8. These findings thus begin to form a signaling pathway between certain TLR and an active caspase complex that may contain components common to other receptor signaling that leads to NF-κB activation.
We recently reported that synovial γδ T cells were capable of activating DC through a Fas/FasL mechanism (33). In this system Vδ1 cells were observed to express high and sustained levels of FasL, whereas myeloid DC were highly resistant to Fas-induced cell death due to their high expression of the caspase-8 inhibitor, c-FLIPL (33). Furthermore, the augmented expression of c-FLIPL was able to divert Fas signals from caspase-8 activation and toward activation of NF-κB and stimulation by DC of cytokine production and expression of surface costimulatory molecules (33). Combined with the current findings, a model emerges in which synovial Vδ1 T cells and myeloid DC can mutually stimulate each other in the presence of B. burgdorferi through a process initiated by Borrelia engagement of TLR signaling. The products of this interaction are also important for activation of the adaptive immune response, placing certain γδ T cells at a juncture between the innate and adaptive immune responses.
Acknowledgments
The authors wish to thank Ms. Colette Charland for technical assistance with flow cytometry, Dr. Matthew Poynter of the Vermont Lung Center for the kind gift of TLR2−/−, TLR9−/−, and MyD88−/− mice, and Dr. Robert Finberg for the blocking anti-TLR2 antibody.
Abbreviations used in this paper
- DC
dendritic cells
- EDCI
1-ethyl-3(3’-dimethylaminopropyl)-carbodiimide
- TLR
Toll-like Receptor.
Footnotes
This work was supported by grants AR43520 and AI 45666 (to R.C.B.), and P30CA22435 (Vermont Cancer Center) from the National Institutes of Health.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 2.Rahn DW. Lyme disease: clinical manifestations, diagnosis, and treatment. Semin Arthritis Rheum. 1991;20:201–218. doi: 10.1016/0049-0172(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorfer W, Barbour AG, Hayes FF, Benach JL, Grunwaldt BE, David JP. Lyme Disease--a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW, Sigal LH, Spieler PN, Stenn KS, Malawista SE. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 6.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Duray PH, Butcher EC. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31:487–497. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 8.Lim LC, England DM, DuChateau BK, Glowacki NJ, Schell RF. Borrelia burgdorferi-specific T lymphocytes induce severe destructive Lyme arthritis. Infect Immun. 1995;63:1400–1408. doi: 10.1128/iai.63.4.1400-1408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigal LH, Steere AC, Freeman DH, Dwyer JM. Proliferative responses of mononuclear cells in Lyme disease. Reactivity to Borrelia burgdorferi antigens is greater in joint fluid than in blood. Arthritis Rheum. 1986;29:761–769. doi: 10.1002/art.1780290609. [DOI] [PubMed] [Google Scholar]
- 10.Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 11.Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978;298:869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- 12.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 13.Wright A, Lee JE, Link MP, Smith SD, Carroll W, Levy R, Clayberger C, Krensky AM. Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor delta chain. J Exp Med. 1989;169:1557–1564. doi: 10.1084/jem.169.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosat JP, MacDonald HR, Louis JA. A role for gamma delta + T cells during experimental infection of mice with Leishmania major. J Immunol. 1993;150:550–555. [PubMed] [Google Scholar]
- 16.Kaufmann SH, Ladel CH. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig RS, Zavala F, Tonegawa S. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc Natl Acad Sci U S A. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mixter PF, Camerini V, Stone BJ, Miller VL, Kronenberg M. Mouse T lymphocytes that express a gamma delta T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect Immun. 1994;62:4618–4621. doi: 10.1128/iai.62.10.4618-4621.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan FM, Londei M, Jackson AM, Hercend T, Brenner MB, Maini RN, Feldmann M. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J Autoimmun. 1988;1:319–326. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 20.Vincent M, Roessner K, Lynch D, Cooper SM, Sigal LH, Budd RC. Apoptosis of Fas high CD4+ Synovial T cells by Borrelia reactive Fas Ligand high gamma delta T cells in Lyme Arthritis. J. Exp. Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rust C, Kooy Y, Pena S, Mearin ML, Kluin P, Koning F. Phenotypical and functional characterization of small intestinal TcR gamma delta + T cells in coeliac disease. Scand J Immunol. 1992;35:459–468. doi: 10.1111/j.1365-3083.1992.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 22.Balbi B, Moller DR, Kirby M, Holroyd KJ, Crystal RG. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest. 1990;85:1353–1361. doi: 10.1172/JCI114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of gamma delta T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 24.Pelegri C, Kuhnlein P, Buchner E, Schmidt CB, Franch A, Castell M, Hunig T, Emmrich F, Kinne RW. Depletion of gamma/delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 1996;39:204–215. doi: 10.1002/art.1780390206. [DOI] [PubMed] [Google Scholar]
- 25.Peng SL, Madaio MP, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by gamma delta T cells. J Immunol. 1996;157:5689–5698. [PubMed] [Google Scholar]
- 26.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- 27.Schoel B, Sprenger S, Kaufmann SH. Phosphate is essential for stimulation of V gamma 9V delta 2 T lymphocytes by mycobacterial low molecular weight ligand. Eur J Immunol. 1994;24:1886–1892. doi: 10.1002/eji.1830240826. [DOI] [PubMed] [Google Scholar]
- 28.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 31.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs MR, Haynes BF. Increase in γδ T lymphocytes in synovia from rheumatoid arthritis patients with active synovitis. J. Clin. Immunol. 1992;12:130–139. doi: 10.1007/BF00918143. [DOI] [PubMed] [Google Scholar]
- 33.Collins C, Wolfe J, Roessner K, Shi C, Sigal LH, Budd RC. Lyme arthritis synovial gammadelta T cells instruct dendritic cells via fas ligand. J Immunol. 2005;175:5656–5665. doi: 10.4049/jimmunol.175.9.5656. [DOI] [PubMed] [Google Scholar]
- 34.Roessner K, Fikrig E, Russell JQ, Cooper SM, Flavell RA, Budd RC. Prominent T lymphocyte response to Borrelia burgdorferi from peripheral blood of unexposed donors. Eur J Immunol. 1994;24:320–324. doi: 10.1002/eji.1830240207. [DOI] [PubMed] [Google Scholar]
- 35.Vincent MS, Roessner K, Sellati T, Huston CD, Sigal LH, Behar SM, Radolf JD, Budd RC. Lyme arthritis synovial gamma delta T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J Immunol. 1998;161:5762–5771. [PubMed] [Google Scholar]
- 36.Lutz MB, Kukutsch N, Ogilvie ALJ, Robner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 37.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 38.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 40.Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 41.Misra RS, Jelley-Gibbs DM, Russell JQ, Huston G, Swain SL, Budd RC. Effector CD4+ T cells generate intermediate caspase activity and cleavage of caspase-8 substrates. J Immunol. 2005;174:3999–4009. doi: 10.4049/jimmunol.174.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [see comments]. [DOI] [PubMed] [Google Scholar]
- 43.Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AT, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor γδ are phenotypically diverse and evenly distributed throughout the lymphoid system. Journal of Experimental Medicine. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 45.O'Neill RM, Hassan J, Reen DJ. IL-7-Regulated Homeostatic Maintenance of Recent Thymic Emigrants in Association with Caspase-Mediated Cell Proliferation and Apoptotic Cell Death. J Immunol. 2003;170:4524–4531. doi: 10.4049/jimmunol.170.9.4524. [DOI] [PubMed] [Google Scholar]
- 46.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, Wallach D. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 50.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]