Abstract
The Arc two-component system, comprising the ArcB sensor kinase and the ArcA response regulator, modulates the expression of numerous genes in response to the respiratory conditions of growth. ArcB is a tripartite histidine kinase whose activity is regulated by the oxidation of two cytosol-located redox-active cysteine residues that participate in intermolecular disulfide bond formation. Here we show that ArcB autophosphorylates through an intramolecular reaction which diverges from the usually envisaged intermolecular autophosphorylation of homodimeric histidine kinases.
The Arc (Anoxic redox control) two-component system is an important element in the complex transcriptional regulatory network that allows facultative aerobic bacteria to sense and respond to various respiratory conditions (9, 18, 25). This system comprises ArcB as the membrane-bound sensor kinase and ArcA as the cognate response regulator (13, 14). ArcA is a typical response regulator that has an N-terminal receiver domain and a C-terminal helix-turn-helix DNA-binding domain, whereas ArcB is a tripartite protein that has, in addition to the primary transmitter domain, a receiver domain and a secondary transmitter or phosphotransfer domain (12, 15). Moreover, in the linker region, there is a putative leucine zipper (6) and a PAS domain (38).
Under anoxic growth conditions, ArcB autophosphorylates, a process that is enhanced by certain fermentative metabolites, such as d-lactate, pyruvate, and acetate (7, 30), and transphosphorylates ArcA through a His292 → Asp576 → His717 → Asp54 phosphorelay (10, 19). Phosphorylated ArcA, in turn, represses the expression of many operons involved in respiratory metabolism and activates others that encode proteins involved in fermentative metabolism (21, 22, 31). Under aerobic growth conditions, the quinone electron carriers inhibit the kinase activity of ArcB (8) through the oxidation of two redox-active cysteine residues that participate in intermolecular disulfide bond formation (24). Under such conditions, ArcB catalyzes the dephosphorylation of ArcA-P via an Asp54 → His717 → Asp576 → Pi reverse phosphorelay (6, 28).
It has been previously reported that histidine kinases act as homodimers (4, 33, 35, 36) and that they autophosphorylate by an intermolecular reaction (32). That is, the γ-phosphoryl group of ATP, which is bound to one monomer in the homodimer, is transferred to the other monomer. Examples include EnvZ (37), NtrB (27), CheA (34), and also the tripartite kinase BvgS (2). Nevertheless, an exception is provided by the HK853 sensor kinase of Thermotoga maritima, which was recently shown to autophosphorylate by an intramolecular reaction (1). Here, the ArcB autophosphorylation reaction was analyzed by using wild-type and mutant proteins unable to bind ATP or blocked at the autophosphorylation site.
(This study was performed in partial fulfillment of the requirements for the Ph.D. degree in Biomedical Sciences of G. R. Peña-Sandoval at the Universidad Nacional Autónoma de México.)
Autophosphorylation of ArcB requires the ATP-binding site and the autophosphorylating site to be present in the same ArcB molecule.
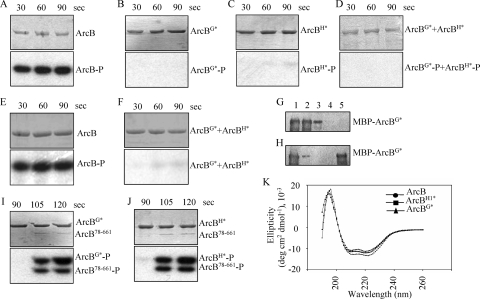
Autophosphorylation in homodimeric histidine kinases is usually envisaged as an intermolecular reaction; that is, one subunit of the dimer binds ATP and phosphorylates the other subunit (32). To test whether this paradigm applies for the ArcB sensor kinase, we generated two mutant ArcB variants, one unable to bind ATP (ArcB78-778,G470A,G472A, hereafter referred to as ArcBG*) and one blocked at the autophosphorylation site (ArcB78-778,H292Q, hereafter referred to as ArcBH*), as His-tagged proteins. For the purposes of this study and to facilitate the purification of the protein variants, amino acid residues 1 to 77, which constitute the transmembrane segments, were omitted. Previous studies on sensor kinases, including ArcB, showed that removal of the transmembrane segments does not affect the processes of autophosphorylation and the subsequent transphosphorylation of the cognate regulator proteins (10, 11, 16, 17, 23, 29). To construct the mutant ArcB-expressing plasmids, we used primer 5′-CCCGGATCCCATATGGAGCAACTGGAGGAGTCACGAC-3′ together with mutagenic primer 5′-CGGCCAGAGCAATAGCGGTGCCGGTGGC-3′ (G470A and G472A) or 5′-GGTGTACGCAATTCTTGACTGATGGTGG-3′ (H292Q) in PCRs with plasmid pQ30ArcB78-778 (10) as the template. The products of these reactions were purified and used as megaprimers in PCRs together with primer 5′-CCCGGATCCATGCATCATTATGATTTACTGTTCTCTTCTGTCGTC-3′ with pQ30ArcB78-778 as the template. The products of the second PCRs were digested with NdeI and NruI and used to replace the NdeI-NruI wild-type fragment of pQ30ArcB78-778, generating plasmids pMX025 (ArcBG*) and pMX028 (ArcBH*). Plasmids pQ30ArcB78-778, pMX025, and pMX028 were used to overexpress and purify ArcB78-778 (hereafter referred to as ArcB), ArcBG*, and ArcBH* as described earlier (10). The purified proteins were incubated with [γ-32P]ATP, and their ability to autophosphorylate was tested. As expected, ArcBG* and ArcBH* failed to phosphorylate, whereas ArcB was rapidly phosphorylated (Fig. 1A to C). We then argued that in a mixture of both defective proteins, hybrid heterodimers should form, and phosphorylation of ArcBG*, which has the conserved H292, would then occur if an intermolecular phosphoryl group transfer were involved. However, no phosphorylation of ArcBG* was observed (Fig. 1D), indicating that ArcB autophosphorylation occurs via an intramolecular reaction or that no heterodimers were formed in the reaction mixture. To ensure protomer exchange and the formation of heterodimers, the two mutant proteins were denatured by urea treatment, mixed in equimolar concentrations, and renatured by gradual urea removal by dialysis against 0.1 M sodium phosphate (pH 8.0)-1 mM Na2EDTA-150 mM NaCl-25% glycerol. As a control, ArcB was subjected to the same treatment. Subsequent phosphorylation assays revealed efficient phosphorylation of ArcB (Fig. 1E) but no phosphorylation of ArcBG* in the reaction mixture containing the two mutant proteins (Fig. 1F). It thus appears that ArcB autophosphorylation does not occur through an intermolecular reaction and thereby diverges from the established paradigm.
FIG. 1.
Testing of the phosphorylation activities of ArcB78-778, ArcBG*, and ArcBH*. Purified ArcB (A), ArcBG* (B), and ArcBH* (C) proteins (∼10 pmol) were incubated in 20-μl reaction mixtures with [γ-32P]ATP, and 5-μl samples were withdrawn at the indicated time intervals for SDS-PAGE analysis. The Coomassie blue-stained gels revealing protein bands are presented in the upper parts of the panels, and the corresponding autoradiograms are presented in the bottom parts of the panels. (D) Probing for intermolecular ArcB autophosphorylation using ArcBG* and ArcBH*. Purified ArcBG* and ArcBH* mutant proteins were added to a 20-μl reaction mixture with [γ-32P]ATP, and 5-μl samples were withdrawn at the indicated time intervals for analysis by SDS-PAGE (top, Coomassie blue-stained gel; bottom, autoradiogram). (E and F) Probing for intermolecular ArcB autophosphorylation using urea-treated and renatured ArcB or a mixture of ArcBG* and ArcBH*. Purified ArcB (E) or a mixture of the ArcBG* and ArcBH* mutant proteins (F) was denatured by urea treatment, renatured by gradual urea removal by dialysis, and added to a 20-μl reaction mixture with [γ-32P]ATP. Five-microliter samples were withdrawn at the indicated time intervals for analysis by SDS-PAGE (top, Coomassie blue-stained gel; bottom, autoradiogram). (G and H) Probing for heterodimer formation. Purified MBP-ArcBG* (G) or a mixture of MBP-ArcBG* and ArcBH* (H) was treated with Q0 and incubated with Ni-nitrilotriacetic acid resin, which interacts with the His6 tag (lane 1). The resin was washed to dispose of unbound proteins (lanes 2 to 4), and ArcBH* bound to the resin was eluted with imidazole (lane 5). The MBP-ArcBG* protein that copurified with ArcBH* was analyzed by Western blotting using MBP-specific antibodies. (I and J) Testing of the phosphoaccepting activity of ArcBG* and ArcBH*. Purified ArcBG* and ArcBH* were incubated with [γ-32P]ATP, and after 90 s, ArcB78-661 (∼1 pmol) was added to the reaction mixtures. Five-microliter samples were withdrawn at the indicated time intervals for analysis (top, Coomassie blue-stained gels; bottom, autoradiograms). (K) CD spectra of ArcB78-778, ArcBG*, and ArcBH*. The mutant ArcB proteins show the same degree of secondary structure as the wild-type protein, indicating that the mutations do not significantly perturb the global fold of the protein.
The properties of the two mutant proteins that have the same size and both of which possess a His6 tag do not permit the experimental identification of heterodimers in the above-described reaction mixtures. Therefore, we created a plasmid carrying an ArcBG* version fused to the maltose-binding protein (MBP-ArcBG*) by cloning the BamHI-HindIII fragment from plasmid pMX025 into pMAL-c2. The constructed plasmid was used to overexpress and purify MBP-ArcBG*, which was mixed with ArcBH*, which possesses a His6 tag, and the mixture was split into two parts. The first one was incubated with [γ-32P]ATP and tested for intermolecular autophosphorylation of MBP-ArcBG*. In agreement with the above-described results, no MBP-ArcBG*-P was detected (data not shown). The second half was used to test whether the MBP-ArcBG* protein forms heterodimers with ArcBH*. To this end, 1 mM ubiquinone 0 (Q0) was added to the mixture containing MBP-ArcBG* and ArcBH* to promote disulfide bond formation between dimers (24), and the protein mixture was incubated with Ni-nitrilotriacetic acid resin, which interacts with the His6 tag. The resin was washed to dispose of unbound proteins, and the ArcBH* bound to the resin was eluted with imidazole. As a control, a mixture containing only MBP-ArcBG* and Q0 was incubated with Ni-nitrilotriacetic acid resin and processed the same way. Eluents of the above-described experiments were analyzed by Western blotting using MBP-specific antibodies. As expected, in the absence of ArcBH*, MBP-ArcBG* failed to interact with the resin and eluted in the washing fractions (Fig. 1G). In contrast, in the presence of ArcBH*, a considerable fraction of the MBP-ArcBG* protein was retained in the column and coeluted with ArcBH* (Fig. 1H), indicating the formation of heterodimers.
Subsequently, we tested whether ArcBG* and ArcBH*are able to receive the phosphoryl group at the conserved Asp576 of their receiver domain and/or His717 of their phosphotransfer domain from an ArcB protein that is able to autophosphorylate. Purified ArcBG* and ArcBH* were incubated with [γ-32P]ATP, and after 90 s of incubation, ArcB78-661 (10) was added to the reaction mixture (Fig. 1I and J). Although ArcBG* and ArcBH* were not able to autophosphorylate, both mutant ArcB proteins were able to rapidly receive the phosphoryl group from ArcB78-661-P at Asp576 and/or His717 through an intermolecular reaction. It therefore appears that ArcB, like other sensor kinases (4, 33, 35, 36), functions as a dimer and that the G470A-G472A and H292Q substitutions do not affect the overall structure of the protein.
The structural integrity of the purified ArcB proteins was also assessed by analysis of their secondary structure by far-UV circular dichroism (CD) spectra. Purified ArcB, ArcBG*, and ArcBH* proteins (1.5 mM) were dialyzed against 9.32 mM Na2HPO4-6.8 mM NaH2PO4-10 mM β-mercaptoethanol, and scans from 190 to 260 nm were performed. The CD spectra showed that all three polypeptides maintain their structural integrity during purification and contain the characteristics of globular proteins (Fig. 1K).
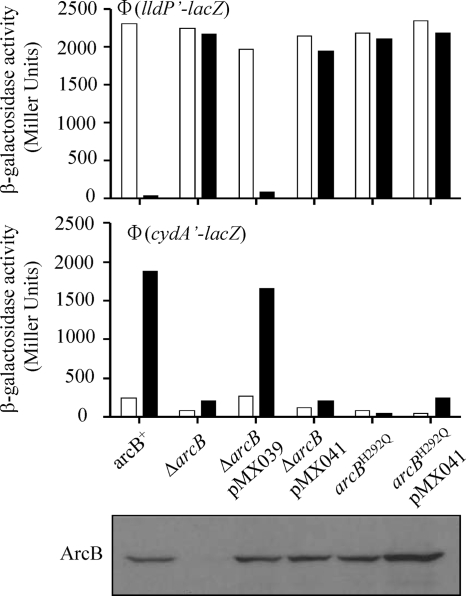
To verify the in vitro results by in vivo experiments, a plasmid-borne arcB1-778,G470A,G472A mutant allele (pMX041) was coexpressed with a chromosomal arcB1-778,H292Q mutant allele (19) and their ability to complement each other was analyzed by monitoring the in vivo levels of phosphorylated ArcA, as indicated by the expression of the λΦ(lldP′-lacZ) and λΦ(cydA′-lacZ) target operons. The lldP′-lacZ and cydA′-lacZ target operons were chosen as an ArcA-P-repressible reporter and as an ArcA-P-activatable reporter, respectively. To construct plasmid pMX041, we first created plasmid pMX712 by cloning the BamHI-HindIII fragment from plasmid pIBW (20), which carries the arcB promoter, the arcB ribosomal binding site, an introduced NdeI site that includes the initiation codon of arcB, and the arcB open reading frame (ORF) and stop codon into pBlueScript II KS+. Subsequently, the MluI-HindIII fragment of pMX025 was used to replace the MluI-HindIII wild-type ArcB fragment of pMX712, generating plasmid pMX040. Finally, the BamHI-HindIII fragment of pMX712 and pMX040 was cloned into low-copy-number plasmid pEXT21 (5) to generate plasmids pMX039 (carrying the wild-type arcB allele) and pMX041 (carrying the arcB1-778,G470A,G472A mutant allele), respectively.
The generated plasmids were transformed into the ΔarcB mutant strains ECL5004 [carrying a λΦ(cydA′-lacZ) operon fusion] and ECL5012 [carrying a λΦ(lldP′-lacZ) operon fusion] and the arcB1-778,H292Q mutant strains ECL5022 and ECL5030 carrying λΦ(cydA′-lacZ) and λΦ(lldP′-lacZ) operon fusions, respectively (19). The transformants were grown aerobically or anaerobically in buffered Luria-Bertani broth containing 100 mM MOPS (morpholinepropanesulfonic acid), pH 7.4, and 20 mM d-xylose. In the case of the λΦ(lldP′-lacZ)-bearing strains, the above-described medium was supplemented with 20 mM l-lactate as the inducer (3). At an optical density at 600 nm (OD600) of ∼ 0.5, the cultures were harvested and β-galactosidase activity was determined (Fig. 2). As expected, plasmid pMX039, carrying the wild-type arcB allele, complemented the ΔarcB mutant strains, restoring regulation of reporter expression (Fig. 2A and B). On the other hand, an arcB-null phenotype was found when the pMX041-borne ArcB1-778,G470A,G472A mutant protein was expressed in either a ΔarcB or an arcB1-778,H292Q mutant background (Fig. 2A and B). Western blot analysis of the cell extracts with polyclonal antiserum raised against purified His6-ArcB78−520 showed that the plasmid-borne arcB alleles did produce wild-type levels of ArcB (Fig. 2C). Thus, the two defective ArcB proteins, when coexpressed in a cell, are not able to complement each other to restore the ArcB kinase activity and regulate reporter expression.
FIG. 2.
Coexpression of arcBG470A,G472A and arcBH292Q does not restore ArcB kinase activity and regulation of reporter expression. The λΦ(lldP′-lacZ)-bearing (top panel) and λΦ(cydA′-lacZ)-bearing (middle panel) strains with the wild-type, ΔarcB, pMX039 (carrying an arcB wild-type allele)-complemented ΔarcB, pMX041 (carrying an arcBG470A,G472A mutant allele)-complemented ΔarcB, arcBH292Q, and pMX041-complemented arcBH292Q genetic backgrounds were grown in Luria-Bertani broth containing 0.1 M MOPS (pH 7.4) and 20 mM d-xylose. In the case of the λΦ(cydA′-lacZ)-bearing strains, the medium was supplemented with 20 mM l-lactate as the inducer (3). For aerobic growth, cells were cultured in 5 ml of medium in 250-ml baffled flasks at 37°C with shaking (300 rpm). For anaerobic growth, cells were cultured in a screw-cap tube filled with medium up to the rim at 37°C and stirred by a magnet. At the mid-exponential growth phase (OD600, ∼0.5) β-galactosidase activity was assayed and expressed in Miller units (26). Empty bars represent aerobic growth, and solid bars represent anaerobic growth. The data are averages from four independent experiments (variations were less than 10% of the mean). (Bottom panel) Western blot analysis. A 1-ml sample of the above-described aerobic cultures was harvested at an OD600 of 0.5. The pelleted cells were solubilized by incubation at 95°C for 10 min in 100 μl of 4× SDS sample buffer. Samples of 5 μl were subjected to electrophoresis in an SDS-8% polyacrylamide gel, and the resolved proteins were electrotransferred to a Hybond-ECL filter (Amersham). Immunoblot analyses were subsequently performed using ArcB polyclonal antibodies as previously described (20).
Increasing amounts of ArcBG* are not able to quench ArcB autophosphorylation.
To provide independent evidence in support of the above conclusion, we argued that if increasing amounts of ArcBG* were added to phosphorylation reaction mixtures of ArcB78-520 (10), heterodimer formation should be favored, resulting in a decline of the ArcB78-520-P levels if an intermolecular autophosphorylation were involved. Also, the phosphoryl group of the ATP bound on ArcB78-520 should be transferred to His292 of ArcBG* and ArcBG*-P should be detected. On the other hand, no change in ArcB78-520-P should be observed and no ArcBG*-P should be detected if an intramolecular autophosphorylation were involved.
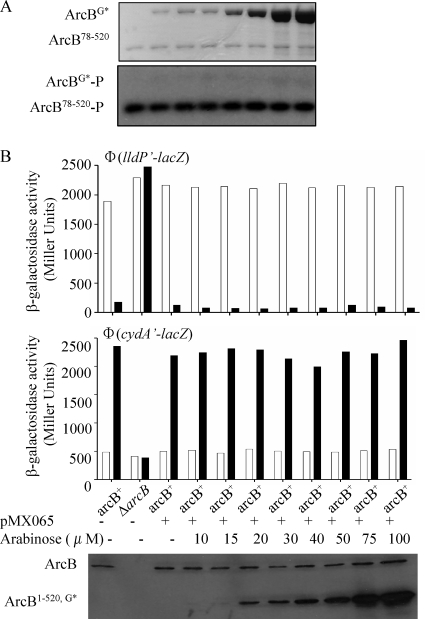
To distinguish between the two possibilities, ArcB78-520 (10) and ArcBG* were purified and used in phosphorylation reaction mixtures with [γ-32P]ATP. ArcB78-520 (the primary transmitter domain) was chosen because it contains the elements necessary for autophosphorylation but not the receiver domain, which is known to have an associated phosphatase activity and rapidly loses the phosphoryl group from Asp576 (6, 10). The purified ArcB78-520 protein (2 pmol) was incubated in nine phosphorylation reaction mixtures containing various amounts of ArcBG* (0 to 200 pmol) for 10 min at room temperature before [γ-32P]ATP was added. Each reaction was carried out for 1 min, terminated by the addition of an equal volume of 4× sodium dodecyl sulfate (SDS) sample buffer, and immediately subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (Fig. 3A). As expected, ArcB78-520 was rapidly phosphorylated. However, the presence of ArcBG*, even at a 100-fold excess, failed to affect the autophosphorylation level of ArcB78-520 (Fig. 3A). Also, no ArcBG*-P was detected (Fig. 3A).
FIG. 3.
Effect of increasing concentrations of ArcBG* on the autophosphorylation of ArcB. (A) Purified ArcB78-520 (∼2 pmol) was incubated with various concentrations of ArcBG* (0 to 200 pmol) in 10-μl reaction mixtures for 10 min. [γ-32P]ATP was added to the reaction mixtures, and after 1 min, the reactions were stopped by the addition of an equal volume of 4× SDS sample buffer and analyzed by SDS-PAGE. The Coomassie blue-stained gel revealing protein bands is at the top, and the autoradiogram is at the bottom. (B) Strains ECL5001 (top) and ECL5002 (middle), carrying λΦ(cydA′-lacZ) and λΦ(lldP′-lacZ), respectively, were transformed with plasmid pMX065 (carrying an arabinose-inducible arcB1-520,G470A,G472A allele) and grown as described above to an OD600 of ∼0.12. The cultures were split among 10 flasks containing the indicated concentrations of arabinose, and part of each culture was transferred to a screw-cap tube (filled to the rim) for anaerobic growth. At the mid-exponential growth phase (OD600, ∼0.5) β-galactosidase activity was assayed and expressed in Miller units. Empty bars represent aerobic growth, and solid bars represent anaerobic growth. The data are averages from four independent experiments (variations were less than 10% of the mean). (Bottom) Western blot analysis of the above-described aerobic cultures using ArcB polyclonal antibodies was done as described in the legend to Fig. 2.
To verify the above result in vivo, plasmid pMX065, carrying an arabinose-induced arcB1-520,G470A,G472A mutant allele, was transformed into arcB+ strains ECL5001 and ECL5002 harboring, respectively, the λΦ(cydA′-lacZ) and λΦ(lldP′-lacZ) reporter fusions. To construct this plasmid, we first generated plasmid pMX517 by replacing the NdeI-HindIII fragment of plasmid pMX020 (28) with the NdeI-HindIII fragment of plasmid pMX712, placing the arcB1-778 wild-type allele under the control of the arabinose promoter. Subsequently, the PstI-HincII ArcB fragment of pMX025, carrying the G470A and G472A mutations, was used to replace the PstI-HincII fragment of pQE30ArcB78-520 (10), generating plasmid pMX064. Finally the NcoI-HindIII fragment of pMX064 was used to replace the NcoI-HindIII fragment of pMX517, generating pMX065, in which the arcB1-520,G470A,G472A mutant allele is under the control of the arabinose promoter.
The transformants were grown aerobically or anaerobically in the presence of various concentrations of arabinose (0 to 100 μM), and at an OD600 of ∼0.5, the cultures were harvested and the β-galactosidase activities were determined (Fig. 3). Western blot analysis of the cell extracts with polyclonal antiserum raised against purified His6-ArcB78-520 showed that the plasmid-borne arcB1-520,G470A,G472A allele produced increasing amounts of protein with increasing amounts of arabinose (Fig. 3C). In agreement with the in vitro result, the increasing amounts of the mutant ArcB protein did not affect the aerobic/anaerobic expression of either reporter (Fig. 3B), providing further support to the intramolecular mode of ArcB autophosphorylation.
Thus, both the in vivo and in vitro results of two independent experimental approaches indicate that ArcB, in contrast to most homodimeric histidine kinases, autophosphorylates through an intramolecular reaction, requiring the ATP-binding site and the site of autophosphorylation (His292) to be present in the same ArcB molecule.
Acknowledgments
We thank Claudia Rodriguez and Javier de la Mora for technical assistance and the Unidad de Biología Molecular of the Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, for oligonucleotide synthesis and sequencing.
This work was supported by grants 37342-N from the Consejo Nacional de Ciencia y Tecnología (CONACyT) and IN221106/17 from DGAPA-PAPIIT, UNAM.
Footnotes
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Casino, P., V. Rubio, and A. Marina. 2009. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 139:325-336. [DOI] [PubMed] [Google Scholar]
- 2.Cotter, P. A., and A. M. Jones. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11:367-373. [DOI] [PubMed] [Google Scholar]
- 3.Dong, J. M., J. S. Taylor, D. J. Latour, S. Iuchi, and E. C. Lin. 1993. Three overlapping lct genes involved in l-lactate utilization by Escherichia coli. J. Bacteriol. 175:6671-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 5.Dykxhoorn, D. M., R. St Pierre, and T. Linn. 1996. A set of compatible tac promoter expression vectors. Gene 177:133-136. [DOI] [PubMed] [Google Scholar]
- 6.Georgellis, D., O. Kwon, P. De Wulf, and E. C. Lin. 1998. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem. 273:32864-32869. [DOI] [PubMed] [Google Scholar]
- 7.Georgellis, D., O. Kwon, and E. C. Lin. 1999. Amplification of signaling activity of the Arc two-component system of Escherichia coli by anaerobic metabolites. An in vitro study with different protein modules. J. Biol. Chem. 274:35950-35954. [DOI] [PubMed] [Google Scholar]
- 8.Georgellis, D., O. Kwon, and E. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 9.Georgellis, D., O. Kwon, E. C. Lin, S. M. Wong, and B. J. Akerley. 2001. Redox signal transduction by the ArcB sensor kinase of Haemophilus influenzae lacking the PAS domain. J. Bacteriol. 183:7206-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgellis, D., A. S. Lynch, and E. C. Lin. 1997. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J. Bacteriol. 179:5429-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igo, M. M., A. J. Ninfa, J. B. Stock, and T. J. Silhavy. 1989. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 3:1725-1734. [DOI] [PubMed] [Google Scholar]
- 12.Ishige, K., S. Nagasawa, S. Tokishita, and T. Mizuno. 1994. A novel device of bacterial signal transducers. EMBO J. 13:5195-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iuchi, S., D. C. Cameron, and E. C. Lin. 1989. A second global regulator gene (arcB) mediating repression of enzymes in aerobic pathways of Escherichia coli. J. Bacteriol. 171:868-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iuchi, S., and E. C. Lin. 1988. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. U. S. A. 85:1888-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iuchi, S., and E. C. Lin. 1992. Mutational analysis of signal transduction by ArcB, a membrane sensor protein responsible for anaerobic repression of operons involved in the central aerobic pathways in Escherichia coli. J. Bacteriol. 174:3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iuchi, S., and E. C. Lin. 1992. Purification and phosphorylation of the Arc regulatory components of Escherichia coli. J. Bacteriol. 174:5617-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin, S. G., R. K. Prusti, T. Roitsch, R. G. Ankenbauer, and E. W. Nester. 1990. Phosphorylation of the VirG protein of Agrobacterium tumefaciens by the autophosphorylated VirA protein: essential role in biological activity of VirG. J. Bacteriol. 172:4945-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung, W. S., Y. R. Jung, D. B. Oh, H. A. Kang, S. Y. Lee, M. Chavez-Canales, D. Georgellis, and O. Kwon. 2008. Characterization of the Arc two-component signal transduction system of the capnophilic rumen bacterium Mannheimia succiniciproducens. FEMS Microbiol. Lett. 284:109-119. [DOI] [PubMed] [Google Scholar]
- 19.Kwon, O., D. Georgellis, and E. C. Lin. 2000. Phosphorelay as the sole physiological route of signal transmission by the arc two-component system of Escherichia coli. J. Bacteriol. 182:3858-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon, O., D. Georgellis, A. S. Lynch, D. Boyd, and E. C. Lin. 2000. The ArcB sensor kinase of Escherichia coli: genetic exploration of the transmembrane region. J. Bacteriol. 182:2960-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, X., and P. De Wulf. 2004. Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J. Biol. Chem. 279:12588-12597. [DOI] [PubMed] [Google Scholar]
- 22.Lynch, A. S., and E. C. Lin. 1996. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J. Bacteriol. 178:6238-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino, K., H. Shinagawa, M. Amemura, T. Kawamoto, M. Yamada, and A. Nakata. 1989. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J. Mol. Biol. 210:551-559. [DOI] [PubMed] [Google Scholar]
- 24.Malpica, R., B. Franco, C. Rodriguez, O. Kwon, and D. Georgellis. 2004. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc. Natl. Acad. Sci. U. S. A. 101:13318-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malpica, R., G. R. Sandoval, C. Rodriguez, B. Franco, and D. Georgellis. 2006. Signaling by the Arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid. Redox Signal. 8:781-795. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 27.Ninfa, E. G., M. R. Atkinson, E. S. Kamberov, and A. J. Ninfa. 1993. Mechanism of autophosphorylation of Escherichia coli nitrogen regulator II (NRII or NtrB): trans-phosphorylation between subunits. J. Bacteriol. 175:7024-7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peña-Sandoval, G. R., O. Kwon, and D. Georgellis. 2005. Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo. J. Bacteriol. 187:3267-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pernestig, A. K., O. Melefors, and D. Georgellis. 2001. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J. Biol. Chem. 276:225-231. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, C., O. Kwon, and D. Georgellis. 2004. Effect of d-lactate on the physiological activity of the ArcB sensor kinase in Escherichia coli. J. Bacteriol. 186:2085-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmon, K. A., S. P. Hung, N. R. Steffen, R. Krupp, P. Baldi, G. W. Hatfield, and R. P. Gunsalus. 2005. Global gene expression profiling in Escherichia coli K12: effects of oxygen availability and ArcA. J. Biol. Chem. 280:15084-15096. [DOI] [PubMed] [Google Scholar]
- 32.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 33.Surette, M. G., M. Levit, Y. Liu, G. Lukat, E. G. Ninfa, A. Ninfa, and J. B. Stock. 1996. Dimerization is required for the activity of the protein histidine kinase CheA that mediates signal transduction in bacterial chemotaxis. J. Biol. Chem. 271:939-945. [DOI] [PubMed] [Google Scholar]
- 34.Swanson, R. V., R. B. Bourret, and M. I. Simon. 1993. Intermolecular complementation of the kinase activity of CheA. Mol. Microbiol. 8:435-441. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka, T., S. K. Saha, C. Tomomori, R. Ishima, D. Liu, K. I. Tong, H. Park, R. Dutta, L. Qin, M. B. Swindells, T. Yamazaki, A. M. Ono, M. Kainosho, M. Inouye, and M. Ikura. 1998. NMR structure of the histidine kinase domain of the E. coli osmosensor EnvZ. Nature 396:88-92. [DOI] [PubMed] [Google Scholar]
- 36.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 37.Yang, Y., and M. Inouye. 1991. Intermolecular complementation between two defective mutant signal-transducing receptors of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 88:11057-11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhulin, I. B., B. L. Taylor, and R. Dixon. 1997. PAS domain S-boxes in Archaea, Bacteria and sensors for oxygen and redox. Trends Biochem. Sci. 22:331-333. [DOI] [PubMed] [Google Scholar]