Abstract
Escherichia coli chemoreceptors can sense changes in temperature for thermotaxis. Here we found that the aerotaxis transducer Aer, a homolog of chemoreceptors lacking a periplasmic domain, mediates thermoresponses. We propose that thermosensing by the chemoreceptors is a general attribute of their highly conserved cytoplasmic domain (or their less conserved transmembrane domain).
Most organisms have evolved mechanisms to sense and respond to changes in temperature since they can live only within a limited temperature range. Although studies of such thermosensing systems are generally difficult, thermotaxis of Escherichia coli is exceptionally well characterized (5, 6). Wild-type E. coli cells are attracted to warmer and repelled by colder environments. These thermotactic behaviors are mediated by the E. coli chemotaxis signaling system, which regulates the cell's direction of flagellar rotation. Attractant increases, sensed by transmembrane chemoreceptors (Tsr, Tar, Trg, and Tap), also known as methyl-accepting chemotaxis proteins (MCPs), promote counterclockwise (CCW) rotation and forward swimming, whereas repellent increases, sensed by the same receptors, promote clockwise (CW) rotation and random turns or tumbles (4). Early studies revealed that the chemoreceptors of E. coli also mediate thermotactic responses (5, 6). Tsr, Tar, and Trg function as warm sensors, which produce CCW signals upon temperature upshift and CW signals upon temperature downshift. In contrast, Tap functions as a cold sensor that produces signals of the opposite output to temperature changes (5, 7, 9). Tsr and Tar show altered thermosensing properties after adaptation to their attractants, serine and aspartate, respectively. Tsr loses temperature-sensing ability, whereas Tar shifts from a warm sensor to a cold sensor (7, 8, 10-12). To obtain deeper molecular insights into the thermosensing mechanism of receptors, in this study we focused on Aer, a redox sensor of E. coli. Aer has a cytoplasmic kinase control module like that of the other chemoreceptors, but instead of a periplasmic domain, Aer has a large amino-terminal, cytoplasmic PAS domain that binds flavin adenine dinucleotide (FAD) (Fig. 1; for a review, see reference 17).
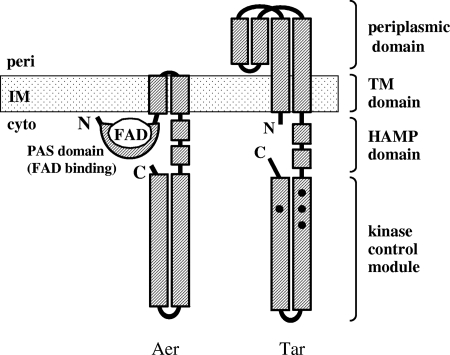
FIG. 1.
Schematic illustration of the domain organizations of the redox sensor Aer and the aspartate chemoreceptor Tar. Tar, like any other MCP, is a homodimeric transmembrane protein, each subunit of which consists of, from the amino terminus to the carboxy terminus, the short cytoplasmic sequence, the first transmembrane helix (TM1), the periplasmic ligand-binding domain, the second transmembrane helix (TM2), the HAMP domain, and the kinase-control module. In contrast, Aer lacks a periplasmic domain but consists of the cytoplasmic PAS domain that binds FAD near the amino terminus, TM1, TM2, and the cytoplasmic domains of high similarity with MCPs. Aer also lacks methylation sites that are conserved in the kinase control modules of MCPs (depicted with closed circles in Tar) and required for chemotactic adaptation. Note that the monomers of Aer and Tar are shown here for simplicity although the minimum functional unit for Aer and Tar is thought to be a homodimer.
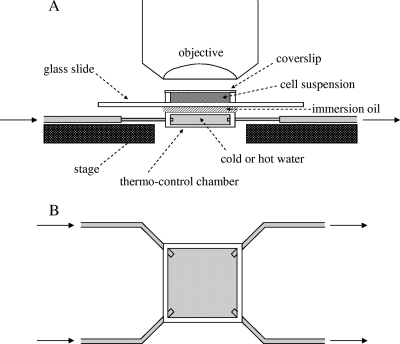
In this study, we developed a new temperature-control device (Fig. 2). It consisted of a chamber formed from two coverslips (18 mm × 18 mm), with short pieces of glass capillaries (1.0 cm in length) fixed with epoxy adhesive serving as spacers between the two slips. Four silicon tubes were placed at the four corners of the chamber and sealed with epoxy adhesive. The two inlet tubes on the left side were connected to one larger tube (2-mm inside diameter) with a Y adapter. The two outlet tubes on the right side were connected together in the same way. Temperature changes inside the chamber were initiated by switching the water flow between two tanks maintained at different temperatures. The water flow was produced by siphon action, and the flow rate was adjusted by shifting the relative height of the tanks. Although the temperature range used in this study varied from 20°C to 30°C, this device could, in principle, be used to vary temperatures over different ranges. With this new device, we observed normal and inverted thermoresponses mediated by Tar (7) and confirmed that the responses were essentially identical to those observed in previous studies (data not shown).
FIG. 2.
Side (A) and top (B) views of the temperature control device used in this study. A glass slide with cell suspension was placed onto the thermo-control chamber mounted on the microscope stage. Immersion oil was applied at the interface between the thermo-control chamber and the glass slide to facilitate effective thermal conduction and to hold the glass slide with surface tension. Arrows indicate the flow of water into and out of the thermo-control chamber. See text for detail.
To test whether Aer can mediate thermotactic responses, we introduced an aer-expressing plasmid, pSB20 (1), into a receptor-less strain, UU2612. (All strains and Aer-carrying plasmids used in this study were kindly provided by J. S. Parkinson.) Expression of Aer was induced with 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG), unless otherwise mentioned, and confirmed by immunoblotting (data not shown). Thermoresponses were measured essentially as described previously (11). Briefly, cells were suspended in motility medium and appropriate concentrations of chemoeffectors were added when necessary. A drop of the cell suspension was placed on a glass slide, sealed with VALAP (equal mixture of petrolatum, lanolin, and paraffin; Wako Chemical, Tokyo, Japan), and mounted on the temperature control device. The aperture between the glass slide and the thermo-control chamber was filled with immersion oil to keep close contact (Fig. 2). Temperature changes were monitored by a thin thermocouple inserted into the cell suspension or immersion oil. Swimming patterns of the cells were measured quantitatively as described earlier (11). The transformants (UU2612/pSB20) swam with extreme smooth bias and did not show any change in swimming behavior upon temperature shifts (Fig. 3A, open circles). It has been known that extreme signaling bias of a receptor sometimes disguises its intrinsic ability to sense a certain stimulus (9, 12). Since glycerol at high concentrations serves as a general repellent for all MCPs (9, 13), we tested whether it also works as a repellent for Aer. When 5% (wt/vol) or higher concentrations of glycerol were added, UU2612/pSB20 cells showed continuous tumbling responses (data not shown). In contrast, cells carrying the parent vector (pCJ30) did not show any response to glycerol (data not shown). In the presence of 5% (wt/vol) glycerol, UU2612/pSB20 cells showed smooth and tumbling responses upon temperature upshift and downshift, respectively (Fig. 3A, closed circles). We also examined another repellent for Aer, 2,3-demethoxy-5-methyl-1,4-benzoquinone (hereinafter referred to as “benzoquinone”) (14). In the presence of 0.7 μM benzoquinone, cells showed similar responses upon temperature shifts (Fig. 3B). In contrast, the same strain carrying the parent vector (pCJ30) did not show any response to the repellents or to subsequent temperature shifts (data not shown). These results suggest that Aer functions as a warm sensor.
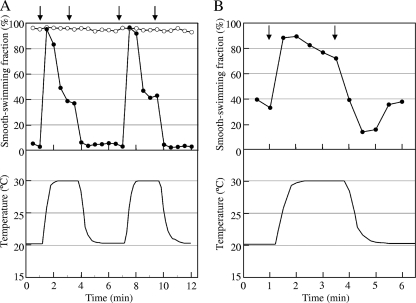
FIG. 3.
Response of otherwise receptorless cells expressing Aer to changes in temperature. Suspensions of UU2612 (ΔMCP Δaer) cells carrying plasmid pSB20 (aer+) in motility medium without (A, open circles) or with 5% glycerol (A, closed circles) or 0.7 μM benzoquinone (B, closed circles) were prepared. The temperature was increased and decreased as shown in lower panels. Arrows indicate the onset of the temperature ramps: A, at 1 and 7 min, 20°C to 30°C, and at 3.5 and 10.5 min, 30°C to 20°C; B, at 1 min, 20°C to 30°C, and at 3.5 min, 30°C to 20°C. Note that temperature changes of the samples were delayed. Swimming behaviors of the cells were recorded, and the time courses of their smooth-swimming fractions were monitored.
Moreover, the cells appeared to adapt to thermal stimulation (Fig. 3A and B): after the early responses, their smooth-swimming fraction tended to resume toward the original values without any further change in temperature. It has been reported that Aer does not have conventional methylation sites and can mediate aerotaxis in a methylation-independent manner (2), whereas methylation and demethylation of sensory receptors, catalyzed by the methyltransferase CheR and the methylesterase CheB, respectively, are required for chemotactic adaptation. To determine whether the adaptation observed in thermoresponses mediated by Aer is methylation dependent, Aer was expressed in a receptorless cheR cheB deletion strain, UU2610. The cells showed clear adaptation of their thermoresponses in the presence of 5% glycerol or 0.7 μM benzoquinone (see Fig. S1 in the supplemental material), suggesting that the adaptation seen in thermoresponses mediated by Aer is methylation independent.
Because Aer is a redox sensor (17), it is important to consider the effect of oxygen depletion on the swimming behavior of the cells under our experimental conditions. When cells were exposed to two sets of identical thermal shifts (from 0 min to 6 min and from 6 min to 12 min), essentially the same response curves were obtained (Fig. 3A). It is therefore unlikely that the responses observed with the device are affected by oxygen depletion, at least under the conditions tested.
To exclude the possibility that repellents are required for thermosensing of Aer, we examined a CW-biased mutant Aer, Aer-S28G. Aer-S28G produces higher CW output than wild-type Aer but can still mediate an aerotactic response (J. S. Parkinson, personal communication). Without glycerol, cells expressing Aer-S28G as a sole receptor showed smooth and tumbling responses upon temperature upshift and downshift, respectively (Fig. 4A). Aer-mediated thermoresponses were also observed without any repellent when wild-type Aer was coexpressed with a CW-biased cytoplasmic Tar fragment that lacks thermosensing ability, though the responses were weak (data not shown). Since changes in temperature can also affect the rate of respiration, it is possible that such upstream elements are involved in Aer thermosensing. To test this possibility, we examined the thermosensing property of an Aer mutant that is defective in FAD binding, Aer-R57H (19). UU2610 cells were transformed with plasmid pSB20 carrying the mutant aer gene. UU2610/pSB20 Aer-R57H cells in which the mutant gene induced with 12 μM IPTG was used for the induction still showed a repellent (tumbling) response to 10% glycerol but did not respond to 100 μM benzoquinone, as expected (data not shown). The cells showed inverted responses upon temperature changes (Fig. 4B), suggesting that Aer-R57H functions as an intrinsic cold sensor and FAD binding is not absolutely required for the thermosensing function of Aer, although the cause of the inversion remains to be elucidated.
FIG. 4.
Thermoresponses of cells expressing the CW-biased Aer mutant (A, S28G) or the FAD-binding Aer mutant (B, R57H) as a sole thermosensor. Suspensions of UU2612 (ΔMCP Δaer) cells carrying plasmid pSB20-Aer-S28G or UU2610 (ΔMCP Δaer ΔcheRB) cells carrying plasmid pSB20-Aer-R57H without any repellent were prepared. The temperature was changed as shown in the lower panel of Fig. 3B; arrows indicate the onsets of the temperature ramps.
It is likely that temperature sensing involves reversible conformational changes of the receptor induced by changes in temperature, a mechanism similar to sensing of chemoeffectors. Although temperature can affect any part of the receptor, there could be a critical domain whose structure is particularly sensitive to temperature. A temperature-induced structural change in such a domain would propagate to the whole protein to switch signal output. Now that the periplasmic domain can be excluded, the most likely candidates for a temperature-sensing element are the transmembrane helices, the HAMP domain, and the kinase control module (Fig. 1). In the previous study, we found that mutations in the second transmembrane domain (TM2) of Tar cause inversion of its thermosensing profile (10), suggesting the importance of the TM segments for thermosensing. Changes in temperature might cause reorientation and/or alteration of packing interactions of the transmembrane helices (TM1 and TM2) that are similar to those caused by aspartate binding to Tar (3). The HAMP domain (named after histidine kinases, adenylyl cyclases, MCPs, and phosphatases), which connects TM2 to the cytoplasmic kinase control module, is commonly used in transmembrane sensors in various species of prokaryotes and thought to function as a flexible sensory transduction module (18). The HAMP domains of chemoreceptors might modulate signal output by undergoing stimulus-induced changes in dynamic behavior (20), a conformational property that should also respond to temperature changes. The conserved kinase control module also seems a likely target for temperature control, because modification of this module by methylation of Tar causes conversion of its thermosensing ability (9, 11, 12). After analyses using Tar mutants with altered methylation sites, we proposed a model that methylation may shift the equilibrium of the signaling states by altering the interaction between α-helices of the kinase control module within a Tar homodimer or between Tar homodimers (11, 12). Similarly, it has been proposed that signaling states of chemoreceptors include modulation of dynamics in adaptation/protein-interaction regions, which constitute the kinase control module (15, 16). Such dynamics can be readily affected by temperature. It should be intriguing to examine Aer/Tar fragments carrying or lacking the TM2 region, the HAMP domain, and/or the kinase control module in various combinations. Finally, it is unlikely that the FAD binding of Aer plays a key role in thermosensing because the FAD-binding mutant Aer-R57H retained the thermosensing function, although it mediated inverted thermoresponses (Fig. 4B).
In conclusion, we found that Aer has an intrinsic thermosensing ability and therefore the periplasmic domain is not essential for the thermosensing function of a receptor. Since changes in temperature are supposed to induce conformational changes of the receptors equivalent to those induced by binding of chemical attractants and repellents, further characterization of temperature sensing of MCPs and Aer should shed new light on molecular mechanisms underlying receptor signaling.
Supplementary Material
Acknowledgments
We thank John S. Parkinson of the University of Utah for kindly providing us with strains and plasmids and Yoshiyuki Sowa and Takehiko Inaba of Hosei University for discussion and encouragement.
This work was supported in part by grants-in-aid for scientific research from the Japan Society for the Promotion of Science to I.K.
Footnotes
Published ahead of print on 22 January 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bibikov, S. I., R. Biran, K. E. Rudd, and J. S. Parkinson. 1997. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 179:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibikov, S. I., A. C. Miller, K. K. Gosink, and J. S. Parkinson. 2004. Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein. J. Bacteriol. 186:3730-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazelbauer, G. L., J. J. Falke, and J. S. Parkinson. 2008. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 33:9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda, K., and Y. Imae. 1979. Thermosensory transduction in Escherichia coli: inhibition of the thermoresponse by L-serine. Proc. Natl. Acad. Sci. U. S. A. 76:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda, K., Y. Imae, J. I. Shioi, and F. Oosawa. 1976. Effect of temperature on motility and chemotaxis of Escherichia coli. J. Bacteriol. 127:1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno, T., and Y. Imae. 1984. Conditional inversion of the thermoresponse in Escherichia coli. J. Bacteriol. 159:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nara, T., I. Kawagishi, S. Nishiyama, M. Homma, and Y. Imae. 1996. Modulation of the thermosensing profile of the Escherichia coli aspartate receptor Tar by covalent modification of its methyl-accepting sites. J. Biol. Chem. 271:17932-17936. [DOI] [PubMed] [Google Scholar]
- 9.Nara, T., L. Lee, and Y. Imae. 1991. Thermosensing ability of Trg and Tap chemoreceptors in Escherichia coli. J. Bacteriol. 173:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiyama, S., I. N. Maruyama, M. Homma, and I. Kawagishi. 1999. Inversion of thermosensing property of the bacterial receptor Tar by mutations in the second transmembrane region. J. Mol. Biol. 286:1275-1284. [DOI] [PubMed] [Google Scholar]
- 11.Nishiyama, S., T. Nara, M. Homma, Y. Imae, and I. Kawagishi. 1997. Thermosensing properties of mutant aspartate chemoreceptors with methyl-accepting sites replaced singly or multiply by alanine. J. Bacteriol. 179:6573-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama, S. I., T. Umemura, T. Nara, M. Homma, and I. Kawagishi. 1999. Conversion of a bacterial warm sensor to a cold sensor by methylation of a single residue in the presence of an attractant. Mol. Microbiol. 32:357-365. [DOI] [PubMed] [Google Scholar]
- 13.Oosawa, K., and Y. Imae. 1983. Glycerol and ethylene glycol: members of a new class of repellents of Escherichia coli chemotaxis. J. Bacteriol. 154:104-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl. Acad. Sci. U. S. A. 94:10541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starrett, D. J., and J. J. Falke. 2005. Adaptation mechanism of the aspartate receptor: electrostatics of the adaptation subdomain play a key role in modulating kinase activity. Biochemistry 44:1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swain, K. E., M. A. Gonzalez, and J. J. Falke. 2009. Engineered socket study of signaling through a four-helix bundle: evidence for a yin-yang mechanism in the kinase control module of the aspartate receptor. Biochemistry 48:9266-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor, B. L. 2007. Aer on the inside looking out: paradigm for a PAS-HAMP role in sensing oxygen, redox and energy. Mol. Microbiol. 65:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams, S. B., and V. Stewart. 1999. Functional similarities among two-component sensors and methyl-accepting chemotaxis proteins suggest a role for linker region amphipathic helices in transmembrane signal transduction. Mol. Microbiol. 33:1093-1102. [DOI] [PubMed] [Google Scholar]
- 19.Wright, S., B. Walia, J. S. Parkinson, and S. Khan. 2006. Differential activation of Escherichia coli chemoreceptors by blue-light stimuli. J. Bacteriol. 188:3962-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou, Q., P. Ames, and J. S. Parkinson. 2009. Mutational analyses of HAMP helices suggest a dynamic bundle model of input-output signalling in chemoreceptors. Mol. Microbiol. 73:801-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.