Abstract
In this work, we report that flavohemoglobin contributes to the azole susceptibility of Staphylococcus aureus. We first observed that deletion of the flavohemoglobin gene leads to an increase in the viability of imidazole-treated S. aureus cells and that reversion to the wild-type phenotype occurs upon expression of flavohemoglobin from a multicopy plasmid. Further spectroscopic analyses showed that miconazole, the most efficient azole antibiotic against S. aureus, ligates to heme of both oxidized and reduced flavohemoglobin. The binding of miconazole to oxidized flavohemoglobin, with an association constant of 1.7 × 106 M−1, typical of a tight, specific binding equilibrium, results in augmentation of the superoxide production by the enzyme. These results are corroborated by in vivo studies showing that imidazole-treated S. aureus cells expressing flavohemoglobin contain a larger amount of reactive oxygen species. Moreover, it was observed that the survival of miconazole-treated S. aureus internalized by murine macrophages is higher for cells lacking flavohemoglobin. Altogether, the present data revealed that in S. aureus, flavohemoglobin enhances the antimicrobial activity of imidazoles via an increase of intracellular oxidative stress.
Staphylococcus aureus is an opportunistic pathogen responsible for a large number of human infections that cause systemic diseases from a mild to life-threatening character. The increasing incidence of methicillin-resistant S. aureus (MRSA) strains observed in the past few years makes S. aureus infections a leading threat to public health, causing more deaths in the United States and Europe than human immunodeficiency virus (AIDS) (11). Like other Gram-positive bacteria, staphylococci are sensitive to imidazoles (27). Imidazoles (such as clotrimazole, miconazole, ketoconazole, and sulconazole) (Fig. 1) represent one of the major classes of azole antifungal that are useful in the treatment of infections, including cutaneous and vaginal candidiasis (8). The activity of these antifungal drugs derives primarily from inhibition of the biosynthesis of ergosterol, an essential component of the fungal plasma membrane, at the level of lanosterol 14-alpha demethylase. Furthermore, in fungi and yeast, azole treatment leads to an increase in the endogenous production of reactive oxygen species (ROS) (12, 25). For example, in Candida albicans and Saccharomyces cerevisiae, the miconazole inhibition of cytochrome c oxidase, peroxidase, and catalase has been reported to be responsible for a high level of ROS production (3, 4). It has also been reported that clotrimazole inhibition of Plasmodium falciparum hemoperoxidase leads to ROS accumulation in this protozoan pathogen (26). For S. cerevisiae, C. albicans, and Escherichia coli, the action of imidazoles was also correlated with the inhibition of the nitric oxide (NO) scavenger activity of flavohemoglobin (7).
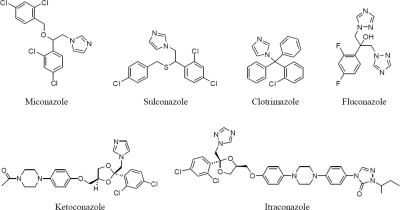
FIG. 1.
Structures of the azole (imidazole; 1,2,4-triazole) antibiotics investigated.
Flavohemoglobins (Hmp) are widespread among bacteria and yeast and contain three domains: C-terminal NAD- and flavin adenine dinucleotide (FAD)-binding domains, which together constitute a ferredoxin-NADP+ oxidoreductase-like domain, and an N-terminal globin domain, which harbors a single B-type heme. The high-spin heme contains one axial histidine and binds small molecules like NO, carbon monoxide (CO), and dioxygen (O2). The heme can also bind bulky aromatic bases, since it is inserted in a large hydrophobic pocket (7). We observed that the binding of imidazoles to S. aureus flavohemoglobin results in an increase in the amount of deleterious reactive oxygen species produced by flavohemoglobin that contributes to the bactericidal effect of azole antibiotics toward S. aureus.
MATERIALS AND METHODS
Reagents.
Miconazole, sulconazole, clotrimazole, ketoconazole, and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) were purchased from Sigma, and 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO) was purchased from Northwest. All of the reagents were dissolved in dimethyl sulfoxide (DMSO), except for ketoconazole and BMPO, which were prepared in methanol and water, respectively. For the UV-visible and resonance Raman (RR) spectroscopic studies, a water-saturated solution of miconazole was used, to avoid the effect of DMSO on the reduced form of flavohemoglobin.
Bacterial strains, culture conditions, and viability assays.
Overnight cultures of S. aureus wild-type (RN4220) and S. aureus Δhmp (LMS800) cells (6) grown in tryptic soy broth (TSB) medium were used to inoculate, to an OD600 of 0.1, Luria-Bertani (LB) medium supplemented with the appropriate antibiotics (2 μM miconazole, 5 μM sulconazole, 12 μM clotrimazole, and 120 μM ketoconazole) and contained in closed flasks. For control purposes, untreated cultures, in which an equal volume of the corresponding antibiotic's solvent was added, were also analyzed. S. aureus viability was then evaluated after a 5-h treatment of the liquid cultures with antibiotic, and the number of viable cells was determined by measuring the CFU per milliliter upon plating 5 μl of each dilution on agar and counting the isolated colonies formed after overnight incubation. The percentage of survival was calculated as the number of cells originated by the treated cultures divided by the number of colonies formed after plating the control cultures.
MICs of the azole antibiotics were determined on 24-well microtiter plates as previously described (19). Assays were conducted in LB medium at 37°C, performed in triplicate and repeated at least twice.
Complementation studies.
For the complementation analysis, a vector expressing S. aureus hmp was constructed. To this end, a fragment containing the complete S. aureus hmp gene was amplified, using oligonucleotides SAHmpFw (5′-TCACATTTTTATTATCATGTTTACTTTTTTCTAGGA-3′) and SAHmpEcoRI (5′-CGTTGATTAAGTTTCATATGAGCACTAATTCTCTTT-3′), and ligated to pMK4 (24). The resulting vector (pHmp) and the empty vector (pMK4) were electroporated into S. aureus Δhmp mutant and wild-type (RN4220) strains. For the mutant strain, the positive transformants were selected on trypticase soy agar (TSA) medium containing 10 μg/ml erythromycin plus 5 μg/ml chloramphenicol, while selection of the wild type was achieved using only chloramphenicol. Cell growth was performed in liquid medium treated with miconazole for 5 h, as described above, and analyzed by serial dilutions plated on agar.
Spectroscopic studies: UV-visible, resonance Raman, and EPR.
S. aureus flavohemoglobin was cloned, expressed and purified as described previously (18). UV-visible spectra were recorded on a Shimadzu UV-1700 spectrophotometer, using 10 μM flavohemoglobin in 10 mM Tris-HCl (pH 7.6) buffer containing 9% glycerol.
Electron paramagnetic resonance (EPR) spectra were obtained on a Bruker EMX spectrometer equipped with an Oxford Instruments continuous-flow helium cryostat. 5-tert-Butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO) was used as a spin trap for the detection of reactive oxygen species, which allows us to distinguish between superoxide anion and the hydroxyl radical (30). These experiments were performed using 10 μM S. aureus flavohemoglobin, 200 μM NADH, 25 mM BMPO, and 50 μM miconazole, in a quartz flat cell, at room temperature.
Resonance Raman spectra were measured using a confocal microscope coupled to a Raman spectrometer (Jobin Yvon U1000) equipped with 1,200 lines/mm grating and a liquid nitrogen-cooled back-illuminated charge-coupled device (CCD) detector. Samples of flavohemoglobin (20 μM) were placed in a quartz rotating cell and excited with the 413-nm line of a krypton ion laser (Coherent Innova 302), with a laser power of 2 to 4 mW and accumulation times of 60 s. After polynomial background subtraction, the positions and line widths of the Raman bands were determined by component analysis using in-house software.
Enzymatic studies.
The equilibrium constant for miconazole binding was determined by titrating a fixed amount of flavohemoglobin with increasing quantities of the antibiotic and monitoring the changes in absorbance in the visible region. The amount of miconazole-protein complex was calculated using a differential absorptivity at 418 − 500 nm, determined from the difference of a spectrum of a solution having excess antibiotic (thus ensuring full complex formation) and the spectrum of the oxidized, isolated protein. A value of Δɛ (418 − 500) = 69,565 M−1 cm−1 was obtained; from this value, the amount of complex at each solution could be determined, and by using the appropriate mass balance equations, the concentrations of free antibiotic and free protein were also calculated. The number of binding sites and the equilibrium constant were then determined by a Scatchard equation (17). The same procedure could not be applied with precision to the reduced protein, due to the interference of DMSO; nevertheless, a lower limit for the binding constant could be determined using a differential absorptivity at 426 − 390 nm. The assays were performed with 5 μM S. aureus flavohemoglobin, and the miconazole concentrations varied between 2 μM and 60 μM. The percentage of DMSO used in all the assays was 0.4% (vol/vol).
Measurement of endogenous ROS production.
Endogenous ROS production was determined by a fluorometric assay according to the method described previously (12). Cells of wild-type S. aureus (RN4220) and the Δhmp (LMS800) mutant were grown for 5 h, in the absence or presence of azoles. Cells were then collected by centrifugation, washed, and resuspended in phosphate-buffered saline (PBS), followed by the addition of 10 μM DCFH-DA. The fluorescence intensities (FIs) were measured on a Varian Eclipse 96-well spectrofluorimeter (excitation at 485 nm and emission at 538 nm). The FIs were normalized in relation to the final OD600 of each culture. To assess the variation of ROS, the FI of control cultures and the FI of azole-treated cultures were subtracted.
Quantitative real-time RT-PCR.
For real-time reverse transcription (RT)-PCR experiments, 2.0 μg of S. aureus total RNA derived from samples grown in LB and treated with 2 μM miconazole for 5 h was used to synthesize cDNA, according to the Transcriptor high-fidelity cDNA synthesis kit protocol (Roche Applied Science). Real-time PCRs were performed in a LightCycler Instrument using LightCycler FastStart DNA master SYBR green I kit according to the manufacturer's instructions (Roche Applied Science). The amplification reactions were carried out with equal amounts of cDNA (100 ng) as the initial template, and each reaction mixture contained 0.5 μM specific primers, 2 mM MgCl2, and the hot-start PCR mix from Roche Applied Science. The expression ratio of the target gene was determined relatively to a reference gene, S. aureus 16S rRNA, whose transcription abundance remains invariant under the tested conditions. The samples were assayed in triplicate.
Assay of intracellular S. aureus viability in J774A.1 macrophages.
Murine macrophages J774A.1 (LGC Promochem) were inoculated with 5 × 105 cells/ml and cultured for 2 days at 37°C in a 5% CO2-air atmosphere in 24-well plates containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 4.5 g/liter glucose, 110 mg/ml sodium pyruvate (DMEM Glutamax), 10% fetal bovine serum, 100 μM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin (all from GIBCO). Prior to infection, macrophages were activated for 5 h with 1 μg/ml gamma interferon (IFN-γ; Sigma) and 5 μg/ml lipopolysaccharides (LPS; Sigma). When required, 800 μM NG-monomethyl-l-arginine acetate salt (L-NMMA; Sigma) was also added to achieve inhibition of the murine macrophage inducible NO synthase (iNOS). S. aureus wild-type and Δhmp strain cells were grown for 5 h in the presence or absence of miconazole (2 μM), washed three times with PBS, and resuspended in DMEM, to obtain for all cultures an initial bacterial concentration of 107 CFU/ml. Macrophages were then infected with these bacterial suspensions, at a multiplicity of infection (MOI) of at least 16, for 30 min at 37°C. The supernatants were then collected to determine the number of bacteria not internalized. Extracellular bacteria were killed by incubation in DMEM supplemented with 50 U/ml penicillin and 50 μg/ml streptomycin for 5 min, and the wells were washed three times with PBS. After that, macrophages were lysed with 2% saponin, and the number of intracellular bacteria was determined by CFU counting of viable bacteria.
RESULTS
S. aureus is susceptible to azole antibiotics.
The susceptibility of S. aureus to several azole antibiotics was analyzed. For S. aureus RN4220, the MICs were of the same order of magnitude for miconazole (15 ± 2 μM), sulconazole (20 ± 0 μM), and clotrimazole (30 ± 3 μM), while a much higher value was determined for ketoconazole (500 ± 70 μM). In previous work, S. aureus viability was also reported to decrease significantly with miconazole, while essentially no effect (>200 μM) was observed with ketoconazole (23). Additionally, we observed that S. aureus is resistant to concentrations of the triazole antibiotics fluconazole and itraconazole (Fig. 1) up to 2 mM.
S. aureus susceptibility to azoles involves flavohemoglobin.
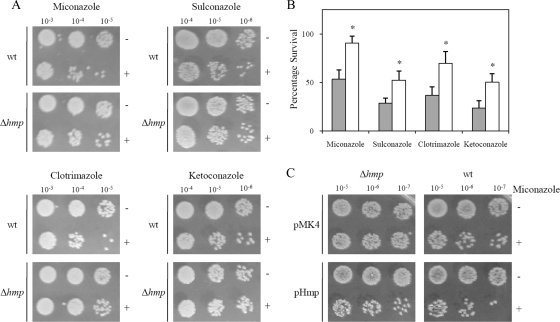
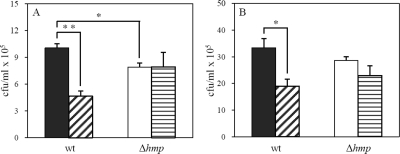
We next investigated the possible role of flavohemoglobin in the sensitivity of S. aureus to imidazoles. To this end, we compared the viability of S. aureus wild-type and Δhmp mutant cells upon treatment with several azole antibiotics. The results showed that inactivation of flavohemoglobin caused an increase in the resistance of S. aureus to imidazoles: i.e., the mutant strain produced higher number of viable cells, a result which was observed for all imidazoles tested (Fig. 2A and B). To confirm that imidazole resistance was a specific consequence of flavohemoglobin gene deletion, the viability of the Δhmp strain expressing flavohemoglobin (from a multicopy plasmid) treated with miconazole was evaluated. As expected, upon complementation, reversion to the wild-type phenotype was observed (Fig. 2C). Furthermore, the overexpression of S. aureus flavohemoglobin in the wild-type strain led to an increase in the sensitivity to imidazoles (Fig. 2C). These results show that flavohemoglobin contributes to the activity of imidazoles against S. aureus, independently of any other stress agent.
FIG. 2.
Flavohemoglobin contributes to imidazole sensitivity of S. aureus. (A) Cell viability of S. aureus wild-type (wt) and Δhmp mutant cells in the absence (−) and in the presence (+) of 2 μM miconazole, 5 μM sulconazole, 12 μM clotrimazole, and 120 μM ketoconazole. (B) The number of viable cells was evaluated by measurement of CFU per milliliter, and the percentage of survival was calculated dividing the number of colonies of treated cultures by that of control cultures (*, P < 0.05). (C) Viability of S. aureus Δhmp and wild-type cells transformed with pHmp and pMK4, untreated (−) and treated (+) with miconazole (2 μM).
To understand the mechanism by which flavohemoglobin affects the susceptibility of S. aureus to imidazoles, we analyzed the binding of miconazole, the most active commercially available azole antibiotic against S. aureus, to flavohemoglobin by UV-visible, EPR, and resonance Raman (RR) spectroscopy.
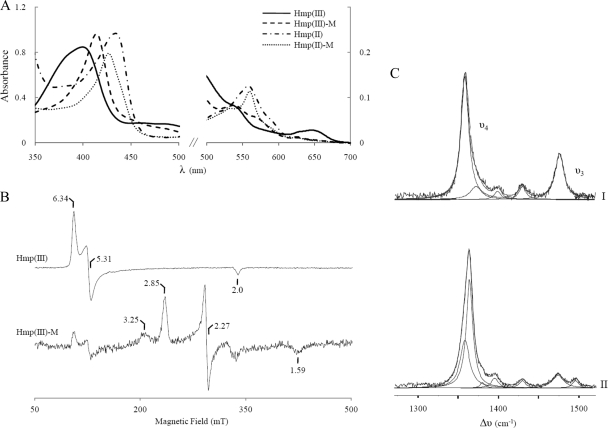
The UV-visible absorption spectra of S. aureus flavohemoglobin in the oxidized and reduced states displayed the characteristic features of high-spin ferric and ferrous B-hemes, respectively (Fig. 3A). Upon addition of miconazole, the spectra of both redox forms changed considerably, indicating binding of the antibiotic to the heme and formation of 6-coordinated, low-spin (6cLS) forms. Miconazole shifts the Soret band of the oxidized form to 414 nm, and a broad band at 538 nm appears; also, the charge-transfer band at 645 nm, characteristic of high-spin hemes, bleaches completely. In the reduced form, the Soret band shifts to 427 nm and bands at 531 and 560 nm are clearly distinguished (Fig. 3A).
FIG. 3.
Spectroscopic analysis of miconazole binding to S. aureus flavohemoglobin. (A) UV-visible spectra of 10 μM oxidized Hmp [Hmp(III)], after binding of miconazole (M) to the ferric protein [Hmp(III)-M] of dithionite-reduced Hmp [Hmp(II)] and upon addition of miconazole to ferrous Hmp [Hmp(II)-M]. (B) EPR spectra of S. aureus Hmp [Hmp(III)] and of flavohemoglobin treated with miconazole [Hmp(III)-M]. At 16 K, spectra were obtained at 9.4-GHz, 2.0-mW microwave power, 1-mT modulation amplitude, and 100-kHz modulation frequency. The spectrum of Hmp(III) shown was divided by 5. (C) RR spectra of flavohemoglobin. I, reduced flavohemoglobin; II, reduced flavohemoglobin upon addition of miconazole. All RR spectra were measured with 413-nm excitation and 20 μM flavohemoglobin (in 10 mM Tris HCl, pH 7.6), in the presence or absence of miconazole, at ambient temperature with a laser power of 2 mW and accumulation times of 60 s.
The binding of the imidazole antibiotic to S. aureus flavohemoglobin was also studied by EPR spectroscopy. In the absence of the antibiotic, the EPR spectrum of oxidized flavohemoglobin exhibits resonances characteristic of a high-spin ferric heme, with g = 6.34, 5.31, and 2.0. Upon addition of miconazole, the high-spin signature almost disappears, being replaced by a set of resonances indicative of the formation of a low-spin ferric heme in a rhombic ligand field, with g values at 2.85, 2.27, and 1.59, compatible with a ligation by two imidazoles from the axial histidine and from miconazole (Fig. 3B). A lower-intensity signal is also observed at gmax = 3.25, which suggests the presence of a second low-spin ferric heme configuration, with a more axial ligand field. The rhombic species (the one with gmax = 2.85) corresponds to a geometry where the two imidazole planes are essentially parallel, while the more axial one (gmax = 3.25) reflects a situation in which the dihedral angle between those two planes is higher (21).
The UV-visible and EPR data show that miconazole binds to the oxidized flavohemoglobin heme, yielding a low-spin ferric species, as expected for ligation through imidazole nitrogens. These results were confirmed by resonance Raman (RR) spectroscopy since the spectra of hemes include marker bands that are sensitive to the oxidation, coordination, and spin state of the heme iron (9, 22, 29), for both paramagnetic and diamagnetic species. We first investigated the ferric protein, in the presence or absence of miconazole. RR spectra of oxidized flavohemoglobin are characteristic of a 5-coordinated, high-spin (5cHS) configuration, with the ν4 andν3 vibrational modes at 1,370 cm−1 and 1,494 cm−1, respectively. This form undergoes a change of spin state upon miconazole binding, as revealed by the shift of the ν3 band to 1,505 cm−1, characteristic of a 6-coordinated, low-spin (6cLS) ferric heme (Table 1). The reduced S. aureus flavohemoglobin is in a 5cHS configuration, with ν4 at 1,357 cm−1 and ν3 at 1,474 cm−1 (Fig. 3C, spectrum I). Component analysis of the spectrum obtained upon addition of miconazole revealed the presence of two species: 5-coordinated ferrous heme, as in the pure protein, and a major component with ν4 upshifted to 1,364 cm−1 (Fig. 3C, spectrum II; Table 1), indicating the formation of a 6-coordinated, low-spin ferrous form. Moreover, since the ν4 band upshifts by 7 cm−1 (in comparison with the ν4 of the unbound ferrous flavohemoglobin), it is apparent that delocalization of the electron cloud from the heme to the miconazole takes place upon binding of the antibiotic (22).
TABLE 1.
Positions of the marker bands ν4 and ν3 in the resonance Raman spectra of oxidized and reduced flavohemoglobin, unbound or bound to miconazole
| Flavohemoglobin species | Position of marker band in spectrum (cm−1) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 5cHS |
6cLS |
|||||||
| Oxidized |
Reduced |
Oxidized |
Reduced |
|||||
| ν4 | ν3 | ν4 | ν3 | ν4 | ν3 | ν4 | ν3 | |
| Oxidized | ||||||||
| Hmp(III) | 1,370 | 1,494 | ||||||
| Hmp(III)-miconazole | 1,372 | 1,505 | ||||||
| Reduced | ||||||||
| Hmp(II) | 1,357 | 1,474 | ||||||
| Hmp(II)-miconazole | 1,364 | 1,494 | ||||||
We have also observed, by both RR and UV-visible spectroscopy that DMSO binds to the ferrous heme, resulting in a distinct low-spin form of flavohemoglobin.
In summary, the spectroscopic results show that miconazole coordinates to the heme moiety of S. aureus flavohemoglobin in the oxidized as well as in the reduced state.
The binding of miconazole to S. aureus flavohemoglobin was also studied by titrating the protein with different antibiotic concentrations and following the absorption change of the Soret band of the flavohemoglobin after ligation of the antibiotic. Following the procedure described in Materials and Methods, we determined an equilibrium association constant of 1.7 × 106 M−1, indicating that one molecule of miconazole binds tightly to oxidized flavohemoglobin. A lower limit for the association constant for the reduced form was determined to be 1.2 × 105 M−1 (data not shown).
Imidazoles increase the ROS production by flavohemoglobin.
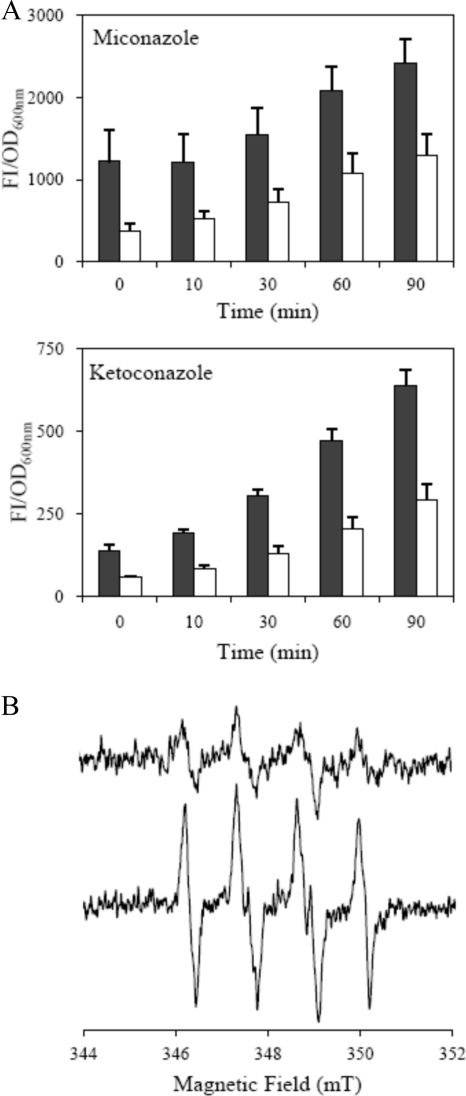
Since, as mentioned before, we detected an increase in imidazole resistance upon inactivation of S. aureus hmp (Fig. 2), we next addressed the origin of this behavior. We first observed that the level of endogenously produced reactive oxygen species augmented in cells of wild-type S. aureus treated with miconazole or ketoconazole (Fig. 4A). More importantly, when the ROS production was evaluated in the Δhmp mutant treated with imidazoles the ROS levels were lower, indicating that the presence of flavohemoglobin contributes to the imidazole-associated ROS generation (Fig. 4A).
FIG. 4.
Imidazoles increase the ROS production by flavohemoglobin. (A) Evaluation of endogenous ROS production of the S. aureus wild-type strain (black bar) and Δhmp mutant (white bar) after treatment with 2 μM miconazole and 120 μM ketoconazole. Each data point represents the average of three independent measurements with the corresponding standard errors. (B) EPR spectra of BMPO-OOH obtained for a sample containing 10 μM Hmp, 200 μM NADH, 25 mM BMPO, and 50 μM miconazole (lower line) and for a sample in which the antibiotic was replaced by an equivalent amount of the antibiotic's solvent (upper line). The spectra were recorded at ambient temperature, at 9.8 GHz, microwave power of 10 mW, and modulation amplitude of 0.1 mT, after 15 min of incubation.
It has been reported that, under certain conditions, E. coli flavohemoglobin produces superoxide ions (16, 28). To investigate the effect of antibiotic binding to flavohemoglobin on radical formation, we used the spin trap BMPO in EPR experiments. In the absence of the antibiotic, when using NADH and under aerobic conditions, the BMPO-OOH adduct was observed (30) being indicative of the formation of superoxide by flavohemoglobin (Fig. 4B, upper line). In the presence of miconazole, the same EPR species was detected but at a significantly higher concentration (Fig. 4B, lower line). By comparing the intensities of the spectra of the BMPO-OOH adduct after 15 min (the time determined to yield the maximum concentration of this adduct), we determined the concentration of BMPO-OOH to be ca. 3-fold higher in samples with miconazole. We thus conclude that not only is superoxide formed by S. aureus flavohemoglobin, since its EPR spectrum is identical to that of the superoxide-BMPO adduct (30), but also that this production increases upon the binding of miconazole to flavohemoglobin. Furthermore, we detected, by mass spectrometry, that the integrity of the imidazole was maintained since no changes occurred in the mass and intensity of the peak of the antibiotic before and after incubation of miconazole with flavohemoglobin in the presence of NADH (data not shown).
Real-time RT-PCR experiments revealed that exposure of S. aureus to miconazole leads to an increase in the expression of the katA gene encoding catalase, a marker of oxidative stress, showing a 11.7 ± 1.4-fold increase in katA expression in the wild-type strain but only a 7.8 ± 0.4-fold increase in expression the hmp mutant: i.e., in the absence of flavohemoglobin, the expression of katA decreased ∼30%. These results are in agreement with a lower production of ROS in the hmp mutant.
Flavohemoglobin decreases survival of miconazole-treated S. aureus in macrophages.
The increased resistance of the S. aureus Δhmp mutant to imidazoles compared to the parent strain (Fig. 2A and B) led us to examine the effect of flavohemoglobin on the survival of miconazole-treated S. aureus cells phagocytized by macrophages. In the absence of the antibiotic, the Δhmp strain was killed more efficiently by activated macrophages (Fig. 5A) due to the lack of the NO detoxifying activity of flavohemoglobin. For miconazole-treated cells, we observed that while incubation in macrophages of antibiotic-treated wild-type cells resulted in a decrease of survival of approximately 50%, the Δhmp cell counts showed no statistical difference between cells unexposed or exposed to miconazole (Fig. 5A). Similar data were obtained in assays performed in the presence of L-NMMA, the mammalian inhibitor of iNOS, which shows that in the presence or absence of NO, flavohemoglobin contributes to the lower survival of azole-treated cells (Fig. 5B). The decreased viability of antibiotic-treated S. aureus in macrophage cell lines can be rationalized taking into consideration that the simultaneous presence of flavohemoglobin and miconazole leads to an increase in the level of deleterious reactive oxygen species, as previously demonstrated by fluorometric and EPR experiments.
FIG. 5.
Intracellular survival in murine macrophages of miconazole-treated S. aureus wild-type (wt) and Δhmp mutant cells. Murine macrophages activated with IFN-γ/LPS (A) and treated with the mammalian iNOS inhibitor L-NMMA (B) were infected with S. aureus wild-type cells in the absence (black bars) and in the presence (diagonal striped bars) of miconazole or incubated with Δhmp cells grown without antibiotic (white bars) or with miconazole (horizontal striped bars). After 30 min of infection, macrophages were lysed and bacterial counts determined. Two independent assays were performed in triplicate, exhibiting the indicated standard errors (**, P < 0.01; *, P < 0.05).
DISCUSSION
In this study, we show that S. aureus is susceptible to several azole antibiotics, with miconazole being, among those tested, the most effective one. The mechanism by which imidazole-treated bacteria undergo growth inhibition was assessed, and the results provide the first evidence that imidazoles induce intracellular ROS production in S. aureus after miconazole and ketoconazole treatment. Remarkably, a strain of S. aureus lacking flavohemoglobin resulted in increased cell viability, while overexpression of flavohemoglobin restored the azole-susceptibility phenotype. These results indicate that flavohemoglobin and imidazoles act together in the killing mechanism.
The binding of miconazole to S. aureus flavohemoglobin was analyzed by UV-visible, EPR, and resonance Raman spectroscopy and revealed that miconazole acts as a strong field heme ligand, since upon miconazole binding the 5-coordinated, high-spin configuration in both oxidation states is converted to a 6-coordinated, low-spin species. Interestingly, the binding of similar-size imidazoles led to similar MIC values, while the larger imidazole ketoconazole exhibits a much higher MIC value. Intriguingly, the two triazoles tested, itraconazole and fluconazole, had no activity against S. aureus. As seen in Fig. 1, this lack of activity cannot solely be related to the size of these molecules since fluconazole is, in fact, the smallest system investigated. Both fluconazole and itraconazole are potent azole antifungals, but rather than having an imidazole side chain, they possess 1,2,4-triazoles. These triazoles do bind well to their P450 targets: since in the P450 enzymes there is an axial cysteine, rather than an axial histidine (as in flavohemoglobin), it seems likely there may simply be large differences between imidazoles and triazoles, binding to P450 or flavohemoglobin, due to electronic effects.
Flavohemoglobins have been reported to have several enzymatic activities, namely, NO dioxygenase (NOD) and alkyl hydroperoxide reductase, as well as production of superoxide (1, 16). We noticed that ligation of imidazoles to S. aureus flavohemoglobin leads to impairment of NOD activity (data not shown), which is in accordance with results described for fungal, yeast, and E. coli flavohemoglobins (7). However, both the susceptibility of wild-type S. aureus to imidazoles and the increase in the resistance of S. aureus to imidazoles upon deletion of the flavohemoglobin gene occur in the absence of any source of nitric oxide. Hence, we tested if binding of imidazoles could interfere with superoxide generation by flavohemoglobin. In fact, EPR spin trap experiments showed that the binding of miconazole magnifies the superoxide production by S. aureus flavohemoglobin. Since the heme is blocked with miconazole, we concluded that the superoxide was generated at the level of the FAD center. In this mechanism, FAD receives electrons from NAD(P)H and reduces oxygen to superoxide. This hypothesis is supported by the ability of several flavin-containing proteins to generate superoxide upon one-electron oxidation by dioxygen (10). Also, it was previously shown that NAD(P)H is oxidized by flavohemoglobin with the electrons transferred via FAD to external acceptors when the ferrous heme is blocked (28).
Therefore, upon ligation of azoles to flavohemoglobin, the S. aureus cells become exposed to larger amounts of deleterious ROS, which explains the lower survival of antibiotic-treated S. aureus wild-type cells and the higher resistance of the Δhmp mutant strain.
Note that in the simultaneous presence of azoles and NO, the higher level of ROS and the inhibition of the NO scavenging activity of flavohemoglobin both will contribute to the more efficient killing of wild-type S. aureus that was indeed detected in activated macrophages (Fig. 5A). Interestingly, the present results may explain the previously observed synergistic antimicrobial action of imidazole antibiotics and NO releasers exerted on Candida species (15), which can be now rationalized considering that in the presence of the two compounds, Candida flavohemoglobin no longer can act as a fungal protective factor.
Finally, we consider future prospects and possible applications. The observation that some azole antifungals also have antibacterial activity is not new, with Janssen et al. (5) reporting as early as 1969 that miconazole had potent (10 nM) activity against Streptococcus hemolyticus (Streptococcus pyogenes). It is also of interest to note that earlier works reported that miconazole killed S. aureus (23) and that cells grown in the presence of miconazole had decreased levels of vitamin K2 and increased levels on octaprenyl diphosphate (2). This would be consistent with targeting MenA biosynthesis, which could also potentially contribute to an increase in ROS levels, and indeed we did observe that in the hmp mutant strain there is still ROS production (Fig. 4A): i.e., even in the absence of flavohemoglobin. So, it is possible that the imidazole antibiotics have more than one target in S. aureus and, potentially, in other bacteria as well.
In S. aureus, the buildup of ROS is of particular interest since S. aureus has a protective golden carotenoid “shield” called staphyloxanthin (20) that protects the bacterium from attack by neutrophil-generated ROS (14). Inhibition of carotenoid biosynthesis (13) results in bacteria that are white (since they lack the carotenoid pigment) and are thus cleared by host defenses. Combining azoles with staphyloxanthin biosynthesis inhibitors may enhance intracellular levels of ROS and NO killing by stripping bacteria of their defenses.
In conclusion, this work revealed for the first time that the binding of azoles to S. aureus flavohemoglobin leads to an increase of the intracellular level of reactive oxygen species, therefore enhancing the antimicrobial activity of these antibiotics.
Acknowledgments
We are grateful to Salomé Gomes and Susana Romão of the Institute for Molecular and Cell Biology, Porto, Portugal, for their support in the manipulation of macrophages. We thank the Mass Spectrometry Laboratory, Analytical Services Unit of ITQB-UNL, for the mass spectrometry studies.
FCT projects POCI/SAU-IMI/56088/2004 and PTDC/BIA-PRO/67263/2006 financed the present work. L.S.N. is the recipient of the Ph.D. grant FCT-SFRH/BD/22425/2005. E.O. was supported by NIH grant AI074233.
Footnotes
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Bonamore, A., P. Gentili, A. Ilari, M. E. Schinina, and A. Boffi. 2003. Escherichia coli flavohemoglobin is an efficient alkylhydroperoxide reductase. J. Biol. Chem. 278:22272-22277. [DOI] [PubMed] [Google Scholar]
- 2.Bossche, H. V., W. Lauwers, G. Willemsens, P. Marichal, F. Cornelissen, and W. Cools. 2006. Molecular basis for the antimycotic and antibacterial activity of N-substituted imidazoles and triazoles: the inhibition of isoprenoid biosynthesis. Pestic. Sci. 15:188-198. [Google Scholar]
- 3.De Nollin, S., H. Van Belle, F. Goossens, F. Thone, and M. Borgers. 1977. Cytochemical and biochemical studies of yeasts after in vitro exposure to miconazole. Antimicrob. Agents Chemother. 11:500-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.François, I. E. J. A., B. P. Cammue, M. Borgers, J. Ausma, G. D. Dispersyn, and K. Thevissen. 2006. Azoles: mode of antifungal action and resistance development. effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti-Infect. Agents Med. Chem. 5:3-13. [Google Scholar]
- 5.Godefroi, E. F., J. Heeres, J. Van Cutsem, and P. A. Janssen. 1969. The preparation and antimycotic properties of derivatives of 1-phenethylimidazole. J. Med. Chem. 12:784-791. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves, V. L., L. S. Nobre, J. B. Vicente, M. Teixeira, and L. M. Saraiva. 2006. Flavohemoglobin requires microaerophilic conditions for nitrosative protection of Staphylococcus aureus. FEBS Lett. 580:1817-1821. [DOI] [PubMed] [Google Scholar]
- 7.Helmick, R. A., A. E. Fletcher, A. M. Gardner, C. R. Gessner, A. N. Hvitved, M. C. Gustin, and P. R. Gardner. 2005. Imidazole antibiotics inhibit the nitric oxide dioxygenase function of microbial flavohemoglobin. Antimicrob. Agents Chemother. 49:1837-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornby, J. M., and K. W. Nickerson. 2004. Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 48:2305-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu, S., K. M. Smith, and T. G. Spiro. 1996. Assignment of protoheme resonance Raman spectrum by heme labeling in myoglobin. J. Am. Chem. Soc. 118:12638-12646. [Google Scholar]
- 10.Imlay, J. A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi, D., K. Kondo, N. Uehara, S. Otokozawa, N. Tsuji, A. Yagihashi, and N. Watanabe. 2002. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob. Agents Chemother. 46:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, C. I., G. Y. Liu, Y. Song, F. Yin, M. E. Hensler, W. Y. Jeng, V. Nizet, A. H. Wang, and E. Oldfield. 2008. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 319:1391-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, G. Y., A. Essex, J. T. Buchanan, V. Datta, H. M. Hoffman, J. F. Bastian, J. Fierer, and V. Nizet. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 202:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElhaney-Feser, G. E., R. E. Raulli, and R. L. Cihlar. 1998. Synergy of nitric oxide and azoles against Candida species in vitro. Antimicrob. Agents Chemother. 42:2342-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Membrillo-Hernandez, J., N. Ioannidis, and R. K. Poole. 1996. The flavohaemoglobin (HMP) of Escherichia coli generates superoxide in vitro and causes oxidative stress in vivo. FEBS Lett. 382:141-144. [DOI] [PubMed] [Google Scholar]
- 17.Miller, A., and J. Tanner. 2008. Essentials of chemical biology structure and dynamics of biological macromolecules. John Wiley & Sons, Hoboken, NJ.
- 18.Nobre, L. S., V. L. Goncalves, and L. M. Saraiva. 2008. Flavohemoglobin of Staphylococcus aureus. Methods Enzymol. 436:203-216. [DOI] [PubMed] [Google Scholar]
- 19.Nobre, L. S., J. D. Seixas, C. C. Romao, and L. M. Saraiva. 2007. Antimicrobial action of carbon monoxide-releasing compounds. Antimicrob. Agents Chemother. 51:4303-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelz, A., K. P. Wieland, K. Putzbach, P. Hentschel, K. Albert, and F. Gotz. 2005. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 280:32493-32498. [DOI] [PubMed] [Google Scholar]
- 21.Salerno, J. C. 1984. Cytochrome electron spin resonance line shapes, ligand fields, and components stoichiometry in ubiquinol-cytochrome c oxidoreductase. J. Biol. Chem. 259:2331-2336. [PubMed] [Google Scholar]
- 22.Siebert, F., and P. Hildebrandt. 2008. Vibrational spectroscopy in life science. Wiley-VCH, Weinheim, Germany.
- 23.Sud, I. J., and D. S. Feingold. 1982. Action of antifungal imidazoles on Staphylococcus aureus. Antimicrob. Agents Chemother. 22:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan, M. A., R. E. Yasbin, and F. E. Young. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29:21-26. [DOI] [PubMed] [Google Scholar]
- 25.Thevissen, K., K. R. Ayscough, A. M. Aerts, W. Du, K. De Brucker, E. M. Meert, J. Ausma, M. Borgers, B. P. Cammue, and I. E. Francois. 2007. Miconazole induces changes in actin cytoskeleton prior to reactive oxygen species induction in yeast. J. Biol. Chem. 282:21592-21597. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi, V., P. Chand, K. Srivastava, S. K. Puri, P. R. Maulik, and U. Bandyopadhyay. 2005. Clotrimazole inhibits hemoperoxidase of Plasmodium falciparum and induces oxidative stress. Proposed antimalarial mechanism of clotrimazole. J. Biol. Chem. 280:41129-41136. [DOI] [PubMed] [Google Scholar]
- 27.Van Cutsem, J. M., and D. Thienpont. 1972. Miconazole, a broad-spectrum antimycotic agent with antibacterial activity. Chemotherapy 17:392-404. [DOI] [PubMed] [Google Scholar]
- 28.Wu, G., H. Corker, Y. Orii, and R. K. Poole. 2004. Escherichia coli Hmp, an “oxygen-binding flavohaemoprotein,” produces superoxide anion and self-destructs. Arch. Microbiol. 182:193-203. [DOI] [PubMed] [Google Scholar]
- 29.Yeh, S. R., M. Couture, Y. Ouellet, M. Guertin, and D. L. Rousseau. 2000. A cooperative oxygen binding hemoglobin from Mycobacterium tuberculosis. Stabilization of heme ligands by a distal tyrosine residue. J. Biol. Chem. 275:1679-1684. [DOI] [PubMed] [Google Scholar]
- 30.Zhao, H., J. Joseph, H. Zhang, H. Karoui, and B. Kalyanaraman. 2001. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 31:599-606. [DOI] [PubMed] [Google Scholar]