Abstract
MreB, the bacterial actin-like cytoskeleton, is required for the rod morphology of many bacterial species. Disruption of MreB function results in loss of rod morphology and cell rounding. Here, we show that the widely used MreB inhibitor A22 causes MreB-independent growth inhibition that varies with the drug concentration, culture medium conditions, and bacterial species tested. MP265, an A22 structural analog, is less toxic than A22 for growth yet equally efficient for disrupting the MreB cytoskeleton. The action of A22 and MP265 is enhanced by basic pH of the culture medium. Using this knowledge and the rapid reversibility of drug action, we examined the restoration of rod shape in lemon-shaped Caulobacter crescentus cells pretreated with MP265 or A22 under nontoxic conditions. We found that reversible restoration of MreB function after drug removal causes extensive morphological changes including a remarkable cell thinning accompanied with elongation, cell branching, and shedding of outer membrane vesicles. We also thoroughly characterized the composition of C. crescentus peptidoglycan by high-performance liquid chromatography and mass spectrometry and showed that MreB disruption and recovery of rod shape following restoration of MreB function are accompanied by considerable changes in composition. Our results provide insight into MreB function in peptidoglycan remodeling and rod shape morphogenesis and suggest that MreB promotes the transglycosylase activity of penicillin-binding proteins.
Most bacteria have characteristic cell morphologies maintained during growth (67). The peptidoglycan (PG) component of the cell wall represents in most cases the physical support of various bacterial shapes. PG is a mesh-like polymeric macromolecule which opposes the osmotic pressure of the bacterial cytoplasm and prevents lysis in hypotonic growth environments (29). Isolated PG cell walls (sacculi) retain the shapes of the cells from which they originate while PG disruption causes the formation of osmotically labile spheroplasts, underscoring PG's essential role in cell shape determination and cellular integrity maintenance. PG is composed of long glycan chains that are oriented roughly along the short axis of rod-shaped Gram-negative bacteria and that are connected by short peptide cross-links (21, 60). Bacterial growth and division necessitate the expansion and division of the PG cell wall, which requires the insertion of new PG material in the preexisting, covalently linked mesh (29). New PG synthesis requires two enzymatic reactions performed by penicillin-binding proteins (PBPs). Glycan chain synthesis is achieved by transglycosylation activity while cross-linkage of glycan chains to the existing mesh is achieved by transpeptidation activity (47). Class A PBPs, called bifunctional or bimodular PBPs (e.g., PBP1a and 1b of Escherichia coli), possess both transpeptidase and transglycosylase domains while class B PBPs, such as PBP2 and PBP3 of E. coli, can perform only transpeptidase reactions (47). Controlled degradation of the PG by cell wall hydrolases is necessary for incorporation of new PG material during growth. Tight coordination between PG synthesis and degradation is required to maintain the integrity of the mesh at all times (29).
The bacterial cytoskeleton also plays a central role in cell shape determination and maintenance (7). MreB is a bacterial actin homolog that forms dynamic helical structures underneath the cytoplasmic membrane in most rod-shaped bacteria (8, 34, 37, 56). In some species, the spatial distribution of MreB varies during the cell cycle, changing from a helical/patchy localization pattern throughout the cell to a ring-like distribution near midcell (20, 22, 50, 58). MreB is required for rod shape maintenance as deletion of the MreB-encoding gene or depletion of MreB causes loss of rod shape and cell rounding (20, 22, 34, 63). Other proteins, including MreC, MreD, RodA, PBP2, and RodZ, function along with MreB to maintain rod shape as loss of their function also results in cell rounding (2, 5, 33, 48, 62). Among these rod-morphogenic proteins, only PBP2 has a known enzymatic function, being involved in PG synthesis as an elongation-specific transpeptidase; the others are membrane-spanning or integral membrane proteins (2, 5, 15, 48). The overall involvement of these morphogenetic proteins in rod shape maintenance has led to a model in which they are part of the elongase complex, a PG synthesizing machine that elongates the PG side wall (2, 5, 15, 48, 59). The elongase complex would include PG lytic enzymes and at least one bifunctional PBP required for glycan strand synthesis (15, 59). In Bacillus subtilis, MreB homologs were found to associate with the bifunctional PBP1 (36) and to regulate the localization of the PG hydrolase LytE (9). However, it is still unclear how MreB functions in the context of the proposed elongase complex to determine and maintain rod shape.
It has been previously shown that repletion of MreB in lemon-shaped, MreB-depleted Caulobacter crescentus cells leads to the formation of cell filaments that present branches and ectopic stalks (64). To examine how MreB can drive de novo rod shape morphogenesis, we followed a similar strategy except that we used drug treatment to interfere with MreB function. The small molecule 3,4-dichlorobenzyl carbamimidothioate, also known as A22, has been shown to rapidly disrupt MreB localization in vivo and to induce growth-dependent rounding in several Gram-negative bacteria (23, 32, 41, 45, 52). Furthermore, genetic and biochemical experiments have shown that MreB is the direct molecular target of A22 and that A22 binds to MreB's ATP-binding pocket, inducing a state with low affinity for polymerization (3, 23). As removal of A22 is followed within minutes by recovery of the normal MreB localization pattern (23), this drug represents a convenient tool for rapid and reversible inhibition of MreB function. However, A22 was found to inhibit the growth of an mreB deletion mutant of E. coli, suggesting that it can have MreB-independent toxic effects (35). In this study, we show that the toxicity of A22 varies with the drug concentration, culture medium conditions, and Gram-negative species tested. We identify a similarly potent but less toxic structural analog, MP265 (4-chlorobenzyl carbamimidothioate), as well as nontoxic concentrations and conditions for both A22 and MP265 that induce loss of rod cell morphology in C. crescentus. We also show that recovery of rod shape after drug removal is accompanied by intensive remodeling of PG morphology and composition.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. To construct pVsigpepCHYN-4, the gene region coding the signal peptide of CC1996 was PCR amplified from C. crescentus genomic DNA using primers 5′-CACATATGATGAGGCAGTTGTGGACGCAAG-3′ and 5′-CACATATGCTGGGCCTGGATCGTCTCACC-3′, digested with NdeI, and inserted into the NdeI site of pVCHYN-4 (53). To construct CJW2959, pVsigpepCHYN-4 was delivered into CB15N by electroporation, and integrants by single crossover into Pvan were selected. To construct CJW3049, pXGFPN-5 (53) was delivered into CJW2959 by conjugation, and integrants by single crossover into Pxyl were selected.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype and/or description | Source or reference |
|---|---|---|
| Strains | ||
| C. crescentus | ||
| CB15N | Also known as NA1000; synchronizable variant of the wild-type strain CB15 | 19 |
| CJW2959 | CB15N Pvan::pVsigpepCHYN-4 | This study |
| CJW3049 | CB15N Pvan::pVsigpepCHYN-4 Pxyl::pXGFPN-5 | This study |
| JG5008 | CB15N recA::Tn5 ΔmreB/pRK290/20R Pxyl-mreB; MreB depletion strain | 20 |
| JS1003 | CB15N rsaA::Kmr; does not produce an S-layer | 65 |
| LS3814 | CB15N Pxyl::pXGFP4-C1mreB; gfp-mreB under the control of Pxyl | 22 |
| MT196 | CB15N Pvan::pMT383; ftsZ-yfp under the control of Pvan | 54 |
| YB1585 | CB15N ftsZ::pBJM1; FtsZ depletion strain | 66 |
| E. coli | ||
| DH5α | Cloning strain | Promega |
| FB9 | PB103 ΔmreB/pFB112 (tet sdiA); stable mreB deletion strain | 4 |
| MC1000 | Wild-type E. coli strain | 10 |
| PB103 | Wild-type E. coli strain | 14 |
| S17-1 | RP4-2, Tc::Mu Km::Tn7; for plasmid mobilization | 49 |
| A. tumefaciens | ||
| 3101 | Wild-type A. tumefaciens strain | S. Dinesh-Kumar |
| S. meliloti | ||
| 1021 | MB501; wild-type S. meliloti strain | Bill Margolin |
| Plasmids | ||
| pVCHYN-4 | Integrating plasmid; contains Pvan-mCherry sequence; for vanillate-inducible expression of mCherry | 53 |
| pVsigpepCHYN-4 | Integrating plasmid; contains Pvan-CC1996sigpep-mCherry; for vanillate-inducible expression of periplasm-exported mCherry | This study |
| pXGFPN-5 | Integrating plasmid; contains Pxyl-gfp sequence; for xylose-inducible expression of GFP | 53 |
C. crescentus strains were grown in peptone-yeast extract (PYE) medium or in defined minimal medium (M2G) with shaking at 30°C (18), with appropriate antibiotics for selection when required. Agrobacterium tumefaciens and Sinorhizobium meliloti were grown in Luria Broth (LB) medium at 30°C. E. coli was grown in LB medium at 37°C. Adjustments of the pH of growth media were done by adding Tris-HCl buffer (pH 8.0) or sodium phosphate buffer (pH 6.1) to a final concentration of 20 mM (to create PYE-TRIS and LB-TRIS or PYE-P and LB-P, respectively). Xylose (0.3%) or vanillic acid (0.5 mM) was added to induce gene expression from the respective inducible promoters while glucose (0.2%) was used to repress the xylose-inducible promoter. Protein synthesis in strain CJW3049 was induced by addition of vanillic acid and xylose for 6 h and 4 h, respectively, prior to imaging. MreB inhibitors were added to the desired concentrations from 1,000× stock solutions in dimethyl sulfoxide (DMSO).
Growth curve experiments were performed using a Bio-Tek Synergy 2 plate reader. In a 96-well plate, 5 μl of three or four independent overnight cultures was inoculated into 195 μl of premade medium containing the desired amount of drugs or their solvent (DMSO). The plates were covered with a lid and incubated at the appropriate temperature with shaking. Absorbance was read at 660 nm (C. crescentus) or 600 nm (A. tumefaciens, S. meliloti, and E. coli) every 2 min (C. crescentus, A. tumefaciens, and E. coli) or every 5 min (S. meliloti). For doubling time and growth rate calculations, the reads from each well were individually plotted in a semilogarithmic scale, and the linear part of the graph was fitted to an exponential curve: y = aebt, where t is time. The doubling time (DT) was calculated as DT = ln2/b and the growth rate r was calculated as r = 1/DT.
Drug synthesis.
A22 and each analog were synthesized according to the following general procedure. Substituted benzyl halide (1.5 Eq) was added to a 0.2 M solution of thiourea in absolute ethanol. The reaction mixture was kept under refluxing conditions for 16 h and then cooled down slowly to room temperature (RT). White crystals were isolated by filtration and washed twice with a cold 1:1 diethyl ether-ethanol mixture. After extensive drying under reduced pressure, the carbamimidothioates were obtained in good to excellent yields (80 to 95%). The compound structures were verified by 1H and 13C nuclear magnetic resonance (NMR) (data not shown). For the synthesis of MP265, a solution of 4-chlorobenzyl chloride (12.92 mmol; 2.08 g) and thiourea (13 mmol; 988 mg) was heated for 12 h in refluxing absolute ethanol (25 ml). After the solution was cooled down slowly to RT, 150 ml of diethyl ether was added slowly, and the mixture was further cooled down to 0°C to induce crystallization of the product. After filtration, two washes with 40 ml of ice-cold diethyl ether, and extensive drying under reduced pressure, 2-(4-chlorobenzyl)isothiouronium chloride was isolated as white crystals (2.81 g; 94% yield). The structure of the product was confirmed using 1H and 13C NMR, and mass spectrometry (MS): 1H NMR (300 MHz, D2O+ ɛ d4-MeOD): δ = 7.34 (s, 4H), 4.31(s, 2H); 13C NMR (75 MHz, D2O + ɛ d4-MeOD): δ = 171.4, 134.8, 134.0, 131.5, 130.1, 35.5; MS (gas chromatography [GC]/MS, electron impact [EI]): 158 (4-ClC6H4CHS+, 40%), 125 (4-ClC6H4CH2+, 100%). MP265 is also available commercially from various vendors (Chemical Abstract Services [CAS] 544-47-8).
Light microscopy.
Light microscopy imaging was performed at RT using 100× differential-interference contrast (DIC) or phase-contrast objectives on an Nikon E1000 microscope equipped with a Hamamatsu Orca-ER LCD camera or a Nikon Eclipse 80i microscope equipped with an Andor iXonEM+ (DU-897E) electron-multiplying charge-coupled-device (EMCCD) camera and Hamamatsu OrcaIIER camera. Cells were placed on 1 to 1.5% agarose pads made with M2G (for single-image acquisitions), with M2G containing 1% PYE (for fluorescence time-lapse microscopy experiments), or with PYE (for DIC time-lapse experiments). For cell width measurements, the cells were fixed in Karnovski's fixative (5% glutaraldehyde, 4% formaldehyde in 0.08 M sodium phosphate buffer, pH 7.2) at RT for 1 h, stored at 4°C, and washed in phosphate-buffered saline (PBS) before imaging. This fixation procedure did not alter the shape or width of the cells (data not shown). Images were acquired and processed using Metamorph software (Molecular Devices). Cellular widths were measured from phase-contrast micrographs using a Matlab-based algorithm.
EM.
For scanning electron microscopy (EM), 1 ml of culture was harvested and centrifuged. Cells were resuspended in 500 μl of Karnovski's fixative and stored at 4°C. Once all samples were collected, the fixed cells were washed twice in PBS and mounted onto poly-l-lysine-coated coverslips. The cells were dehydrated by passage through increasing concentrations of ethanol, ending in 100% ethanol. Finally, the samples were critical point dried and gold coated before being examined using a FEI XL-30 ESEM FEG microscope with the following settings: acceleration voltage, 10.0 kV; spot size, 3; working distance, 7.5 mm (17).
For transmission EM, the cells were pelleted, washed in PBS, and fixed for 1 to 2 h in Karnovski's fixative at RT. The cells were washed twice in PBS and fixed in aqueous 2% osmium tetroxide for 1 to 2 h at RT. Next, the cells were washed three times in PBS, mixed with 2% agarose solution, and allowed to solidify at 4°C. The agarose-trapped cells were dehydrated through an ascending series of ethanol solutions and embedded in EMbed812 (Electron Microscopy Sciences) according to the manufacturer's instructions. In a variation of the procedure, the agarose-trapped cells were stained en bloc prior to the ethanol dehydration steps by treating them for 2 h with a 1% uranyl acetate solution in 50% aqueous ethanol (26). Ultrathin sections (40 to 100 nm) were cut with a diamond knife on an MT-1 Sorvall ultramicrotome, mounted onto copper grids, stained for 1 min each with aqueous uranyl acetate and lead citrate, and visualized with a JEOL JEM-1230 transmission electron microscope operating at 80 kV. Digital images were acquired with a Hamamatsu ORCA-HR camera.
Isolation and analysis of peptidoglycan sacculi.
PG sacculi were isolated as previously described (24, 39). Five hundred milliliters of cell culture in exponential growth phase (optical density at 600 nm [OD660] of 0.2 to 0.5) was cooled for 10 min on ice and centrifuged at 10,400 × g for 10 min at 4°C. The pellet was resuspended in 8 ml of ice-cold PBS and added dropwise under vigorous stirring to 8 ml of boiling 8% SDS. The mixture was boiled for 30 min with stirring while water was added throughout to maintain a constant volume. Sacculi were pelleted at 440,000 × g in a Beckman Coulter Optima TLX Ultracentrifuge for 15 min. The pellet was washed by sequential resuspension in water, followed by ultracentrifugation until the supernatant was free of SDS as evaluated by the Hayashi test (27). The sacculi were resuspended in 10 mM Tris-HCl buffer (pH 7.0), incubated with 100 μg/ml amylase for 2 h at 37°C, followed by incubation for 1 h with 200 μg/ml pronase at 60°C (pronase was preincubated for 2 h at 60°C), and boiled for 30 min in 4% SDS. The sacculi were washed free of SDS and stored at 4°C with 0.02% sodium azide in water. For transmission EM, the sacculi suspensions were diluted 1:5 in water, mounted onto glow-discharged Formvar carbon-coated copper grids, stained with 1% uranyl acetate for 1 min, and visualized similarly to the thin sections.
Muropeptide preparation and analysis.
Muropeptide preparation was achieved as described previously (24), with some modifications. Briefly, PG sacculi were isolated as described above and digested at 37°C overnight with 20 μg/ml cellosyl in 20 mM sodium phosphate, pH 4.8. Cellosyl was removed by boiling the mixture at 100°C for 10 min and centrifugation at 15,000 × g for 30 min. The muropeptide mixture was reduced with sodium borohydride in 0.25 M (final concentration) sodium borate, pH 9.0, for 30 min; then the pH was adjusted to 3 to 4.5 using 20% phosphoric acid, and the samples were concentrated under vacuum at RT in a Rotovap. The muropeptides were separated with an Agilent 1200 series HPLC system on a 250-mm by 4.6-mm, 3-μm C18 Hypersyl ODS column. The solvent gradient was linear from 50 mM sodium phosphate, pH 4.31, to 70% (75 mM sodium phosphate, pH 4.95)-30% methanol in 135 min. The column was maintained at 55°C, and UV detection was performed at 205 nm. Fractions corresponding to peaks of interest were collected manually and analyzed by mass spectrometry as previously described (6). All muropeptide-related calculations were performed as previously described (24).
RESULTS
A22 can have an MreB-independent toxic effect on bacterial growth.
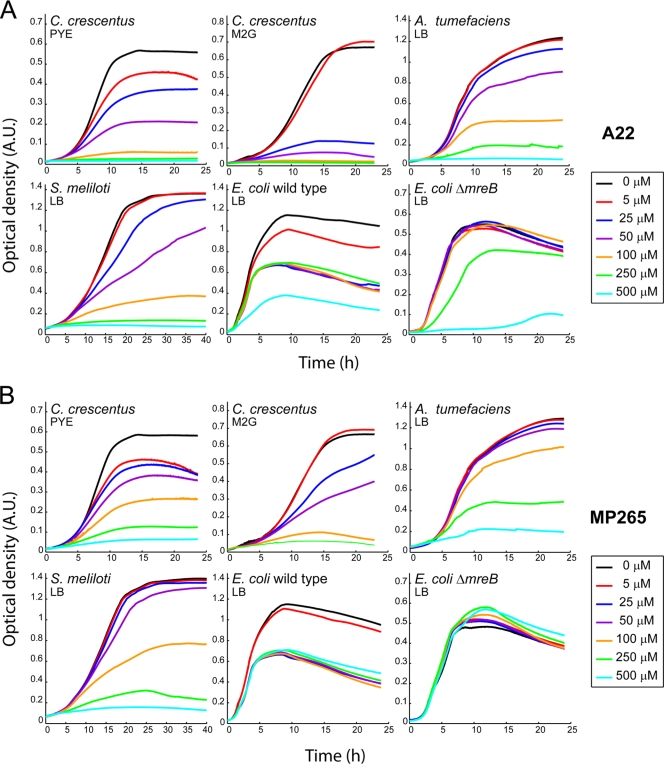
A22 has been used to reversibly disrupt the localization and function of MreB in C. crescentus and other Gram-negative bacteria (23, 32, 41, 45, 52). However, A22 is known to reduce the growth rate of an E. coli mutant that lacks MreB at concentrations of 50 μg/ml (212 μM) and higher (35), suggesting that A22 inhibits cellular function(s) other than that of MreB. Given the rapidly increasing use of A22 in bacterial studies, we tested whether this potentially toxic effect of A22 varied with the bacterial species and/or growth conditions used. We first examined the effects of different concentrations of A22 on the growth of three rod-shaped alphaproteobacteria: C. crescentus, S. meliloti, and A. tumefaciens. S. meliloti and A. tumefaciens do not have an MreB homolog (11) while MreB is present and essential for viability in the closely related C. crescentus (20, 22). We found that A22 affected the rate and extent of growth of all three species in a concentration-dependent manner (Fig. 1A and 2; see also Table S1 in the supplemental material). A culture medium effect was also observed as the growth-inhibitory effect was significantly more pronounced when C. crescentus was grown in minimal M2G medium than when it was grown in rich PYE medium (Fig. 1A and 2). Treatment with the commonly used concentration of 10 μg/ml (∼50 μM) of A22 reduced the growth rates not only of C. crescentus but also of A. tumefaciens and S. meliloti (Fig. 1A and 2). Consistent with the absence of mreB from the genomes of these last two species, their rod morphologies were not affected by A22 treatment (data not shown). A ΔmreB E. coli strain (FB9) and its wild-type parent strain (PB103) also exhibited slower growth rates in an A22 concentration-dependent fashion (Fig. 1A and 2 and Table S1), confirming the previous study (35). E. coli was, however, considerably more resistant than C. crescentus, A. tumefaciens, or S. meliloti. These results argue that A22 has an MreB-independent toxic effect on bacterial growth that varies among species. This toxicity is consistent with the finding that A22 binds to MreB as a low-affinity ADP mimic (dissociation constant [Kd] of ∼1.32 μM) (3) and may therefore affect other nucleotide-binding processes.
FIG. 1.
Inhibitory effects of A22 and MP265 on bacterial growth. C. crescentus CB15N, A. tumefaciens 3101, S. meliloti 1021, and E. coli PB103 and FB9 (PB103 ΔmreB) were grown in the presence of the noted concentrations of A22 or MP265, and the optical densities of the cultures were recorded. Cells were grown in the media indicated on the figure. Each curve represents the average optical density of at least three independent cultures.
FIG. 2.
Effects of A22 and MP265 on bacterial growth rates. The growth rate of each bacterial culture grown in the presence of a 0, 5, 25, 50, 100, 250, or 500 μM concentration of either A22 or MP265 was calculated from the exponential part of each individual growth curve (shown in Fig. 1), and divided by the average growth rate of the respective culture in the absence of any drug. The obtained relative growth rates were plotted as a function of the drug concentration added to the culture (average ± standard deviation of at least three independent values).
MP265 (4-chlorobenzyl carbamimidothioate) is an A22 analog with reduced toxicity on cell growth.
As A22 affected MreB-independent processes, we searched for other drugs that were more specific in disrupting MreB function. Sceptrin, a naturally occurring compound that was shown to pull down MreB in vitro (46), did not disrupt the rod shape of C. crescentus when used at 1 or 5 μg/ml (1.6 or 8.1 μM, respectively) and completely blocked cell growth at 5 μg/ml (data not shown).
Structural analogs of A22 have been shown to induce cell rounding in E. coli by presumably acting on MreB (30, 31). We therefore synthesized and tested a small library of A22 analogs in assays designed to evaluate the anti-MreB effects of these compounds (see Table S2 in the supplemental material). We scored their ability to disrupt green fluorescent protein (GFP)-MreB localization and to induce cell rounding when the drugs were added at a concentration of 50 μM to a growing culture of wild-type C. crescentus cells (Table S2). Under these experimental conditions, A22-treated cells displayed a largely diffuse GFP-MreB localization and adopted a lemon-shaped morphology (23). Of the A22 analogs tested, MP265 and MP253 exhibited anti-MreB activities comparable with those of A22 (Table S2). As MP265 appeared to be less toxic than either A22 or MP253 in preliminary growth curve experiments (data not shown), we decided to further characterize its effects on MreB and bacterial growth.
In C. crescentus, MreB localization alternates between patchy/helical and midcell ring patterns during the cell cycle (20, 22). Asynchronous cell populations treated with only the drug solvent (DMSO) for 1 h displayed the expected patchy/banded localization of GFP-MreB (Fig. 3A, Untreated), as previously reported (22). Under similar conditions, 5 μM A22 or MP265 caused partial delocalization of the GFP-MreB signal (Fig. 3A), and at 50 μM (Fig. 3A), both drugs delocalized most of the GFP-MreB signal although some weak GFP-MreB bands could still be observed in some cells, as previously noted for A22 (1). We concluded that MP265 is equivalent to A22 in its ability to disrupt MreB filaments in live C. crescentus cells. However, MP265 has the advantage of being less toxic than A22. Invariably, higher concentrations of MP265 than of A22 were needed to reduce growth rates and to increase the generation times of the tested bacterial cultures (Fig. 1B and 2; see also Table S1 in the supplemental material). When used at 50 μM, a commonly employed concentration for A22 treatment, MP265 barely decreased the growth rates of the MreB-lacking bacteria A. tumefaciens and S. meliloti, and even at 500 μM, MP265 had no detectable inhibitory effect on the growth of the tested ΔmreB E. coli strain under our experimental conditions (Fig. 2 and Table S1).
FIG. 3.
Disruptive effects of A22 and MP265 treatments on GFP-MreB localization and rod morphology of C. crescentus. (A) Synthesis of GFP-MreB was induced for 2 h in cultures of strain LS3814; then the cells were treated for 1 h with the indicated drug concentrations while induction was maintained, placed on agarose pads containing the same drug concentrations used for treatment, and imaged by light microscopy. Treatment with the 5 μM drug concentrations was performed in PYE-TRIS medium while treatment with the 50 μM drug concentrations was performed in PYE medium. (B) Wild-type CB15N cultures growing in PYE or PYE-TRIS were treated with the indicated drug concentrations. Cells harvested before the addition of the drugs and after 3 and 6 h of growth in the presence of the indicated amounts of drugs were fixed and imaged by phase-contrast microscopy. The maximum width of each cell was measured using a Matlab-based algorithm, and the mean width of 400 to 2,000 cells from each culture was determined. The plot depicts the average of the mean cell width from three independent experiments for each treatment condition as a function of the duration of the treatment.
Basic pH of the growth medium enhances the rod shape-disrupting effect of A22 and MP265.
Treatment with A22 or MP265 in PYE medium resulted in a gradual widening of C. crescentus cells over time (Fig. 3B; see also Fig. S1 in the supplemental material). At 5 μM, both drugs were particularly potent in disrupting rod cell morphology in denser cultures, and this effect was correlated with an increase in the pH of the culture medium during growth (data not shown). Sterile PYE medium had a pH of 6.6 to 6.8 while medium from stationary-phase cultures of C. crescentus had a pH of 8.5. To eliminate the effect of pH variation during growth, we buffered the medium at pH 7.8 (PYE-TRIS medium). Treatment of PYE-TRIS cultures with either A22 or MP265 at 5 μM resulted in the most significant increases in cell widths (Fig. 3B and S1), confirming an enhancement of drug activity by basic pH similar to that documented for other amine-containing drugs acting on cytoplasmic targets (28). This effect was likely due to increased concentration of the membrane-diffusible, nonprotonated form of the drug in the basic medium, leading to increased intracellular accumulation of the drug. Conversely, treatment with a 5 μM concentration of either drug in medium buffered at an acidic pH of 6.6 (PYE-P) caused no significant increase in cell widths (data not shown). The pH dependence of the rod shape-disrupting effect of 5 μM A22 also occurred with E. coli cells (see Fig. S2 in the supplemental material). Treatment of PYE-TRIS C. crescentus cultures with 50 μM MP265 or A22 produced smaller cell width increases than treatment with 5 μM, with A22 being less potent (Fig. 3B and S1). This effect was likely due to enhanced growth inhibition by A22 and MP265 under these treatment conditions, with A22 being more toxic than MP265 (Fig. 3B, S1, and S3 and Table S3). Cell widening induced by MreB inhibition is growth dependent (23). At 5 μM, A22 or MP265 did not affect the growth of bacteria lacking MreB when bacteria were grown in the basic medium LB-TRIS (see Fig S3 and Table S3 in the supplemental material), prompting us to use a 5 μM concentration of either MP265 or A22 on C. crescentus growing in PYE-TRIS (pH 7.8) to disrupt rod shape in all of the following experiments.
Morphological changes associated with recovery of C. crescentus cells from A22 or MP265 treatment comprise cell thinning, filamentation, branching and shedding of outer membrane vesicles.
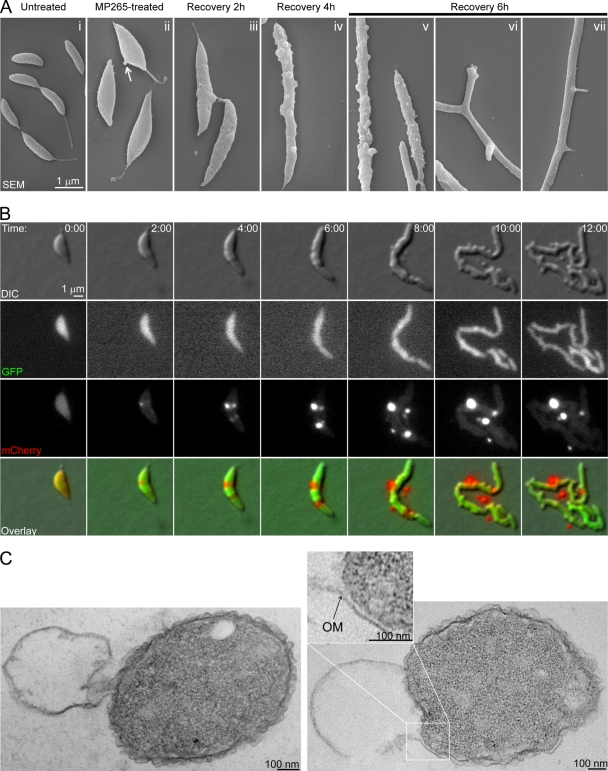
After 4 h of treatment with 5 μM MP265 in PYE-TRIS medium, the cells acquired wide, lemon-like shapes (Fig. 4Aii), occasionally presenting a bump on one lateral side. To monitor their recovery after dug removal, lemon-shaped, MP265-treated cells were washed and imaged by time-lapse DIC microscopy. Immediately after removal of the drug, a fraction of the lemon-shaped cells gradually became thinner until they reached normal cell widths (see Movie S1 in the supplemental material). The cell-thinning process was striking as it occurred along the entire lateral wall of lemon-shaped C. crescentus cells, including where the cells were originally wide. Cell thinning was also accompanied by cell elongation, producing long filamentous cells (Movie S1). Other cells within the population also began to thin and elongate but eventually lysed on the agarose growth pad (Movie S2). Thinning and elongation occurred concomitantly (see Fig. S4 in the supplemental material). These dramatic morphology changes required de novo PG synthesis since treatment with the PG synthesis inhibitor fosfomycin prevented cell shape recovery after removal of the MreB inhibitor (data not shown).
FIG. 4.
Phenotypes associated with recovery from MP265 and A22 treatment. (A) Scanning EM images of CB15N cells before MP265 treatment (i); after 4 h of treatment with 5 μM MP265 in PYE-TRIS (ii); or at 2 h (iii), 4 h (iv), and 6 h (v to vii) after removal of MP265. (B) Selected images of CJW3049 cells from a time-lapse sequence of recovery from 4 h of 5 μM MP265 treatment. Synthesis of cytoplasmic GFP and periplasm-exported mCherry was achieved by growing cells in the presence of 0.3% xylose and 0.5 mM vanillic acid for 4 h and 6 h, respectively, prior to imaging on agarose pads containing xylose and vanillic acid. Time is given as hours:minutes. (C) Transmission EM images of CB15N cells after 6 h of recovery from 4 h of 5 μM A22 treatment. OM, outer membrane.
The processes of cell thinning and filamentation were also accompanied by abundant shedding of cellular particles from the sides of the growing cells (Movies S1 and S2, arrowheads). We obtained similar results with cells recovering from A22 treatment (data not shown). Cell thinning, filamentation, and shedding of cellular particles could also be observed when the MP265-treated cells were washed and allowed to grow in the absence of the drug in liquid culture, as shown by scanning EM (Fig. 4A). Untreated and MP265-treated C. crescentus cells had curved-rod and lemon-like shapes, respectively, and largely smooth cell surfaces (Fig. 4Ai and ii; see also Fig. S5i and ii in the supplemental material). After removal of the drug, the lemon-shaped cells became increasingly thinner and longer (Fig. 4Aiii to vii and S5iii to vii). Their surfaces became progressively more irregular, and extensive blebbing could be observed on the surfaces of many of the recovering cell filaments (Fig. 4Aiv and v and S5iv to vii). By the time the cells had reached widths similar to those of wild-type cells, fractions of the cell population had grown cell branches or ectopic stalks positioned along the side wall (18% and 10%, respectively; n = 108) (Fig. 4Avi to vii and S5vi). These phenotypes are reminiscent of cells recovering from MreB depletion, as previously described (64).
A surprising and previously unreported phenotype associated with the recovery process was the profuse shedding of cellular vesicles. We also observed this shedding in cells recovering from MreB depletion (see Movie S3 in the supplemental material), indicating that it is not due to destabilization of the outer membrane by MP265 or A22 independent of their action on MreB. To identify the contents of the shedding particles, we constructed a C. crescentus strain (CJW3049) (see Materials and Methods) that produces the fluorescent proteins GFP and mCherry in the cytoplasm and periplasm, respectively. Lemon-shaped, MP265-treated CJW3049 cells growing after drug removal elongated, became thinner, occasionally grew small branches, and shed cellular particles (Fig. 4B and Movie S4), as expected. The cytoplasmic GFP signal remained contained within the cell body (including the branches) throughout the recovery process and was completely absent from the shed particles, suggesting that they do not contain cytoplasm (Fig. 4B and Movie S4). The periplasmic mCherry signal, on the other hand, became concentrated at distinct locations where shedding occurred and formed distinct bright foci that colocalized with the shed particles (Fig. 4B and Movie S4). That the mCherry signal was higher in the shed vesicles than in the nonblebbing cell periphery is likely due to the large volume of periplasm enclosed within vesicles relative to the rest of the periplasm. These observations strongly suggest that the shed particles are large outer membrane vesicles (OMVs).
We confirmed this result by using transmission EM of ultrathin sections through fixed, plastic-embedded cells. Under our fixation and imaging conditions, the outer membrane (OM) appeared frizzled, and the S-layer was not visible (see Fig. S6 in the supplemental material), consistent with previous reports (42, 51). The peptidoglycan layer was particularly well stained in our preparations, and the inner membrane was easier to detect (Fig. S6) when we used a uranyl acetate en bloc staining step prior to the ethanol dehydration procedure (26). Transmission EM images of ultrathin sections of cells recovering from treatment with A22 or MP265 revealed that the shedding particles were bound by a single-layered structure continuous with the outer membrane of the cell, confirming that they are OMVs (Fig. 4C and data not shown).
Given the filamentation defect associated with recovery from drug treatment, we examined the localization of the major cell division protein FtsZ. Fluorescence microscopy of a strain producing FtsZ fused to yellow fluorescent protein (FtsZ-YFP) showed that FtsZ formed a ring (which appears as a band) in untreated cells but failed to form a ring in lemon-shaped A22-treated cells and, instead, largely localized in a tight focus either within the bumps observed by DIC microscopy or at one pole of the cells (see Fig. S7A in the supplemental material). The bumps were also noticeable in PG sacculi isolated from A22-treated cells (Fig. S7B, arrowhead). As FtsZ-driven cell division results in the synthesis of new poles in daughter cells, it is conceivable that the observed local concentration of FtsZ on the side of lemon-shaped A22-treated cells drives local PG synthesis, resulting in the creation of an ectopic pole. This was supported by the observation that bumps occasionally developed into cell branches (see Movie S5, arrow, in the supplemental material). Shortly after removal of A22, FtsZ-YFP formed a ring in the recovering cells; however, the ring showed instability and moved along the elongating filament until late into the recovery process when stable FtsZ rings drove division of the filament (Movie S5).
The morphology of the peptidoglycan cell walls changes during recovery from A22 treatment.
Bacterial cell morphology is thought to be determined by the shape of the peptidoglycan cell wall. As recovery from A22 or MP265 treatment was accompanied by extensive cell shape remodeling, we inquired whether any changes in the peptidoglycan accompanied this process. Invariably, isolated PG sacculi retained the shape of the cells from which they had been isolated (Fig. 5; note that the spheres in the EM images represent polyhydroxybutyrate [PHB] storage granules trapped inside the PG mesh) (43). Uranyl acetate staining of sacculi isolated from untreated or A22-treated cells resulted in a uniform, light stain (Fig. 5A and B). Sacculi isolated from cells early during the process of recovery from A22 treatment (at 1, 2, and 3 h after removal of A22) and stained with uranyl acetate under identical conditions invariably presented darker-stained striations that crossed the otherwise lightly stained sacculi along their short axes (Fig. 5C to E). These striations were most dense in images of sacculi isolated 1 h after removal of A22 (Fig. 5C). As the cells progressed through the recovery process and the sacculi became thinner, the striations were spread farther apart and were fainter (Fig. 5C to E). They were completely absent from sacculi that had reached normal widths (Fig. 5F) (5 h after removal of A22). These observations suggested that the striations might represent changes in the PG cell walls associated with, and presumably important for, the recovery of the rod shape. Importantly, these dark striations were distinct from regular folds of the sacculi that can occur as the sacculi lay down on the EM grids (see Fig. S8 in the supplemental material) (16).
FIG. 5.
Transmission electron micrographs of C. crescentus sacculi isolated at different time points during recovery from A22. (A) Untreated cells. (B) Cells treated for 4 h with 5 μM A22 in PYE-TRIS. (C to E) Cells after 1 h (C), 2 h (D), and 3 h (E) of recovery from a 4-h treatment with 5 μM A22 in PYE-TRIS. (F) Cells after 5 h of recovery from a 5-h treatment with 5 μM A22 in PYE-TRIS. (G) High-magnification image of a sacculus containing strongly stained striations, isolated after 3 h of recovery from a 4-h treatment with 5 μM A22 in PYE-TRIS. PHB, polyhydroxybutyrate granule trapped inside the sacculus.
As noted above, not all cells in a population were able to fully recover after removal of A22 or MP265. Consistent with this, a fraction of the sacculi from the samples harvested at 3 or 5 h into the recovery process remained aberrantly wide and retained an elongated lemon shape (11% of sacculi [n = 341] for 3 h and 5.5% of sacculi [n = 475] for 5 h). These sacculi invariantly displayed thick, dark-stained, discrete striations along the length of the sacculus (Fig. 5G; see also Fig. S9 in the supplemental material). These striations were generally slightly tilted with respect to the short axis of the sacculi and often connected with other striations, forming V-like structures that crossed each other along the length of the sacculi (Fig. 5G and S9).
Changes in PG composition during recovery from A22 treatment.
Given that A22 treatment and recovery from A22 treatment involve extensive changes in the morphology of PG sacculi, we determined whether these changes were associated with changes in PG composition. The muropeptide composition of the C. crescentus PG has only been roughly described previously (39). We therefore used an HPLC-based protocol to separate and quantify the reduced muropeptide building blocks of the C. crescentus PG (24, 39). In order to unambiguously assign molecular structures to the separated muropeptides, we collected the fractions containing the major muropeptide peaks and analyzed them by MS (6). The overall muropeptide profile of C. crescentus differed significantly from that of E. coli (24, 25) although the PG type was the same and typical for Gram-negative bacteria, and major muropeptides with the same structure were present in both species (Fig. 6A; see also Table S4 in the supplemental material). In contrast to E. coli PG, C. crescentus PG did not contain detectable amounts of covalently linked Braun's lipoprotein (Lpp) (39, 43). This is consistent with the absence from the C. crescentus genome of the gene encoding this outer membrane anchor. Second, a variant of the normal pentapeptide with Gly replacing the terminal d-Ala [penta(Gly5)] was highly abundant in both free and cross-linked forms, consistent with previously published data (39) (Table 2 and Fig. 6B). Third, cross-linked muropeptides were more abundant, including trimers and tetramers, leading to a high overall degree of cross-linkage of the C. crescentus PG (34%) (Table 2 and Fig. 6B) compared to that of E. coli PG (23%) (24) (note that the degree of cross-linkage is a numerical value and is not the same as the molar percentage of peptides present in cross-links). We also identified by MS a muropeptide dimer carrying an l,d-cross-link (m-Dap-mDap) (Fig. 6A and Table S4). l,d-Cross-links were thought to be absent from the C. crescentus PG (39); their presence, however, is not surprising, given that the C. crescentus genome encodes a putative l,d-transpeptidase (locus cc_1511; BLASTP value of 4.4 × e−33 against the E. coli K12 l,d-transpeptidase YcbB) (38). Fourth, 1,6-anhydro-MurNAc glycan chain ends were abundant (Table 2 and Table S5), including as part of di-anhydro cross-linked species, which are extremely rare in E. coli (24, 25). This correlated with average glycan chain lengths (∼9 disaccharide subunits/glycan chain) (Table 2) that were significantly shorter than those of E. coli (∼30 disaccharide subunits/glycan chain) (24).
FIG. 6.
Muropeptide composition of C. crescentus PG during A22 treatment and recovery from A22 treatment. (A) UV chromatogram of HPLC-separated muropeptides isolated from the PG of CB15N cells. Peak identities determined by MS/MS: Tri, GlcNAc-MurNAc-l-Ala-l-γ-Glu-m-Dap; tetra, Tri-d-Ala; Penta, Tetra-d-Ala; Penta(Gly5), Tetra-Gly; Anh, 1,6-anhydro-MurNAc; (d,l), m-Dap-m-Dap cross-link. (B) Composition (in molar percentage) of various muropeptide species in the PG of untreated CB15N cells (mean values ± standard deviation of measurements from three independent samples). (C) Changes in the composition of C. crescentus PG during A22 treatment and recovery from treatment. The molar percentage of each species in a given sample is expressed as a percentage of the average molar percentage of the species in the PG from untreated cells. Treatment conditions are as indicated on the figure. Bars represent mean values ± standard deviation of three (U), two (T, 1, and 2), and one (3) independent PG samples.
TABLE 2.
Summary of C. crescentus PG composition during A22 treatment and recovery from treatment
| Muropeptide species or feature | PG composition (molar % ± SD) of cells |
||||
|---|---|---|---|---|---|
| Without treatmenta | With A22 treatmentb | During recovery at: |
|||
| 1 hb | 2 hb | 3 hc | |||
| Monomer | 41.54 ± 1.23 | 40.39 ± 0.40 | 36.20 ± 0.15 | 38.81 ± 0.03 | 38.57 |
| Dimer | 38.68 ± 1.35 | 38.04 ± 0.89 | 47.77 ± 0.14 | 46.96 ± 0.03 | 46.60 |
| Trimer | 17.23 ± 0.69 | 17.98 ± 1.31 | 14.48 ± 0.56 | 12.70 ± 0.18 | 13.10 |
| Tetramer | 4.10 ± 0.40 | 6.10 ± 0.15 | 2.91 ± 0.31 | 2.14 ± 0.15 | 2.23 |
| Cross-linkaged | 33.89 ± 0.72 | 35.59 ± 0.55 | 35.72 ± 0.21 | 33.55 ± 0.02 | 33.71 |
| Pentapeptide (Ala5) | 20.33 ± 0.44 | 19.01 ± 0.37 | 18.31 ± 0.07 | 17.91 ± 0.05 | 19.18 |
| Pentapeptide (Gly5) | 14.70 ± 1.08 | 14.94 ± 1.18 | 22.21 ± 0.04 | 23.13 ± 0.18 | 21.73 |
| Pentapeptide (Total) | 35.03 ± 1.33 | 33.94 ± 0.81 | 40.51 ± 0.12 | 41.04 ± 0.23 | 40.91 |
| Tetrapeptide | 63.75 ± 1.05 | 64.86 ± 1.06 | 58.53 ± 0.17 | 58.03 ± 0.48 | 58.18 |
| Tripeptide | 1.09 ± 0.56 | 1.06 ± 0.26 | 0.90 ± 0.04 | 0.89 ± 0.25 | 0.85 |
| Anhydro | 10.66 ± 0.50 | 13.50 ± 0.005 | 6.46 ± 0.34 | 5.28 ± 0.15 | 5.66 |
| Average glycan chain lengthe | 9.40 ± 0.45 | 7.41 ± 0.003 | 15.50 ± 0.83 | 18.96 ± 0.55 | 17.66 |
Results of three independent experiments.
Results of two independent experiments.
Results of one experiment.
Calculated as in described in reference 26.
Values represent number of disaccharide subunits/glycan chain.
Having identified the major muropeptides present in the C. crescentus PG, we then characterized and compared the muropeptide composition of PG isolated from untreated cells, A22-treated cells, and cells recovering from A22 treatment (see Table S5 in the supplemental material). A22 treatment caused a 20% decrease in the average glycan chain length (Table 2 and Fig. 6C). A similar decrease of the average glycan chain length has been observed in the PG of A22-treated E. coli cells (57). The amount of highly cross-linked tetrameric muropeptides was increased by about 50% after A22 treatment (Table 2 and Fig. 6C). The degree of cross-linkage, however, was not significantly changed in A22-treated cells compared to untreated cells (Table 2 and Fig. 6C). This suggests that the A22-induced loss of the rod morphology was not due to reduction of the number of PG cross-links.
Recovery from A22 treatment was accompanied by considerable changes in PG composition, pointing toward enhanced PG synthesis. Most importantly, the average glycan chain length was increased up to 2.5-fold compared to the glycan chain length of A22-treated cells and up to 2-fold compared to the value obtained from untreated cells (Table 2 and Fig. 6C). The amount of un-cross-linked, glycine-containing pentapeptide, penta(Gly5), in the muropeptide side chains was increased by ∼50% compared to the amounts of the same species in PG from untreated or A22-treated cells. Similarly, recovery from A22 treatment was accompanied by ∼20% increase in the amount of dimeric muropeptides, which are directly formed in the transpeptidation reactions that cross-link newly inserted precursors to existent PG. Conversely, trimeric and tetrameric muropeptides, which are formed in reactions of pentapeptides with previously formed dimeric and trimeric acceptors, were decreased by at least 20% and 50%, respectively, in PG of cells recovering from A22 treatment compared to PG from A22-treated cells (Table 2 and Fig. 6C). Recovery of the rod shape following A22 treatment is therefore accompanied by extensive remodeling of the PG at both morphological and structural levels.
DISCUSSION
We show here that the widely used MreB inhibitor A22 affects bacterial growth by also acting on an essential target(s) other than MreB. This MreB-independent toxic effect varies depending on the species subjected to treatment and on the treatment conditions used (e.g., medium and pH). Our results are consistent with previous documentation of MreB-independent toxic effects of A22 on the growth of E. coli (35) or of A22 structural analogs on the growth of the Gram-positive Staphylococcus aureus (31), which lacks MreB (11). The species-dependent variation in toxicity levels requires careful examination of the growth-inhibitory effect of A22 or its analogs whenever such drugs are used in the study of a new bacterial species. Furthermore, the discovery of MP265 as a compound with anti-MreB activity similar to that of A22, but with lower toxicity, provides a better tool for reversible disruption of MreB function in Gram-negative bacteria. Another important consideration is the pH of the culture medium since it has a significant effect on the activity and toxicity of both A22 and MP265.
Cells recovering from A22 or MP265 treatment thinned until they reached normal widths. Such a remarkable change in cell width implies extensive PG remodeling within the wide sacculi of MreB-disrupted cells. Transient, dark, uranyl acetate-stained striations on sacculi appeared during this thinning process. In nonrecovering cells, the striations were exacerbated and displayed clear V-shaped patterns, which may be related to the suggested helical localization of MreB filaments in live cells. The projection of three-dimensional helices on a planar surface, such as the EM grids, would resemble crossing Vs. These striations were present only in sacculi from cells recovering from A22 treatment which had not yet returned to normal widths. They were also distinct from previously described folded PG (16), and they displayed highly regular patterns. It is therefore highly unlikely that they are folding or staining artifacts. Previously, thicker, multilayered PG found in the septa of PG hydrolase mutants of E. coli was shown to also stain darker with uranyl acetate than the rest of the sacculi (44). Thus, while we cannot completely rule out other explanations, we believe that the striations represent areas of thicker, multilayered PG, likely where new insertion took place. We assume that the disappearance of the striations from sacculi at late time points of recovery is due to the action of PG hydrolases. An endopeptidase activity, for example, could account for the observed disappearance of the striations and concomitant decrease in the amounts of trimeric and tetrameric muropeptides and increase of the dimeric species (Fig. 6C). The transient presence of V-shaped striations during rod shape recovery correlates with a considerable increase in glycan chain length. PG synthesis is thought to occur along helical paths within the side wall (15, 59), and the glycan strands are thought to be oriented similarly (21, 61). Our results are consistent with this model, and the longer glycans found in the PG of recovering cells may be present within the striations and may be required to reduce the width of the wide sacculi. Conversely, loss of rod shape following MreB disruption may be due to loss of transverse/helical orientation of newly inserted glycan strands.
Our findings that MreB inactivation results in a decrease of the glycan strand length and that restoration of MreB function results in a considerable increase of the glycan length suggest that MreB may either promote the glycan strand-synthesizing activity of the PBPs, inhibit the strand-cutting activity of the lytic transglycosylases, or both. A careful biochemical analysis of PG synthesis in E. coli has shown that MreB inactivation by A22 significantly inhibits the synthesis of new glycan strands and causes accumulation of the soluble UDP-MurNAc-pentapeptide PG precursor in the cytoplasm (55). The same study also demonstrated that lytic transglycosylase activity was not affected by A22 treatment, and another study showed that A22 treatment causes a decrease of the average glycan strand length in E. coli, consistent with our observations in C. crescentus (55, 57). These corroborated findings argue that MreB promotes, directly or indirectly, PBP transglycosylase activity. This function may be achieved via protein-protein interactions within the elongase complex or through regulation of the delivery of PG precursors to periplasmic PBPs. MreB may also affect PBP activity and PG growth by altering the mechanical properties of the cell wall (13).
The shedding of OMVs was another surprising phenotype associated with recovery from A22 or MP265 treatment. A difference between the growth rate of the outer membrane and that of the PG during recovery may be responsible for an accumulation of excess outer membrane and blebbing. Another, perhaps more likely, possibility is that the intense PG remodeling associated with recovery of rod morphology causes the loss of PG material that served as points of anchorage for outer membrane proteins. Local detachment of the outer membrane from the PG layer would favor blebbing. Indeed, in Salmonella, loss of attachments between PG and outer membrane results in increased shedding of OMVs (12), consistent with a proposed model for the formation of such vesicles (40).
Supplementary Material
Acknowledgments
We are grateful to L. Shapiro, P. de Boer, Y. Brun, J. Smit, J. Gober, S. Dinesh-Kumar, and W. Margolin for providing strains and plasmids; to B. Piekos, M. Mooseker, and Z. Jiang for support with the EM; to K. Bui and J. Gray for support with HPLC and tandem MS (MS/MS) analyses; to O. Sliusarenko for the Matlab-based software; and to H. Lam and the members of the Jacobs-Wagner laboratory for critical reading of the manuscript.
This work was supported by the National Institutes of Health (GM076698 and GM065835 to C.J.-W.) and the European Commission within the EUR-INTAFAR project (to W.V.). C.N.T. was supported in part by a Yale College Dean's Office Science, Technology and Research Scholars (STARS) II fellowship; M. P. was supported by a Human Frontier Science Program Organization for a Cross Disciplinary Fellowship, and S.P. was supported by a PEW Latin American Fellowship. C.J.-W. is a Howard Hughes Medical Institute investigator.
Footnotes
Published ahead of print on 18 December 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aaron, M., G. Charbon, H. Lam, H. Schwarz, W. Vollmer, and C. Jacobs-Wagner. 2007. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol. Microbiol. 64:938-952. [DOI] [PubMed] [Google Scholar]
- 2.Alyahya, S. A., R. Alexander, T. Costa, A. O. Henriques, T. Emonet, and C. Jacobs-Wagner. 2009. RodZ, a component of the bacterial core morphogenic apparatus. Proc. Natl. Acad. Sci. U. S. A. 106:1239-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bean, G. J., S. T. Flickinger, W. M. Westler, M. E. McCully, D. Sept, D. B. Weibel, and K. J. Amann. 2009. A22 disrupts the bacterial actin cytoskeleton by directly binding and inducing a low-affinity state in MreB. Biochemistry 48:4852-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendezu, F. O., and P. A. de Boer. 2008. Conditional lethality, division defects, membrane involution, and endocytosis in mre and mrd shape mutants of Escherichia coli. J. Bacteriol. 190:1792-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendezu, F. O., C. A. Hale, T. G. Bernhardt, and P. A. de Boer. 2009. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J. 28:193-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bui, N. K., J. Gray, H. Schwarz, P. Schumann, D. Blanot, and W. Vollmer. 2009. The peptidoglycan sacculus of Myxococcus xanthus has unusual structural features and is degraded during glycerol-induced myxospore development. J. Bacteriol. 191:494-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabeen, M. T., and C. Jacobs-Wagner. 2007. Skin and bones: the bacterial cytoskeleton, cell wall, and cell morphogenesis. J. Cell Biol. 179:381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carballido-Lopez, R., and J. Errington. 2003. The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev. Cell 4:19-28. [DOI] [PubMed] [Google Scholar]
- 9.Carballido-Lopez, R., A. Formstone, Y. Li, S. D. Ehrlich, P. Noirot, and J. Errington. 2006. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev. Cell 11:399-409. [DOI] [PubMed] [Google Scholar]
- 10.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 12.Deatherage, B. L., J. C. Lara, T. Bergsbaken, S. L. Rassoulian Barrett, S. Lara, and B. T. Cookson. 2009. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72:1395-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer, P. A. 2009. Bend into shape. EMBO J. 28:1193-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer, P. A., R. E. Crossley, and L. I. Rothfield. 1988. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J. Bacteriol. 170:2106-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Blaauwen, T., M. A. de Pedro, M. Nguyen-Disteche, and J. A. Ayala. 2008. Morphogenesis of rod-shaped sacculi. FEMS Microbiol. Rev. 32:321-344. [DOI] [PubMed] [Google Scholar]
- 16.de Pedro, M. A., J. C. Quintela, J. V. Holtje, and H. Schwarz. 1997. Murein segregation in Escherichia coli. J. Bacteriol. 179:2823-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebersbach, G., A. Briegel, G. J. Jensen, and C. Jacobs-Wagner. 2008. A self-associating protein critical for chromosome attachment, division, and polar organization in Caulobacter. Cell 134:956-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ely, B. 1991. Genetics of Caulobacter crescentus. Methods Enzymol. 204:372-384. [DOI] [PubMed] [Google Scholar]
- 19.Evinger, M., and N. Agabian. 1977. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figge, R. M., A. V. Divakaruni, and J. W. Gober. 2004. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol. Microbiol. 51:1321-1332. [DOI] [PubMed] [Google Scholar]
- 21.Gan, L., S. Chen, and G. J. Jensen. 2008. Molecular organization of Gram-negative peptidoglycan. Proc. Natl. Acad. Sci. U. S. A. 105:18953-18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitai, Z., N. Dye, and L. Shapiro. 2004. An actin-like gene can determine cell polarity in bacteria. Proc. Natl. Acad. Sci. U. S. A. 101:8643-8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gitai, Z., N. A. Dye, A. Reisenauer, M. Wachi, and L. Shapiro. 2005. MreB actin-mediated segregation of a specific region of a bacterial chromosome. Cell 120:329-341. [DOI] [PubMed] [Google Scholar]
- 24.Glauner, B. 1988. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem. 172:451-464. [DOI] [PubMed] [Google Scholar]
- 25.Glauner, B., J. V. Holtje, and U. Schwarz. 1988. The composition of the murein of Escherichia coli. J. Biol. Chem. 263:10088-10095. [PubMed] [Google Scholar]
- 26.Graham, L., and J. M. Orenstein. 2007. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc. 2:2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi, K. 1975. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal. Biochem. 67:503-506. [DOI] [PubMed] [Google Scholar]
- 28.Hille, B. 1977. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J. Gen. Physiol. 69:475-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtje, J. V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:181-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwai, N., T. Ebata, H. Nagura, T. Kitazume, K. Nagai, and M. Wachi. 2004. Structure-activity relationship of S-benzylisothiourea derivatives to induce spherical cells in Escherichia coli. Biosci. Biotechnol. Biochem. 68:2265-2269. [DOI] [PubMed] [Google Scholar]
- 31.Iwai, N., T. Fujii, H. Nagura, M. Wachi, and T. Kitazume. 2007. Structure-activity relationship study of the bacterial actin-like protein MreB inhibitors: effects of substitution of benzyl group in S-benzylisothiourea. Biosci. Biotechnol. Biochem. 71:246-248. [DOI] [PubMed] [Google Scholar]
- 32.Iwai, N., K. Nagai, and M. Wachi. 2002. Novel S-benzylisothiourea compound that induces spherical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicillin-binding protein 2. Biosci. Biotechnol. Biochem. 66:2658-2662. [DOI] [PubMed] [Google Scholar]
- 33.Iwaya, M., R. Goldman, D. J. Tipper, B. Feingold, and J. L. Strominger. 1978. Morphology of an Escherichia coli mutant with a temperature-dependent round cell shape. J. Bacteriol. 136:1143-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, L. J., R. Carballido-Lopez, and J. Errington. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913-922. [DOI] [PubMed] [Google Scholar]
- 35.Karczmarek, A., R. Martinez-Arteaga, S. Alexeeva, F. G. Hansen, M. Vicente, N. Nanninga, and T. den Blaauwen. 2007. DNA and origin region segregation are not affected by the transition from rod to sphere after inhibition of Escherichia coli MreB by A22. Mol. Microbiol. 65:51-63. [DOI] [PubMed] [Google Scholar]
- 36.Kawai, Y., R. A. Daniel, and J. Errington. 2009. Regulation of cell wall morphogenesis in Bacillus subtilis by recruitment of PBP1 to the MreB helix. Mol. Microbiol. 71:1131-1144. [DOI] [PubMed] [Google Scholar]
- 37.Kruse, T., J. Moller-Jensen, A. Lobner-Olesen, and K. Gerdes. 2003. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 22:5283-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnet, S., L. Dubost, A. Marie, M. Arthur, and L. Gutmann. 2008. Identification of the l, d-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J. Bacteriol. 190:4782-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markiewicz, Z., B. Glauner, and U. Schwarz. 1983. Murein structure and lack of dd- and ld-carboxypeptidase activities in Caulobacter crescentus. J. Bacteriol. 156:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashburn-Warren, L. M., and M. Whiteley. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61:839-846. [DOI] [PubMed] [Google Scholar]
- 41.Noguchi, N., K. Yanagimoto, H. Nakaminami, M. Wakabayashi, N. Iwai, M. Wachi, and M. Sasatsu. 2008. Anti-infectious effect of S-benzylisothiourea compound A22, which inhibits the actin-like protein, MreB, in Shigella flexneri. Biol. Pharm. Bull. 31:1327-1332. [DOI] [PubMed] [Google Scholar]
- 42.Poindexter, J. L. S., and G. Cohen-Bazire. 1964. The fine structure of stalked bacteria belonging to the family Caulobacteraceae. J. Cell Biol. 23:587-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poindexter, J. S., and J. G. Hagenzieker. 1982. Novel peptidoglycans in Caulobacter and Asticcacaulis spp. J. Bacteriol. 150:332-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Priyadarshini, R., M. A. de Pedro, and K. D. Young. 2007. Role of peptidoglycan amidases in the development and morphology of the division septum in Escherichia coli. J. Bacteriol. 189:5334-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson, G. T., T. B. Doyle, Q. Du, L. Duncan, K. E. Mdluli, and A. S. Lynch. 2007. A novel indole compound that inhibits Pseudomonas aeruginosa growth by targeting MreB is a substrate for MexAB-OprM. J. Bacteriol. 189:6870-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez, A. D., M. J. Lear, and J. J. La Clair. 2008. Identification of the binding of sceptrin to MreB via a bidirectional affinity protocol. J. Am. Chem. Soc. 130:7256-7258. [DOI] [PubMed] [Google Scholar]
- 47.Sauvage, E., F. Kerff, M. Terrak, J. A. Ayala, and P. Charlier. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234-258. [DOI] [PubMed] [Google Scholar]
- 48.Shiomi, D., M. Sakai, and H. Niki. 2008. Determination of bacterial rod shape by a novel cytoskeletal membrane protein. EMBO J. 27:3081-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering-transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 50.Slovak, P. M., G. H. Wadhams, and J. P. Armitage. 2005. Localization of MreB in Rhodobacter sphaeroides under conditions causing changes in cell shape and membrane structure. J. Bacteriol. 187:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smit, J., D. A. Grano, R. M. Glaeser, and N. Agabian. 1981. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J. Bacteriol. 146:1135-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava, P., G. Demarre, T. S. Karpova, J. McNally, and D. K. Chattoraj. 2007. Changes in nucleoid morphology and origin localization upon inhibition or alteration of the actin homolog, MreB, of Vibrio cholerae. J. Bacteriol. 189:7450-7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thanbichler, M., A. A. Iniesta, and L. Shapiro. 2007. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 35:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thanbichler, M., and L. Shapiro. 2006. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126:147-162. [DOI] [PubMed] [Google Scholar]
- 55.Uehara, T., and J. T. Park. 2008. Growth of Escherichia coli: significance of peptidoglycan degradation during elongation and septation. J. Bacteriol. 190:3914-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Ent, F., L. A. Amos, and J. Lowe. 2001. Prokaryotic origin of the actin cytoskeleton. Nature 413:39-44. [DOI] [PubMed] [Google Scholar]
- 57.Varma, A., M. A. de Pedro, and K. D. Young. 2007. FtsZ directs a second mode of peptidoglycan synthesis in Escherichia coli. J. Bacteriol. 189:5692-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vats, P., and L. Rothfield. 2007. Duplication and segregation of the actin (MreB) cytoskeleton during the prokaryotic cell cycle. Proc. Natl. Acad. Sci. U. S. A. 104:17795-17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vollmer, W., and U. Bertsche. 2008. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta 1778:1714-1734. [DOI] [PubMed] [Google Scholar]
- 60.Vollmer, W., D. Blanot, and M. A. de Pedro. 2008. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32:149-167. [DOI] [PubMed] [Google Scholar]
- 61.Vollmer, W., and J. V. Holtje. 2004. The architecture of the murein (peptidoglycan) in gram-negative bacteria: vertical scaffold or horizontal layer(s)? J. Bacteriol. 186:5978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wachi, M., M. Doi, Y. Okada, and M. Matsuhashi. 1989. New mre genes mreC and mreD, responsible for formation of the rod shape of Escherichia coli cells. J. Bacteriol. 171:6511-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wachi, M., and M. Matsuhashi. 1989. Negative control of cell division by mreB, a gene that functions in determining the rod shape of Escherichia coli cells. J. Bacteriol. 171:3123-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner, J. K., C. D. Galvani, and Y. V. Brun. 2005. Caulobacter crescentus requires RodA and MreB for stalk synthesis and prevention of ectopic pole formation. J. Bacteriol. 187:544-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker, S. G., D. N. Karunaratne, N. Ravenscroft, and J. Smit. 1994. Characterization of mutants of Caulobacter crescentus defective in surface attachment of the paracrystalline surface layer. J. Bacteriol. 176:6312-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, Y., B. D. Jones, and Y. V. Brun. 2001. A set of ftsZ mutants blocked at different stages of cell division in Caulobacter. Mol. Microbiol. 40:347-360. [DOI] [PubMed] [Google Scholar]
- 67.Young, K. D. 2006. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 70:660-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.