Abstract
Endoglin, a transmembrane glycoprotein that acts as a transforming growth factor-β (TGF-β) coreceptor, is downregulated in PC3-M metastatic prostate cancer cells. When restored, endoglin expression in PC3-M cells inhibits cell migration in vitro and attenuates the tumorigenicity of PC3-M cells in SCID mice, though the mechanism of endoglin regulation of migration in prostate cancer cells is not known. The current study indicates that endoglin is phosphorylated on cytosolic domain threonine residues by the TGF-β type I receptors ALK2 and ALK5 in prostate cancer cells. Importantly, in the presence of constitutively active ALK2, endoglin did not inhibit cell migration, suggesting that endoglin phosphorylation regulated PC3-M cell migration. Therefore, our results suggest that endoglin phosphorylation is a mechanism with relevant functional consequences in prostate cancer cells. These data demonstrate for the first time that TGF-β receptor-mediated phosphorylation of endoglin is a Smad-independent mechanism involved in the regulation of prostate cancer cell migration.
Introduction
Growth factors from the transforming growth factor-β (TGF-β) family play a critical role in prostate cancer progression (1,2). This family includes the TGF-βs, the bone morphogenetic proteins (BMPs), activin A (ActA) and the anti-Mullerian hormone (AMH). These ligands specifically bind and activate different complexes of TGF-β type I and type II receptors, which then activate the signaling pathway mediated by the Smad proteins: TGF-βs activate Smad2 and 3, whereas BMPs activate Smad1, 5 and 8. Once phosphorylated, these Smads interact with Smad4 and translocate to the nucleus where they regulate gene expression (3). In addition, TGF-β receptors can activate Smad-independent signaling pathways (4), thus highlighting the potential for multiple pathway responses to individual TGF-β ligands.
Signaling by the TGF-β family factors is modulated by additional accessory proteins. Endoglin is a transmembrane protein that acts as a TGF-β coreceptor. The predominant L- or long isoform of endoglin, L-endoglin, contains a large extracellular domain, a transmembrane domain and a 47 amino acid cytosolic domain (CD) (5–7). Endoglin interacts with the TGF-β type II receptor TβRII, and the TGF-β type I receptors ALK1 and ALK5, and it binds TGF-β1 and 3, ActA and BMP2 and 7 (7). Endoglin is implicated in the endothelial cell response to TGF-β-related ligands (8) and is required for vascular development (9–11). Recent studies support the view that endoglin regulates diverse tissue properties, including endothelial cell-dependent regulation of vascular smooth muscle cell recruitment and differentiation (12), maintenance of vascular smooth muscle cell myogenic potential (13) and the epithelial–mesenchymal transformation during cardiac valve formation (14). Endoglin may also function as a regulator of the cell–extracellular microenvironment interaction. ALK1, the type I receptor specifically expressed in endothelial cells, phosphorylates endoglin on CD threonine residues (15). The functional consequences of endoglin phosphorylation include prevention of the ALK1-induced cell growth arrest and upregulation of proteins involved in cell–microenvironment interactions (15,16). Several studies show that endoglin is involved in regulating cell adhesion and migration independently of canonical TGF-β family signaling, potentially via interaction of its CD with multiple proteins (15,17–20). For example, the endoglin CD specifically interacts with zyxin and zyxin-related protein, two LIM domain proteins that regulate the dynamics of the actin cytoskeleton (19,20). This interaction, therefore, may be regulated by endoglin phosphorylation. These studies suggest a mechanism for the previously described inhibitory role of endoglin in cell migration and detachment in a variety of cell types (15,20–22). From these results, we propose that endoglin acts as a Smad-independent target of TGF-β receptors that regulates cell adhesion and migration.
Endoglin has an emerging role as a regulatory protein in cancer (23). Two independent groups reported a correlation between endoglin expression and inhibition of carcinogenesis. Quintanilla and coworkers described that endoglin attenuates malignancy in an in vivo model of mouse skin carcinogenesis (24). Liu et al. (25) found that endoglin expression is downregulated in metastatic human prostate cancer cells, which is associated with increased invasiveness. In these cells, endoglin inhibits TGF-β-induced cell migration by switching the ALK5-Smad3 response to ALK2-Smad1 (2). We now provide evidence for a novel mechanism by which endoglin is a Smad-independent substrate for ALK2 and ALK5 that regulates cell migration in prostate cancer cells.
Materials and methods
Plasmids and viral constructs
Human endoglin constructs cloned in the pWzl vector, constitutively active (ca, Q207D) and kinase-dead (kd, K233R) ALK2 and caALK5 (T204D), were described previously (2,15,20,26). kdALK5 was obtained by mutagenesis of ALK5 in K232R using the Quickchange kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The mutations were confirmed by sequencing analysis.
Sequences targeted against human ALK2 (ACTCTACATGTGTGTGTGT) and human ALK5 (AGACTTAATTTATGATATG) were cloned in pSilencer 5.1 (Ambion, Austin, TX). A control pSilencer vector containing a non-specific sequence was purchased from the same company.
Cell culture and growth factor treatment and construction of retrovirus-transduced cell lines
PC3 (American Type Culture Collection, Rockville, MD) and PC3-M cells (27) were grown in RPMI medium with 10% fetal bovine serum and antibiotics (Gibco, Carlsbad, CA). Retrovirus-transduced cell lines were constructed as described previously (20). Normal human prostate epithelial cells (Clonetics, Lonza, Walkersville, MD) were grown in prostate epithelial growth media (Clonetics, Lonza). All cell lines were kept at 37°C with 5% CO2.
Human recombinant TGF-β1, BMP7, ActA or the AMH (R&D Systems, Minneapolis, MN) was added to serum-starved subconfluent cell cultures at the indicated final concentrations.
Luciferase assay
For luciferase reporter analysis of Smad activation, the cells were transfected using Effectene (Qiagen, Valencia, CA). The BRE2 and CAGA luciferase reporter constructs (28,29) were provided by Dr Peter ten Dijke (Leiden University, Leiden, The Netherlands).
Immunoprecipitation and western blot analysis
Cells were transiently transfected with the indicated constructs using Effectene (Qiagen) for 48 h or treated with different growth factors at the indicated concentrations (R&D Systems). Endoglin immunoprecipitation and western blot analysis were performed as described previously (15). Quantitation of immunoblot data was performed essentially as described previously (20), except that endoglin phosphorylation was normalized to total endoglin levels.
Reverse transcription–polymerase chain reaction analysis
Total RNA isolated with RNAeasy (Qiagen) was transcribed into complementary DNA with Superscript (Invitrogen, Carlsbad, CA). The oligonucleotide pairs and polymerase chain reaction programs used to amplify fragments of several different genes are summarized in supplementary Table 1 (available at Carcinogenesis Online).
Migration assay
Cells were transfected transiently with the indicated constructs using Effectene. Twenty-four hours later, the cells were seeded in 8 μm pore uncoated inserts (Becton Dickinson, Franklin Lakes, NJ) in serum-free media, with or without growth factors, and allowed to migrate toward media with 5% serum. After 18 h, the inserts were fixed in methanol and stained with 4′,6-diamidino-2-phenylindole (Sigma, St Louis, MO), and the number of migrated cells was determined by fluorescence microscopy.
Tumorigenicity assay
Mice were maintained according to the National Institutes of Health standards established in the ‘Guidelines for the Care and Use of Experimental Animals’. Protocols and procedures were approved by the Institutional Animal Care and Use Committee at the Maine Medical Center Research Institute. Suspensions of 1 × 106 cells were injected subcutaneously in both flanks of 7- to 10-week-old female NOD-CB17-Prkdcscid-J mice (The Jackson Laboratories, Bar Harbor, ME). The animals were periodically examined for the presence of tumors.
Tumor analysis
Mice were killed 19–22 days after inoculation. Tumors were harvested and fixed in 4% paraformaldehyde. Hematoxylin and eosin and platelet endothelial cell adhesion molecule staining were analyzed as described previously (13). Ki67 was detected with an antibody from DakoCytomation (Glostrup, Denmark). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling analysis was performed with a kit (Roche, Mannheim, Germany).
Results
Endoglin CD threonine residues are involved in the inhibition of PC3-M cell migration and tumorigenicity
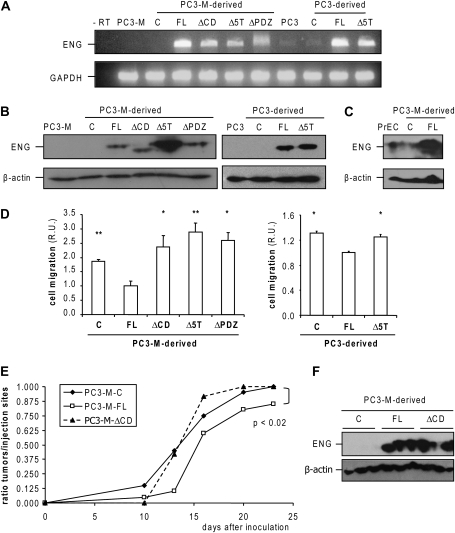
Endoglin function was previously studied in PC3 cells and their metastatic derivatives, PC3-M (25). Compared with PC3-M cells, PC3 cells are less invasive and metastatic (30) and have a lower propensity to detach (27). Consistent with previously reported results (25), we detected a low level of endoglin messenger RNA in PC3 cells, but we did not detect endoglin messenger RNA in PC3-M cells (Figure 1A). The protein levels were undetectable in both cell lines, as determined by western blotting (Figure 1B). Using retroviral transduction, we generated stable polyclonal PC3-M cell populations expressing wild-type L-endoglin (FL, full length) or the following CD mutations: ΔCD, a CD deletion mutant; Δ5T, in which five threonine residues in the CD are replaced by non-phosphorylatable residues; and ΔPDZ, a putative PSD-95/discs large/zonula-occludens-1 (PDZ)-binding motif deletion mutant (15). The resulting polyclonal cell populations expressed equivalent levels of endoglin RNA (Figure 1A) and protein (Figure 1B), with the exception of Δ5T-endoglin-expressing cells. A control cell line (PC3-M-C) was generated by transduction with the empty retroviral vector. A subset of these constructs was used to generate stable PC3-derived cells expressing FL and Δ5T-endoglin, as well as a control cell line (Figures 1A and B). The endoglin protein levels achieved by retroviral transduction in PC3-M-FL cells were higher than the endogenous levels of endoglin expressed by human normal prostate epithelial cells (Figure 1C). Although this is a limitation of this experimental approach, we chose PC3-M cells as the primary model for our studies because of the absence of endogenous endoglin in the parental cell line. Therefore, in PC3-M-derived cells, the effect of wild-type or mutated endoglin can be fully attributed to the expression of the transfected endoglin protein.
Fig. 1.
Endoglin expression affects cell migration and tumorigenicity in PC3-M cells. (A) Reverse transcription–polymerase chain reaction and (B) western blot analysis of endoglin expression in PC3-M and PC3 cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A) and β-actin (B): controls. (C): PC3-M- or PC3-derived control cells. (C) Western blot analysis of endoglin expression in prostate epithelial cell (PrEC), PC3-M-C and FL cells. β-Actin: protein loading control. In order to obtain a detectable endoglin signal in prostate epithelial cell, the film exposure was longer than in the films shown in panel (B). (D) The effect of endoglin in the basal migration of PC3-M- and PC3-derived cells was determined as described in Material and Methods. Cells were allowed to migrate for 18 h. Five different fields per sample (n = 6) were quantified by microscopy. R.U., relative units. *P < 0.05; **P < 0.005 (Student's t-test), expressed relative to PC3-M-FL or PC3-FL. (E) In total, 1 × 106 PC3-M-control, FL- or ΔCD-endoglin-expressing cells were injected subcutaneously in both flanks of SCID mice (C and FL, n = 10; ΔCD, n = 6). The data sets were compared using the chi-square test. (F) Western blot analysis of endoglin expression in tumors harvested 19–22 days after inoculation. β-Actin: protein loading control. A minimum of three tumors per group were analyzed.
It was shown previously that endoglin inhibits migration and invasion of PC3-M and PC3 cells (2,25). Endoglin's inhibition of cell migration was less pronounced in PC3 cells than in PC3-M cells, probably due to endogenous endoglin expression in these cells. Importantly, all the endoglin CD mutants tested showed a reduced capacity to inhibit cell migration in these cell lines when compared with wild-type endoglin (Figure 1D). To determine if this effect could be attributed to a difference in cell proliferation, we analyzed whether FL endoglin or the ΔCD deletion mutant affected PC3-M cell growth. Neither wild-type nor mutated endoglin expression affected the proliferation rate of these cells (supplementary Figure 1 is available at Carcinogenesis Online). Therefore, our cell migration results suggest that phosphorylation of threonine residues in an intact endoglin CD are necessary for endoglin-dependent inhibition of cell migration.
To determine the influence of endoglin on tumor growth in vivo, we injected PC3-M-control, FL and ΔCD endoglin-expressing cells subcutaneously in SCID mice (Figure 1E). At the time of injection, all the cell lines were growing exponentially and did not appear to be quiescent or detaching. The tumorigenicity of PC3-M-FL cells was significantly delayed as compared with control and ΔCD cells (P < 0.02, chi-square test). Tumor incidence was 87.5% for PC3-M-FL cells versus 100% for PC3-M-control and ΔCD cells. This trend was observed in three independent experiments. Western blot analysis of the tumor-derived total protein lysates confirmed that endoglin expression had not been gained in those derived from injection of control cells nor lost in those derived from injection of FL- or ΔCD-endoglin-expressing cells (Figure 1F). The average size of PC3-M-FL-derived tumors was slightly smaller than PC3-M-control and ΔCD-derived tumors, though this trend did not achieve statistical significance (supplementary Table 2 is available at Carcinogenesis Online). We did not find significant differences in the histology or the vascularization pattern of the tumors. Moreover, the proliferation and apoptotic rates of PC3-M-FL and ΔCD-derived tumors were similar and not significantly different than the proliferation and apoptotic rates of PC3-M-control-derived tumors (supplementary Table 2 is available at Carcinogenesis Online). Taken together, these results suggest that endoglin attenuates the tumorigenicity of PC3-M cells in SCID mice in an endoglin CD-dependent fashion.
Signaling through ALK2 and ALK5 leads to phosphorylation of endoglin CD threonine residues in PC3 and PC3-M prostate cancer cells
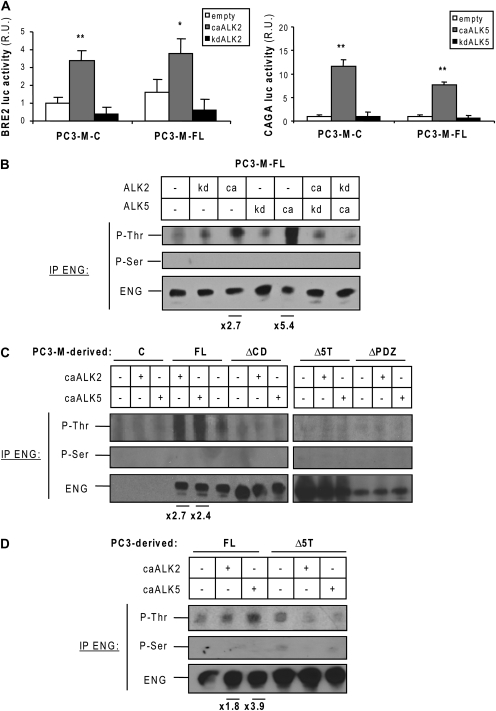
We hypothesized that endoglin is phosphorylated in PC3-M cells that express ALK2 and ALK5 (2). Using specific luciferase reporter constructs, we confirmed that both caALK2 and caALK5 activate Smad signaling independently of endoglin expression in PC3-M cells (Figure 2A). Conversely, kinase-dead type I receptor mutants failed to activate Smad signaling. Therefore, these constructs were used to analyze endoglin phosphorylation in PC3-M-FL. caALK2 phosphorylated endoglin on threonine but not on serine residues (Figure 2B). Phosphorylation of stably expressed endoglin was attributable to ALK2 because kdALK2 eliminated endoglin phosphorylation. In these cells, caALK5 also phosphorylated endoglin on threonine residues, with no effect on basal endoglin phosphorylation for kdALK5 (Figure 2B), though quantitative analysis of immunoblotting for endoglin phosphorylation, normalized to total endoglin levels, indicated substantially stronger phosphorylation of endoglin by caALK5 in PC3M-FL cells. The combinations caALK2/kdALK5 or caALK5/kdALK2 did not lead to significant changes in endoglin phosphorylation when compared with the activated receptors alone. This result suggests that ALK2 and ALK5 independently phosphorylate endoglin (Figure 2B). The lack of detectable phosphorylation by caALK2 and caALK5 on ΔCD and Δ5T-endoglin indicates that these kinases phosphorylate threonine residues from endoglin CD (Figure 2C). Furthermore, the result obtained with ΔPDZ-endoglin-expressing cells suggests that an intact PDZ-binding motif is required for endoglin phosphorylation (Figure 2C).
Fig. 2.
Endoglin phosphorylation by TGF-β type I receptors in prostate cancer cells. (A) PC3-M-control and FL cells were transfected with the BRE2 and CAGA luciferase reporter constructs, which were specifically activated by Smad1, 5 and 8 or Smad2 and 3, respectively, and the different receptor constructs for 48 h. The luciferase activities measured in duplicate samples were normalized and plotted (n = 6). R.U., relative units. *P < 0.05 and **P < 0.005 (Student's t-test). (B) PC3-M-FL cells were transfected with different combinations of caALK2 or caALK5, kdALK2 and kdALK5 or with an empty vector. Endoglin was immunoprecipitated and western blot was performed with P-Thr, P-Ser or total endoglin-specific antibodies. Values indicated at bottom of panel: P-Thr relative signal intensity increase compared with control. The band intensity values were quantified using ImageJ software (NIH Image), and P-Thr levels were normalized to total endoglin levels. These measurements are representative of a minimum of three independent experiments. (C) Endoglin phosphorylation by caALK2 and caALK5 in PC3-M-control, FL-, ΔCD-, Δ5T- and ΔPDZ-endoglin-expressing cells. (D) Endoglin phosphorylation by caALK2 and caALK5 in PC3 control, FL- and Δ5T-endoglin-expressing cells.
To determine whether endoglin phosphorylation occurs in other human prostate cancer cell lines, PC3-FL and PC3-Δ5T cells were transfected with caALK2 or caALK5, and endoglin phosphorylation was analyzed. Endoglin was basally phosphorylated on threonine residues in PC3 cells (Figure 2D). Both caALK2 and caALK5 induced threonine phosphorylation of FL endoglin. Consistent with previous data, quantitation of endoglin phosphorylation relative to total endoglin indicated stronger phosphorylation by caALK5 in PC3 cells. The results obtained with Δ5T-endoglin-expressing PC3 cells suggest that ALK2 and ALK5 phosphorylate threonine residues from endoglin's CD in PC3 cells, as we have previously observed in PC3-M cells (Figure 2D). Therefore, endoglin phosphorylation by TGF-β receptors is not a cell line-restricted phenomenon.
TGF-β1 and BMP7 induce endoglin phosphorylation in PC3-M cells
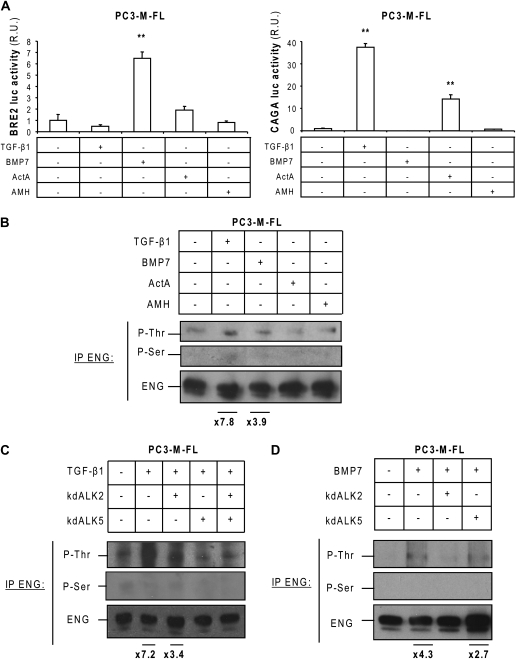
Our next objective was to analyze the effect of TGF-β family ligands on endoglin phosphorylation. TGF-β1 activates ALK5 and BMP7, ActA and the AMH activate ALK2. The effect of these ligands on Smad signaling in PC3-M cells was evaluated using luciferase reporters (Figure 3A). As expected, TGF-β1, BMP7 and ActA activated Smad signaling. AMH did not activate Smad signaling, indicating that PC3-M cells were not responsive to this growth factor.
Fig. 3.
Endoglin phosphorylation by TGF-β family ligands in PC3-M cells. (A) PC3-M-FL cells were transfected with the BRE2 and CAGA luciferase reporters, and 24 h later they were treated for an additional 24 h with 5 ng/ml TGF-β, 50 ng/ml BMP7, 20 ng/ml ActA or 500 ng/ml AMH. The luciferase activities were measured as described for Figure 2A (n = 6). R.U., relative units. **P < 0.005 (Student's t-test). (B) Serum-starved PC3-M-FL cells were treated for 30 min with 5 ng/ml TGF-β1, 50 ng/ml BMP7, 20 ng/ml ActA or 500 ng/ml AMH. Endoglin phosphorylation was analyzed as described for Figure 2B. (C and D) PC3-M-FL cells were transfected with kdALK2 and/or kdALK5 or with an empty vector. Twenty-four hours later, they were serum-starved overnight. In total, 5 ng/ml TGF-β1 (C) or 50 ng/ml BMP7 (D) was added for 30 min, and endoglin phosphorylation was analyzed. Fold increases for P-Thr were determined as described above.
The ligands TGF-β1 and, to a minor extent based on quantitation of endoglin phosphorylation, relative to total endoglin levels, BMP7 induced endoglin phosphorylation on threonine residues (Figure 3B). Interestingly, TGF-β1 treatment also induced weak phosphorylation of endoglin on serine residues, with quantitative phosphothreonine increases equivalent to those seen above. kdALK5 completely blocked TGF-β1-induced endoglin phosphorylation on threonine residues, whereas the effect of kdALK2 on TGF-β1-induced endoglin phosphorylation was partial (Figure 3C). Therefore, these data suggest that both ALK2 and ALK5 are involved in endoglin phosphorylation in response to TGF-β1 but that ALK5 is the main mediator of this effect. The use of kd receptors indicated that BMP7-induced endoglin phosphorylation depends only on ALK2 activity (Figure 3D).
TGF-β1 suppresses endoglin inhibition of cell migration through an ALK2-dependent mechanism
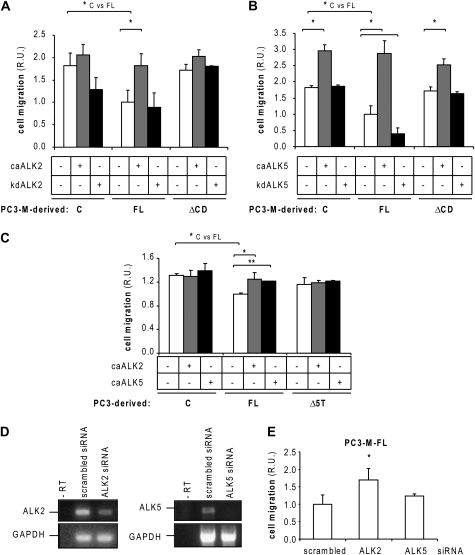
Endoglin inhibits PC3-M cell migration by a CD-dependent mechanism (Figure 1C). Therefore, we investigated whether endoglin phosphorylation could be involved in the regulation of PC3-M cell migration. We tested the effect of caALK2 and caALK5 in PC3-M cell migration. caALK2 stimulated cell migration of PC3-M-FL cells and had no effect on PC3-M-control or ΔCD cell migration (Figure 4A). caALK5 strongly activated cell migration, independently of endoglin expression (Figure 4B). Together, these results support the view that ALK2 is the type I receptor involved in endoglin-dependent inhibition of cell migration.
Fig. 4.
Effect of endoglin phosphorylation in prostate cancer cell migration. (A) Effect of caALK2 and kdALK2 or (B) caALK5 and kdALK5 in cell migration of PC3-M-control, FL and ΔCD cells. Migration assays were performed as described previously. Five different fields per sample (n = 6) were quantified by microscopy. R.U., relative units. *P < 0.05 (Student's t-test). (C) Effect of caALK2 and caALK5 in cell migration of PC3 control, FL and Δ5T cells. Migration assays were performed as described previously. Five different fields per sample (n = 6) were quantified by microscopy. R.U., relative units. *P < 0.05 (Student's t-test). (D) PC3-M-FL cells were transfected with small interfering ribonucleic acids (siRNAs) directed against ALK2, ALK5 or a scrambled sequence (control) for 48 h. ALK2 and ALK5 expression was analyzed by reverse transcription–polymerase chain reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): control. (E) PC3-M-FL cells were transfected with siRNAs directed against ALK2, ALK5 or a control scrambled sequence. Twenty-four hours after transfection, they were allowed to migrate for 18 h. Cell migration assays were performed as described previously (n = 6). R.U., relative units. *P < 0.05 (Student's t-test).
We described previously that endoglin effect in prostate cancer cell migration is not restricted to PC3-M cells and can also be observed in PC3 cells (2,25). Therefore, we analyzed the effect of caALK2 and caALK5 expression on PC3-derived cell migration (Figure 4C). Similar to PC3-M cells, basal cell migration was inhibited by endoglin in a mechanism dependent on the five phosphorylatable threonine residues from the CD. caALK2 and caALK5 stimulated cell migration of PC3-FL cells, but had no effect on PC3-control or Δ5T cell migration. These results confirm that endoglin phosphorylation by ALK2 and ALK5 in prostate cancer cells blocks its inhibitory effect in cell migration.
Our previous RNA interference experiments showed that ALK2 signaling inhibits cell migration in PC3-M cells (2). To further investigate this apparent contradiction with the current data, we quantified the migratory ability of PC3-M-FL-endoglin-expressing cells transfected with small interfering ribonucleic acids against ALK2 or ALK5 (Figure 4D and E). In agreement with our previous observations, small interfering ribonucleic acid-mediated suppression of ALK2 stimulated cell migration of PC3-M-FL cells, whereas suppression of ALK5 had no significant effect. These results support the view that TGF-β receptors have to be considered carefully because of their potential promiscuity when forming complexes.
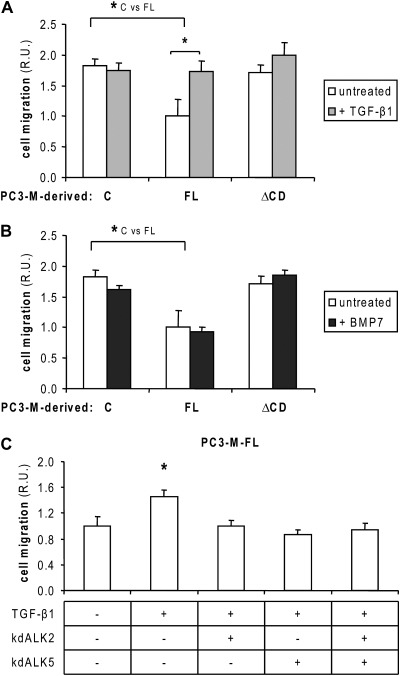
A limitation of the experimental approaches used above (mutant receptor overexpression and small interfering ribonucleic acid silencing) is that they constitute strong gain- and loss-of-function manipulations of receptor function, respectively. Therefore, we also analyzed the effect of TGF-β- and BMP-related ligands on PC3-M cell migration. TGF-β1 did not significantly affect control or ΔCD cell migration, but it stimulated migration of PC3-M-FL cells and neutralized endoglin's inhibitory effect in cell migration, similar to caALK2 (Figure 5A). In contrast, BMP7 did not have a significant effect on PC3-M-control, FL or ΔCD-cell migration (Figure 5B). Together, these results point to TGF-β1 as the ligand involved in endoglin regulation of PC3-M cell migration. kdALK2 and kdALK5 (alone or combined) blocked TGF-β1-induced PC3-M-FL cell migration, indicating that both type I receptors mediate this response (Figure 5C).
Fig. 5.
Effect of TGF-β1 and BMP7 in PC3-M cell migration. (A and B) PC3-M-FL cells in suspension in serum-free media were supplemented with 5 ng/ml TGF-β1 (A) or 50 ng/ml BMP7 (B). Cell migration assay was performed as described previously (n = 6). R.U., relative units. *P < 0.05 (Student's t-test). (C) PC3-M-FL cells were transfected with kdALK2 and/or kdALK5 or with an empty vector. Twenty-four hours later, they were prepared as a cell suspension in serum-free media and supplemented with 5 ng/ml TGF-β1. Cell migration assay was performed as described previously (n = 6). R.U., relative units. *P < 0.05 (Student's t-test).
Discussion
The alteration of the cellular response to TGF-β is a key step in the development of prostate cancer (1,2,31,32). The loss of the cellular response to specific BMPs is also implicated in prostate carcinogenesis (33–35). Although the Smad pathway is the canonical signaling mechanism activated by these factors (3), the present study provides evidence for a novel Smad-independent TGF-β effector that regulates cell migration via phosphorylation of endoglin by ALK2. We previously demonstrated that ALK1 directly phosphorylates endoglin in human umbilical vein endothelial cells and, as a consequence, the ALK1-dependent inhibition of cell adhesion and proliferation in these cells is inhibited (15,16). We now show that endoglin is phosphorylated in prostate cancer cells and, as in endothelial cells, the phosphorylated residues are predominantly the five threonines in the CD. Importantly, this finding confirms that endoglin phosphorylation is not an isolated mechanism that operates only in endothelial cells but supports the view that it is a more broadly significant mechanism for the regulation of endoglin function.
Despite the foregoing similarities, we observed striking differences between endoglin phosphorylation in endothelial and prostate cancer cells. First, detection of endoglin phosphorylation in prostate cancer cell lines is much more technically demanding than for endothelial cells (15), probably reflecting lower levels and higher rates of turnover of phosphorylated endoglin serine and threonine residues in non-endothelial cells. The lower levels of endoglin in PC3 cells also suggests a closer stoichiometric relationship between endoglin in TGF-β receptors in prostate cancer cells than is the case in endothelial cells, where endoglin is probably in excess of TGF-β ligand and receptor levels (6). Second, in prostate cancer cells, we did not detect basal phosphorylation of endoglin on serine residues, suggesting that the turnover of serine-linked phosphates is higher in these cells than in endothelial cells. Third, different type I receptors phosphorylate endoglin in endothelial cells (ALK1) versus prostate cancer cells (ALK2 and ALK5). Finally, threonine phosphorylation of ΔPDZ-endoglin is dramatically enhanced in endothelial cells (15), whereas it was virtually abolished for the same mutated protein in prostate cancer cells. This result suggests that the PDZ-binding motif, which has been implicated in endoglin interaction with other cellular proteins including β-arrestin (18), is involved in cell type-specific processes.
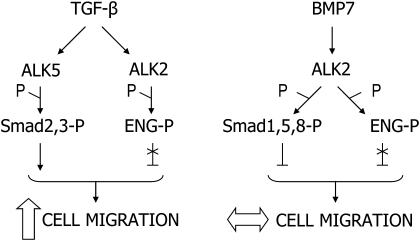
In the present study, we determined that ALK2 and ALK5 phosphorylated endoglin in prostate cancer cells. Based on these results, and on the described Smad specificities of ALK2 and ALK5 (3), we propose that upon TGF-β1 stimulation ALK5 phosphorylates Smad2 and 3 with a negative impact on ALK2-Smad1, 5 and 8 signaling. Therefore, our data suggest that ALK2 phosphorylates endoglin as an alternative substrate, and upon BMP7 stimulation, ALK2 phosphorylates endoglin without a requirement for ALK5 participation (depicted in Figure 6).
Fig. 6.
Proposed model for endoglin phosphorylation by TGF-β1 and BMP7 in prostate cancer cells and its effect in cell migration.
We demonstrate that endoglin phosphorylation affects prostate cancer cell migration. The results of our tumorigenicity experiments suggest that endoglin attenuates the progression of prostate carcinogenesis. Moreover, the tumorigenic potential of prostate cancer cells expressing an endoglin mutant that cannot be phosphorylated was the same as the tumorigenicity of endoglin-deficient cells. This result is consistent with the observations of Perez-Gomez et al. (36). These authors demonstrated that L-endoglin inhibits keratinocyte-induced tumorigenicity in mice, whereas the short, S-endoglin isoform, which lacks the L-endoglin isoform CD, does not inhibit tumorigenicity, thus supporting the view that TGF-β receptor-dependent endoglin phosphorylation regulates tumorigenicity in vivo.
Another functional consequence of endoglin phosphorylation is that phosphorylated endoglin no longer exerts its inhibitory effect on prostate cancer cell migration (2,25). The present study supports a role for endoglin as a novel element in TGF-β1-dependent regulation of cancer cell migration. TGF-β1 stimulates cell migration via ALK5/Smad2 and 3 (2). In addition, in response to TGF-β1, endoglin is phosphorylated and its inhibitory effect in cell migration is blocked (Figure 6).
This work advances the hypothesis that ALK2 plays a dual role in prostate cancer cell migration depending on the available substrates for its kinase activity. When ALK2 phosphorylates Smad1, the net result is the inhibition of cell migration (2). However, when ALK2 phosphorylates endoglin, cell migration is promoted. Thus, there is an implied balance between endoglin expression levels and phosphorylation, ALK2 activation and Smad1 availability, which may explain the differences between endoglin anti-invasive action in prostate cancer versus the observation that endoglin expression on metastatic breast cancer cells promotes their invasive character (37). Therefore, additional effects of local ligand activation and availability, receptor-substrate affinities and potential interactions between the cells and the extracellular matrix remain to be elucidated.
The present study describes for the first time that TGF-β receptor-mediated phosphorylation of endoglin is a Smad-independent mechanism involved in the regulation of prostate cancer cell migration and tumor progression. We are currently exploring novel animal models to further examine how endoglin expression in tumor cells and their microenvironment affects prostate cancer progression.
Supplementary material
Supplementary Figure 1 and Tables 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health National Center for Research Resources (P20 RR 15555); Maine Cancer Foundation.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Igor Prudovsky (Center for Molecular Medicine, Maine Medical Center Research Institute, Scarborough, ME) for many helpful discussions and suggestions and Kathleen Carrier (Center for Molecular Medicine, Maine Medical Center Research Institute, Scarborough, ME) for her excellent technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ActA
activin A
- AMH
anti-Mullerian hormone
- BMP
bone morphogenetic protein
- ca
constitutively active
- CD
cytosolic domain
- FL
full length
- kd
kinase-dead
- PDZ
PSD-95/discs large/zonula-occludens-1
- TGF-β
transforming growth factor-β
References
- 1.Ao M, et al. Transforming growth factor-beta promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66:8007–8016. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craft CS, et al. Endoglin inhibits prostate cancer motility via activation of the ALK2-Smad1 pathway. Oncogene. 2007;26:7240–7250. doi: 10.1038/sj.onc.1210533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massague J, et al. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 4.Derynck R, et al. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 5.Bernabeu C, et al. Novel biochemical pathways of endoglin in vascular cell physiology. J. Cell. Biochem. 2007;102:1375–1388. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheifetz S, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 7.Barbara NP, et al. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 8.Lastres P, et al. Endoglin modulates cellular responses to TGF-beta 1. J. Cell Biol. 1996;133:1109–1121. doi: 10.1083/jcb.133.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourdeau A, et al. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc. Med. 2000;10:279–285. doi: 10.1016/s1050-1738(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 10.Arthur HM, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 11.Li DY, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 12.Mancini ML, et al. Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev. Dyn. 2009 doi: 10.1002/dvdy.22066. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancini ML, et al. Endoglin is required for myogenic differentiation potential of neural crest stem cells. Dev. Biol. 2007;308:520–533. doi: 10.1016/j.ydbio.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercado-Pimentel ME, et al. Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev. Biol. 2007;304:420–432. doi: 10.1016/j.ydbio.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koleva RI, et al. Endoglin structure and function: determinants of endoglin phosphorylation by transforming growth factor-beta receptors. J. Biol. Chem. 2006;281:25110–25123. doi: 10.1074/jbc.M601288200. [DOI] [PubMed] [Google Scholar]
- 16.Lamouille S, et al. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood. 2002;100:4495–4501. doi: 10.1182/blood.V100.13.4495. [DOI] [PubMed] [Google Scholar]
- 17.Meng Q, et al. Identification of Tctex2beta a novel dynein light chain family member interacting with different TGF-beta receptors. J. Biol. Chem. 2006;281:37069–37080. doi: 10.1074/jbc.M608614200. [DOI] [PubMed] [Google Scholar]
- 18.Lee NY, et al. The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J. Biol. Chem. 2007;282:21507–21517. doi: 10.1074/jbc.M700176200. [DOI] [PubMed] [Google Scholar]
- 19.Sanz-Rodriguez F, et al. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the lim family of proteins. J. Biol. Chem. 2004;279:32858–32868. doi: 10.1074/jbc.M400843200. [DOI] [PubMed] [Google Scholar]
- 20.Conley BA, et al. Endoglin controls cell migration and composition of focal adhesions: function of the cytosolic domain. J. Biol. Chem. 2004;279:27440–27449. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- 21.Muenzner P, et al. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J. Cell Biol. 2005;170:825–836. doi: 10.1083/jcb.200412151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerrero-Esteo M, et al. Endoglin overexpression modulates cellular morphology, migration, and adhesion of mouse fibroblasts. Eur. J. Cell Biol. 1999;78:614–623. doi: 10.1016/S0171-9335(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 23.Bernabeu C, et al. The emerging role of Tgf-beta superfamily co-receptors in cancer. Biochim. Biophys. Acta. 2009 doi: 10.1016/j.bbadis.2009.07.003. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Quintanilla M, et al. Expression of the TGF-beta coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis. Oncogene. 2003;22:5976–5985. doi: 10.1038/sj.onc.1206841. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene. 2002;21:8272–8281. doi: 10.1038/sj.onc.1206117. [DOI] [PubMed] [Google Scholar]
- 26.Craft CS, et al. Genistein induces phenotypic reversion of endoglin deficiency in human prostate cancer cells. Mol. Pharmacol. 2008;73:235–242. doi: 10.1124/mol.107.038935. [DOI] [PubMed] [Google Scholar]
- 27.Bergan R, et al. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin. Exp. Metastasis. 1996;14:389–398. doi: 10.1007/BF00123398. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro RM, et al. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J. Cell Sci. 2004;117:4653–4663. doi: 10.1242/jcs.01337. [DOI] [PubMed] [Google Scholar]
- 29.Piek E, et al. Expression of transforming-growth-factor (TGF)-beta receptors and Smad proteins in glioblastoma cell lines with distinct responses to TGF-beta1. Int. J. Cancer. 1999;80:756–763. doi: 10.1002/(sici)1097-0215(19990301)80:5<756::aid-ijc21>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, et al. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65:3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 31.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 32.Tu WH, et al. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia. 2003;5:267–277. doi: 10.1016/S1476-5586(03)80058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim IY, et al. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer Res. 2000;60:2840–2844. [PubMed] [Google Scholar]
- 34.Miyazaki H, et al. BMP signals inhibit proliferation and in vivo tumor growth of androgen-insensitive prostate carcinoma cells. Oncogene. 2004;23:9326–9335. doi: 10.1038/sj.onc.1208127. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, et al. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65:5769–5777. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Gomez E, et al. A role for endoglin as a suppressor of malignancy during mouse skin carcinogenesis. Cancer Res. 2007;67:10268–10277. doi: 10.1158/0008-5472.CAN-07-1348. [DOI] [PubMed] [Google Scholar]
- 37.Oxmann D, et al. Endoglin expression in metastatic breast cancer cells enhances their invasive phenotype. Oncogene. 2008;27:3567–3575. doi: 10.1038/sj.onc.1211025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.