Abstract
Epidemiological studies showed that high levels of oxidized low-density lipoproteins (oxLDLs) are associated with increased cancer risk. We examined the direct effect of physiologic concentrations oxLDL on cancer cells. OxLDLs were cytotoxic and activate both apoptosis and autophagy. OxLDLs have ligands for peroxisome proliferator-activated receptor gamma and upregulated proline oxidase (POX) through this nuclear receptor. We identified 7-ketocholesterol (7KC) as a main component responsible for the latter. To elucidate the role of POX in oxLDL-mediated cytotoxicity, we knocked down POX via small interfering RNA and found that this (i) further reduced viability of cancer cells treated with oxLDL; (ii) decreased oxLDL-associated reactive oxygen species generation; (iii) decreased autophagy measured via beclin-1 protein level and light-chain 3 protein (LC3)-I into LC3-II conversion. Using POX-expressing cell model, we established that single POX overexpression was sufficient to activate autophagy. Thus, it led to autophagosomes accumulation and increased conversion of LC3-I into LC3-II. Moreover, beclin-1 gene expression was directly dependent on POX catalytic activity, namely the generation of POX-dependent superoxide. We conclude that POX is critical in the cellular response to the noxious effects of oxLDL by activating protective autophagy.
Introduction
The epidemiologic association of obesity with increased cancer risk has been reported (1), but the underlying mechanism for this association remains obscure. In the case of breast cancer the increase of adipose tissue mass and cumulative aromatase activity can affect the metabolism of estrogens, a critical factor in breast carcinogenesis. However, for gastrointestinal as well as for other cancers, no plausible mechanism has been elucidated. Some investigators have pointed out the parallels between atherosclerosis and cancer in their metabolic derangements (2). Obese subjects have not only increased levels of cholesterol/low-density lipoprotein (LDL) and triglycerides but also, more importantly, increased oxidative stress (3) leading to LDL conversion into oxidized low-density lipoprotein (oxLDL) (4). This process of LDL oxidation plays a key role in the development of atherosclerosis in both obese and lean patients. It is important to emphasize the positive correlation between increased serum oxLDL level and an increased risk of colon, breast and ovarian cancer (5).
Pathophysiologic effects of oxLDL in atherosclerosis are firmly established. The lowering of plasma oxLDL results in a marked retardation of atherosclerotic progression (6). However, atherosclerosis has been an insufficient model for carcinogenesis. In spite of accumulating ‘procarcinogenic’ epidemiologic data, there are no studies on the direct effect of oxLDL on cancer development. On the one hand, components of oxLDL stimulate cell proliferation (7,8) and mutagenesis in vitro (9). In contrast, it can also arrest cell growth (10), activate p53-dependent apoptosis (11,12) and initiate autophagy (13,14). Clearly, additional work is needed to elucidate context-dependent metabolic signaling responsible for the effects of oxLDL on carcinogenic processes.
We considered proline oxidase (POX) an ideal candidate to execute oxLDL-dependent signaling. POX is a mitochondrial enzyme that oxidizes proline, an unique imino acid, which may be derived from microenvironmental sources under conditions of metabolic stress (15). OxLDLs are a source of endogenous ligands for peroxisome proliferator-activated receptor gamma (PPARγ) (16) and POX responds to PPARγ (17). More importantly, POX is a downstream target of p53 and generates proline-dependent reactive oxygen species (ROS) (18) to induce apoptosis. Overexpression of POX initiates apoptosis in p53-deficient cells (19–21). The expression of POX is decreased in renal carcinomas (19) and also in carcinomas of the digestive tract (22). On the other hand, POX responds to nutrient stress in an mammalian target of rapamycin (mTOR)-dependent process and increases the generation of adenosine triphosphate (ATP) (23). This last fact links POX to autophagy, a process regulated mainly through the mTOR pathway.
Macroautophagy, which we refer to here as autophagy, is a process of ‘self-eating’ (24), which serves a housekeeping role and degrades damaged or unwanted organelles (25). Autophagy also serves to derive energy during stress conditions (26). Beyond a threshold, this process may eventually lead to cell death (27). Therefore, increased but not excessive autophagy can benefit cancer cells and promotes their survival.
In this study we show for the first time that oxLDLs have cytotoxic effects on cancer cells in vitro and activate both apoptosis and autophagy. POX is upregulated by oxLDL through PPARγ and plays a key role in the regulation of protective autophagy in cancer cells. We propose that POX-dependent autophagy is activated as a prosurvival response to the noxious stimuli of oxLDL. The mechanism we describe may allow selection and survival of cancer cells.
Materials and methods
Cell culture
Human cancer cells HT29, HeLa, OVCAR3, OVCAR5, MCF7, A549 and PC3 were supplied by the National Cancer Institute repository and cultured in Dulbecco's modified Eagle's medium (Quality Biologicals, Gaithersburg, MD) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT) and 2 mM L-glutamine at 37°C and 5% CO2. Human umbilical vein endothelial cells (HUVECs) were purchased from PromoCell (Heidelberg, Germany) and cultured in Endothelial Cell Growth Medium MV 2 supplemented with 10% fetal bovine serum (HyClone Laboratories) at 37°C and 5% CO2. DLD-1 Tet-off POX cells, stably overexpressing POX under the doxycycline depletion, were cultured as described (18). All cell lines tested negative for mycoplasma contamination.
Lipoproteins and experimental design
OxLDL and non-oxidized low-density lipoprotein (nLDL) were purchased from Intracel (Frederick, MD). The level of oxidation was assessed using a thiobarbituric acid reactive substances assay kit (Cayman, Ann Arbor, MI) and estimated as 59.5 ± 0.5 nmol malondialdehyde per mg ApoB protein for oxLDL and non-detectable for nLDL.
Cells were treated with oxLDL at physiological ranges (28,29). NLDL and untreated samples have been used as controls. Since Intracel supplied lipoproteins in the NaCl/ethylenediaminetetraacetic acid, pH 7.2, we tested this buffer and it had no effect on the POX promoter activity. Residual amounts of copper in the lipoproteins were determined with atomic absorption spectrophotometry, and addition of this amount of single copper sulfate had no effect on POX promoter activity.
POX promoter activity
Before the assay, the cells were cotransfested with the POX-luciferase (PxP) and pRL-null renilla construct (17). The cells were treated with various concentrations of oxLDL and nLDL at 6–10 h post-transfection. POX promoter activity was assessed with Dual-Luciferase reporter Assay (Promega, Madison, WI) in accordance with the manufacturer's instruction. All experiments were done in triplicates.
Western immunoblotting
The technique was done as described previously (17). Each experiment has been done at least twice and representative scan is shown. Cytochrome c-treated Jurkat cell extracts were used as positive controls for cleaved PARP (Cell Signaling, Danvers, MA). Light-chain 3 protein (LC3)-positive controls were from MBL (Woburn, MA).
POX enzyme activity
POX enzymatic activity was measured using O-aminobenzaldehyde assay (17). Briefly, pyrroline-5-carboxilate, the product of proline degradation, was bound with the O-aminobenzaldehyde and then amount of this complex was detected spectrophotometrically. The amount of pyrroline-5-carboxilate was determined using a pyrroline-5-carboxilate standard curve and calculated as nanomolar per minute per microgram of protein.
Reverse transcription–polymerase chain reaction
Total RNA was isolated using RNeasy Kit (Qiagen, Valencia, CA). The two-step reverse transcription (RT)–polymerase chain reaction (PCR) was performed with RT–PCR beads (GE Healthcare, Piscataway, NJ). The specific primers for the POX were as follows: forward, 5′-GCCATTAAGCTCACAGCACTGGG-3′; reverse, 5′-CTGATGGCCGGCTGGAAGTAG-3′ with a resulting product of 300 bp. β-Actin was amplified as a loading control with primers: forward, 5′-GATTCCTATGTGGGCGACGA-3′; reverse, 5′-CCATCTCTTGCTCGAAGTCC-3′ with a resulting product of 500 bp. PPARγ was amplified as recommended using RT–PCR primer from Santa Cruz Biotechnology (Santa Cruz, CA), PPARγ (h)-PR with a resulting product of 596 bp.
Quantitative PCR
Total RNA was extracted as described for RT–PCR and complementary DNA was synthesized using SuperScript™ III First-Strand Synthesis SuperMix (Invitrogen, Carlsbad, CA).
Expression of the gene of interest was measured by TaqMan quantitative PCR (Chromo4 Real-Time PCR detector; Bio-Rad, Hercules, CA). Inventoried Taqman primers for genes of interest and β-actin labeled with FAM and VIC fluorescent dyes, respectively, were from (Applied Biosystems, Foster City, CA). The samples were run in triplicates for target and endogenous β-actin control. The results were analyzed using MJ Opticon Monitor analysis software 3.1 (Bio-Rad).
Knockdown experiments
For POX knockdown, we used Stealth Select RNA interference (Invitrogen; target accessions no. NM 016335). Stealth RNA interference negative control Duplex (Invitrogen) was used as a control. Transfection efficiency was monitored using a fluorescent oligonucleotide (BLOCK-iT fluorescent oligonucleotide; Invitrogen) and estimated as 80–90%. PPARγ expression was knocked down with small interfering RNA (Santa Cruz Biotechnology) according to the manufacturer's instructions. Knockdown efficiency was analyzed by RT–PCR as described above for both POX Stealth RNA interference and PPARγ small interfering RNA (data not shown). Both transfections were done using Lipofectamine2000 (Invitrogen).
Plasmids and viral vectors
MCherry-LC3 construct, cloned from the original enhanced green fluorescent protein-LC3 (Addgene plasmid 11546; Addgene, Cambridge, MA) was used for autophagosomes visualization. We replaced green fluorescent protein sequence by mCherry sequence to be able to use this vector in DLD Tet-off POX cells, which already express green fluorescent protein under the conditions of doxycycline removal to report efficient POX gene expression. Recombinant adenoviral green fluorescent protein construct (Ad-GFP), manganese superoxide dismutase construct (Ad-MnSOD) and empty vector (Ad-Empty) were from Gene Therapy Vector Core Facility of the University of Iowa. The infection was done as described previously using 1000 multiplicity of infection (20).
Proliferation and viability assay
The effect of lipoproteins on cell viability/proliferation was estimated with CellTiter 96®Aqueous Solution Cell proliferation Assay (Promega).
ROS measurement
To measure intracellular ROS 2,7-dichlorohydrofluorescein diacetate (Sigma, St Louis, MO) was used. Cells were washed and exposed to serum-free, phenol red-free medium containing 50 μM 2,7-dichlorohydrofluorescein diacetate for 1 h in the darkness and the fluorescent intensity was measured using a spectrofluorometer (Jovin Yvon Specs2) with excitation and emission wavelengths of 485 and 530 nm, respectively.
Microscopy and visualization
Morphological changes in cancer cells treated with lipoproteins were estimated using an Axiovert 200M Zeiss microscope. Autophagosomes formation was visualized using LSM 510 laser scanning microscope (Zeiss, Thornwood, NY).
Data analysis
The results are reported as a mean ± SD from at least three experiments. Statistical differences between two groups were calculated by the unpaired Student's t-test, *P ≤ 0.05, **P ≤ 0.01.
Results
OxLDLs have cytotoxic effects on cancer cells in vitro and activate apoptosis and autophagy
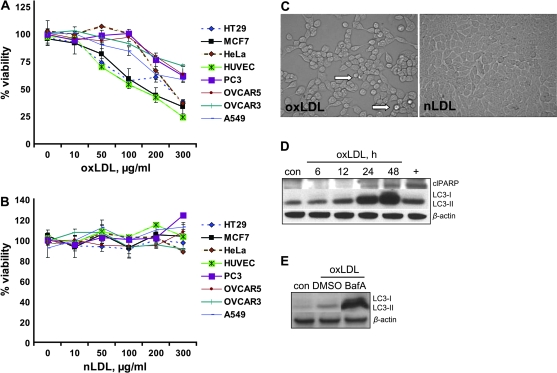
The effects of oxLDL were examined in several cancer cell lines: HT29 (colon), OVCAR3 and OVCAR5 (ovarian), HeLa (cervical), MCF7 (breast), A549 (lung) and PC3 (prostate). OxLDL, applied at physiologic concentrations, decreased cell viability and proliferation in a dose-dependent manner in all cell lines tested (Figure 1A), whereas native nLDL had no effect (Figure 1B).
Fig. 1.
Cytotoxic effect of oxLDL. (A) The effect of oxLDL and (B) nLDL on viability measured in various cancer cells and HUVEC treated for 24 h with lipoproteins as specified. (C) Phase-contrast microscopy picture shows morphology of HT29 cells treated with oxLDL versus nLDL. White arrow shows rounded-up cells. (D) Western immunoblotting was done for apoptotic and autophagic markers in HT29 cells treated with 200 μg/ml oxLDL for the indicated time. Plus sign represents positive control; ‘clPARP’ designates cleaved PARP. (E) HT29 cells were treated with either bafilomycin A1 (BafA) or dimethyl sulfoxide (DMSO) as a solvent control simultaneously with 200 μg/ml oxLDL for 24 h. Western immunoblotting for LC3-I and II was done to confirm oxLDL-dependent autophagic flux. (A and B) Data presented as mean ± SD. (D and E) β-Actin serves as loading control.
We also investigated the morphology of the cells treated with lipoproteins. NLDL (Figure 1C) and untreated control (data not shown) did not have any microscopically discernible adverse effects on HT29 cells. In contrast, oxLDL decreased the cell number per field and caused the cells to round up (Figure 1C, white arrow). Numerous non-adherent cells were observed and many neighboring cells lost their cell–cell contacts (data not shown).
To elucidate the process underlying the decrease in cell viability, we monitored apoptotic markers. First, in HT29 cells, the native 116 kDa PARP was cleaved to 85 kDa in a process dependent on duration of exposure to 200 μg/ml of oxLDL (Figure 1D). Since autophagy is often activated as a prosurvival mechanism during metabolic stress and, if excessive, may lead to programmed cell death type II, we also assessed markers of the autophagic process. The processing of microtubule-associated LC3 from the 18 kDa cytosolic LC3-I into the 16 kDa autophagosome-bound LC3-II lipidated form is a reliable marker of autophagy (30). We found a time-dependent increase of LC3-II peaking at 48 h of exposure to oxLDL (Figure 1D). To ensure that this is a dynamic and complete autophagic process and not just the accumulation of endocytosed oxLDL in autophagic vesicles, we blocked the fusion of the autophagosome with the lysosome using bafilomycin A1. Bafilomycin A1 led to a dramatic accumulation of the unprocessed LC3-II (Figure 1E). This observation provided convincing evidence that oxLDL indeed caused autophagic flux (30).
OxLDLs upregulate POX in cancer cells
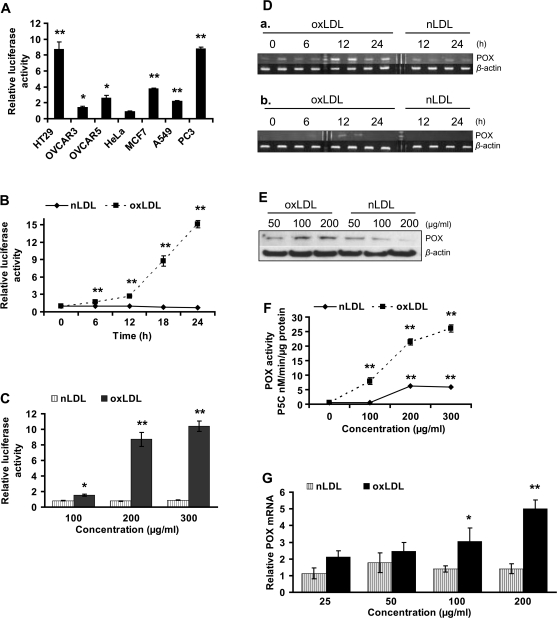
To ascertain whether POX is involved in oxLDL-mediated signaling, we sought oxLDL-dependent effects on POX promoter activity in cancer cells (Figure 2A). Among the tested cells, OVCAR3, OVCAR5, MCF7 and A549 presented modest activation of POX promoter. In these cell lines, POX promoter activity increased up to 4-fold with 200 μg/ml oxLDL relative to nLDL-treated controls. HT29 and PC3 cells showed strikingly high activity of the POX promoter, rising up to 9-fold. Using HT29 cells, we defined the time- and dose-dependent response of the POX promoter to oxLDL treatment (Figure 2B and C). POX promoter activity increased 2-fold by 6 h and almost up to 15-fold at 24 h relative to nLDL-treated controls. The dose-dependent response was found to be 1.6-fold with 100, 8.7-fold with 200 and 10.4 with 300 μg/ml oxLDL, respectively (Figure 2C). Native LDL did not cause significant POX promoter activation relative to untreated controls. OxLDL concentrations <100 μg/ml had no effect on POX promoter activity in HT29 (data not shown).
Fig. 2.
OxLDL upregulate POX in cancer and normal cells. (A) POX promoter activity was measured in various cancer cell lines as indicated after 18 h incubation with 200 μg/ml oxLDL. The graph represents fold change in relative luciferase activity of oxLDL-treated cells over the nLDL-treated cells. Untreated controls were run in parallel. NLDL-treated samples showed no POX promoter activation as compared with the untreated samples. (B and C) The graphs represent a fold change in relative luciferase activity in the oxLDL- or nLDL-treated cells over the untreated control. (B) Time- and (C) dose-dependent effect of 200 μg/ml oxLDL on POX promoter activity in HT29 cells. In (C), cells were treated for 18 h. (D) RT–PCR analysis of POX mRNA from (a) HT29 and (b) PC3 cells treated with 200 μg/ml oxLDL for the time specified. The samples were run in duplicates. (E) Western immunoblot analysis of POX protein levels in HT29 cells treated with oxLDL or nLDL as specified. (F) The graph shows POX enzymatic activity, expressed as amount of generated pyrroline-5-carboxylate (P5C) in nanomolar per minute per microgram protein under the influence of lipoproteins, representing dose-dependent effect. (G) The effect of oxLDL on POX expression in HUVEC was estimated with quantitative PCR. The cells were treated for 6 h with various concentrations of lipoproteins. Data are shown as relative POX mRNA level normalized to β-actin in oxLDL/nLDL-treated samples over untreated controls. (A–C, F and G) All data presented as a mean ± SD, *P ≤ 0.05, **P ≤ 0.01. (D and E) β-Actin serves as loading control.
To solidify the aforementioned finding, we further assessed oxLDL-dependent POX gene expression by determining POX messenger RNA (mRNA) with RT–PCR. POX gene expression increased 3.5-fold in HT29 (Figure 2Da, S. 1A) and 10-fold in PC3 (Figure 2Db, S. 1B) at 12 h with 200 μg/ml of oxLDL. Native LDL did not affect POX mRNA levels. Consistently, POX protein levels on western immunoblots increased in a dose-dependent fashion in HT29 treated with oxLDL, whereas it decreased in nLDL-treated cells (Figure 2E). We also found a consistent dose-dependent increase in POX enzymatic activity in the cells treated with oxidized lipoproteins (Figure 2F). In this experiment, native LDL had a slight effect on POX activity at higher concentrations, presumably due to partial oxidation of LDL during in vitro experiments.
Oxidized LDL activate POX gene expression in HUVEC
Endothelial cells are commonly studied as targets of oxLDL because they are most frequently exposed to oxLDL in the human organism. Thus, we used HUVEC to examine the effect of oxLDL on cellular viability and POX expression in normal untransformed cells. Testing the effect of lipoproteins on HUVEC viability, we found that oxLDL has a dose-dependent cytotoxic effect compared with nLDL (Figure 1A and B). This drastic effect on viability was preceded by the increase of POX mRNA levels in a dose-dependent manner in the cells treated with oxLDL for 6 h (Figure 2G). There is a stable trend of increasing POX gene expression starting at a concentration of oxLDL as low as 25 μg/ml, reaching almost 5-fold at 200 μg/ml compared with untreated control; nLDL had no significant effect.
7-ketocholesterol, the major component of oxLDL, has a similar effect on POX in cancer cells
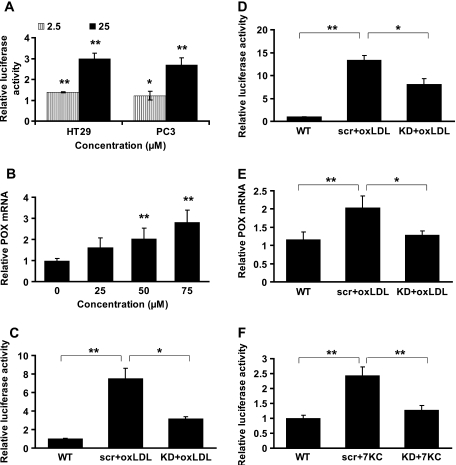
We asked whether the intact oxLDL particle was uniquely necessary for its molecular signaling (31) or whether one of its chemical components could substitute for oxLDL. We tested several oxLDL constituents, including hydroxynonenal, hydroperoxynonenal, 3-chlorothyrosine, 15-hydroxyeicosatetraenoic acid (15-HETE), and none had a significant effect on POX promoter activity (data not shown). Surprisingly, we found that 7-ketocholesterol (7KC), a major oxysterol of oxLDL, uniquely activated POX in a manner similar to oxLDL. 7KC at 2.5 and 25 μM significantly increased POX promoter activity in both HT29 and PC3 cells (Figure 3A). Consistently, 7KC also increased POX gene expression in a dose-dependent manner in HT29 (Figure 3B).
Fig. 3.
The effect of 7KC on POX and the role of PPARγ. (A) POX promoter activity was estimated in cancer cells after the treatment with 7KC at specified concentrations for 18 h. The graph depicts fold increase in relative luciferase activity in oxLDL-treated cells over nLDL-treated cells. 7KC solvent ethanol controls were run in parallel. NLDL-treated samples showed no POX promoter activation as compared with the solvent controls. (B) Quantitative PCR results plotted as relative POX mRNA levels normalized to β-actin mRNA from HT29 cells treated with increasing concentrations of 7KC for 24 h. (C–F) The cells were either transfected with PPARγ small interfering RNA (KD) or scrambled RNA (scr) (data not shown). Both samples were treated with oxLDL or 7KC as indicated. Each experiment had intact untreated wild-type (WT) as reference. (C) POX promoter activity in HT29 and (D) PC3 cancer cells treated with 200 μg/ml oxLDL for 18 h. (E) Quantitative PCR was done to estimate the level of POX mRNA in HT29 treated with 200 μg/ml oxLDL for 12 h. (F) POX promoter activity in HT29 treated with 25 μM 7KC for 18 h. (A–F) All data presented as mean ± SD, *P < 0.05, **P < 0.01.
OxLDL and 7KC activate POX through PPARγ
Since both HT29 and PC3 showed robust responses of POX promoter activity to oxLDL, and both have wild type PPARγ (17,32), we wondered whether the effect of oxLDL on POX is mediated by this nuclear receptor. To test this, we knocked down PPARγ (data not shown) and measured POX promoter activity in the cells treated with lipoproteins. PPARγ knockdown significantly decreased the response of POX promoter to oxLDL in both HT29 and PC3 (Figure 3C and D, respectively). The same effect was found at the level of gene expression in HT29 (Figure 3E). Additionally, we found that PPARγ knockdown decreased 7KC-mediated POX promoter activation in HT29 almost to the level of untreated control (Figure 3F).
POX is necessary to activate protective autophagy in cancer cells exposed to oxLDL
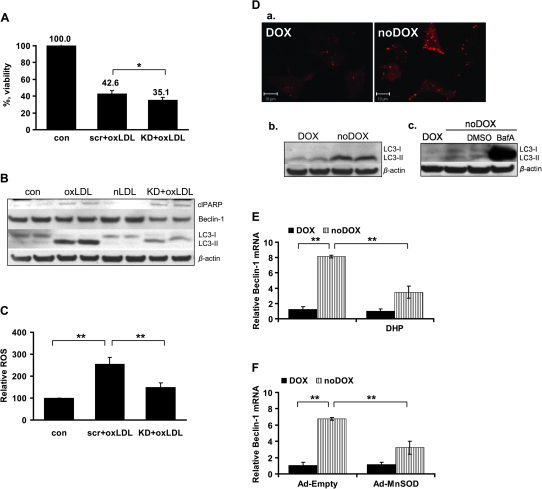
In an effort to decipher the exact function of POX in oxLDL-mediated cytotoxicity, we established small interfering RNA-based POX knockdown (data not shown). After transfection, cells were treated with 200 μg/ml oxLDL for 24 h. First, we checked whether POX knockdown may affect cellular viability of cancer cells treated with oxLDL and found that POX knockdown moderately but significantly decreased cellular viability, suggesting that POX provided protection against oxLDL-mediated cytotoxicity under these conditions (Figure 4A).
Fig. 4.
Role of POX in oxLDL-mediated cytotoxicity. For (A–C), cells were transfected with either POX RNA interference (KD) or scrambled RNA (scr) (data not shown). Both samples were treated with 200 μg/ml oxLDL for 24 h. Untreated intact and/or nLDL-treated sample were run as control in each experiment. (A) Cellular viability was measured with MTS in HT29. (B) Western immunoblotting for apoptotic and autophagic markers. ‘clPARP’ designates cleaved PARP. (C) Relative ROS were measured as fluorescence intensity and expressed relative to total protein amount in the sample. (D) (a) Confocal microscopy image of the DLD-1 Tet-off POX cells with and without doxycycline. Autophagosomes accumulation is tracked by the transient transfection of mCherry-LC3 vector (red fluorescence). (b) Western immunoblotting for autophagic LC3-I and II in DLD-1 Tet-off POX cells with and without doxycycline. (c) Western immunoblotting showing the presence of the autophagic flux in the DLD-1 Tet-off POX cells withdrawn from doxycycline, which were treated with either bafilomycin A1 (BafA) or DMSO (solvent control). (E) Quantitative PCR data presenting relative beclin-1 mRNA expression in DLD Tet-off POX cells with and without doxycycline, as well as with and without inhibitor of POX catalytic activity dehydroproline (DHP). (F) Quantitative PCR data presenting relative beclin-1 mRNA expression in DLD-1 Tet-off POX cells with and without doxycycline, as well as infected either with an empty control virus (Ad-Empty) or with an adenoviral MnSOD (Ad-MnSOD). (A, C, E and F) Data presented as mean ± SD, *P ≤ 0.05, **P ≤ 0.01. (B and D) β-Actin represents loading control.
Since we found that oxLDL activated apoptosis and autophagy in cancer cells, we wondered whether any of these cellular responses could be affected by POX knockdown. We found no effect on PARP cleavage. Instead, we discovered a dramatic decrease in the autophagy-related conversion of LC3-I to LC3-II as a result of POX knockdown (Figure 4B). Scramble RNA alone had no effect (data not shown). Therefore, based on these findings we conclude that POX is responsible for protective autophagy activation in cancer cells exposed to oxLDL.
POX regulates autophagy through beclin-1
A recent report suggests that oxLDL may regulate cellular fate through mTOR (33), an important player in cellular energy homeostasis and a major regulator of autophagy (34). However, it was determined that POX responds to metabolic stress downstream of mTOR (23). Therefore, we asked whether POX, in turn, may regulate oxLDL-dependent autophagy indirectly through mTOR. The marked decrease in both total and phosphorylated mTOR with oxLDL treatment and the inability of POX knockdown to mitigate this oxLDL effect led us to exclude mTOR involvement in oxLDL/POX-dependent regulation of autophagy (data not shown).
To address the question of how autophagy is regulated in this situation, we also examined the effect of POX knockdown on beclin-1, which is one of the central regulators of autophagy in mammalian cells (35,36). First, we found that oxLDL increased beclin-1 gene expression at the mRNA level (data not shown), but did not change the amount of beclin-1 protein. The latter may be explained by the rapid protein turnover in the cell undergoing active autophagy. The important fact that POX knockdown was able to decrease beclin-1 protein level (Figure 4B) in cells treated with oxLDL provided evidence that the effect of POX on autophagy is possibly mediated through beclin-1.
To confirm this finding we used DLD-1 Tet-off POX cells, which stably overexpress POX under doxycycline control (18). We found that POX expression accompanying doxycycline withdrawal triggers autophagosome accumulation (Figure 4Da), and conversion of LC3-I into LC3-II (Figure 4Db), as well as increases expression of beclin-1 (Figure 4E, left side). The presence of autophagic flux was confirmed using bafilomycin A1, which led to a striking accumulation of the unprocessed LC3-II (Figure 4Dc). Therefore, we conclude that not only did oxLDLs mediate POX upregulation but also POX overexpression, by itself, is sufficient to upregulate autophagy.
OxLDL triggers POX-mediated ROS generation in cancer cells
Autophagy is a prosurvival response to metabolic stress (37), the survival of even a few cancer cells enables tumor regrowth. Thus, the mechanism by which POX mediates the demonstrated regulation is of considerable interest. There are two major pathways for POX-dependent regulation. Metabolizing proline, POX can generate ATP or ROS (38). To distinguish between these possibilities, we first measured ATP levels in oxLDL-treated cells. A decrease in ATP would fit into the finding described above, in which POX knockdown resulted in a decreased viability. However, we found no effect on ATP levels with POX knockdown in this setting (data not shown). It is noteworthy, nevertheless, that oxLDL did decrease intracellular ATP almost 2-fold compared with nLDL or an untreated control. Several studies reported that oxLDL and its components impair cell energy metabolism by downregulating variety of metabolic enzymes (39–41), which may be the explanation as to why ATP was unaffected by POX knockdown. Further, we examined the alternative mechanistic possibility of POX-dependent ROS generation. Remarkably, we found that oxLDL markedly increased intracellular ROS generation (254.9%) and, more importantly, nearly two-thirds of the oxLDL-stimulated ROS was POX dependent (Figure 4C).
POX-generated superoxide is essential for beclin-1 upregulation
Having shown that POX is activated by oxLDL through PPARγ, and POX initiates autophagy through the induction of beclin-1, the mechanism for the POX-mediated effect becomes critically important. We found that POX regulates autophagy and hypothesized that this occurs via POX-dependent ROS generation. To confirm this we aimed to establish a direct link between POX enzymatic activity and beclin-1 expression. In DLD-1 Tet-off POX cells the expression of POX triggered beclin-1 upregulation (Figure 4E, left side). That this was dependent on POX catalytic activity, but not due to accumulation of the excess of protein, was shown using dehydroproline, a competitive inhibitor of POX. Inhibition of POX activity in the face of POX overexpression consistently and markedly mitigated the increase in beclin-1 expression accompanying the withdrawal of doxycycline (Figure 4E, right side). POX mRNA levels were measured in the same samples to ensure the overexpression of POX (data not shown). Therefore, we conclude that POX catalytic activity is required to upregulate beclin-1. Since we found that oxLDL-dependent regulation of autophagy depends on POX-generated ROS, we aimed to test whether it is specific for POX-generated superoxide (20). We infected DLD-1 Tet-off POX cells with adenoviral vector containing MnSOD (data not shown), which is responsible for POX-dependent superoxide detoxification (20), and found that it was able to reduce beclin-1 expression under docycycline withdrawal similar to dehydroproline (Figure 4F). These results confirm our hypothesis that POX regulates autophagy by superoxide-dependent induction of beclin-1.
Discussion
A number of reports have described the dichomatous properties of oxLDL. Our studies clearly show that oxLDLs have cytotoxic effects concurrently activating both apoptosis and autophagy in cancer cells. We establish here for the first time a link between metabolism of blood-borne lipids and amino acids. Specifically, we show that oxidized cholesterol-bearing lipoproteins applied in physiological concentrations (28,29) upregulate a key enzyme of proline metabolism, POX, in cancer cells and normal HUVECs. In contrast, native nLDL had no effect on POX promoter activity and mRNA levels, suggesting that oxidized components play a pivotal role in POX regulation. NLDL was able to decrease POX protein levels because they serve as a major energy source and may compete with proline, an alternative energy source. This is consistent with a previous report that POX activity was inhibited by long-chain acyl coenzyme A generated presumably during lipolysis (42). These findings once again emphasize pathophysiologic alterations of lipid and amino acid metabolism in both atherosclerosis and cancer. We show here for the first time that those alterations may be a direct result of oxLDL toxicity.
LDL is a complex nanoparticle with a cholesterol ether core enveloped by phospholipids, non-esterified cholesterol and apolipoprotein B100. Subjected to oxidative stress, each and all LDL components may be oxidized (43). It was not known whether it is the complex structure (31) of the oxLDL particle in toto or its specific components that are responsible for its interaction with molecular targets. To identify a component of oxLDL responsible for POX activation, we tested several constituents of oxLDL and found that only 7KC activates in a manner similar to total oxLDL. 7KC is present in human atherosclerotic plaques and plays an active role in plaque development through the induction of apoptosis in vascular endothelial cells (44). Oxysterols are elevated in human LDL and their higher plasma levels is associated with an increased risk of atherosclerosis (43). In regard to cancer, oxysterols had a cytotoxic effect on human neuroblastoma cells (45). This fact is consistent with our observation that oxLDL, bearing 7KC, is cytotoxic to cancer cells.
The presence of PPARγ ligands in oxLDL (16) and the robust POX response to PPARγ signaling (17,46) strongly suggested that the oxLDL effects on POX involve PPARγ. Our knockdown experiments showed that oxLDL indeed regulate POX through the PPARγ nuclear receptor in both HT29 and PC3. For the 7KC effect on POX, however, the dependence on PPARγ is evident in HT29, but not in PC3. The knockdown of PPARγ in HT29 colon cancer cells completely abolished the 7KC-dependent increase in POX, but did not block the 7KC effect on POX in PC3 cells (data not shown). We can only speculate that the effect of 7KC on POX in PC3 prostate cancer cells may involve additional signaling pathways.
Although our laboratory previously described mainly a proapoptotic function of POX (21), POX knockdown did not affect oxLDL-dependent apoptosis. This may be due to the complex structure of oxLDL and synergistic or overlapping effects of various oxLDL components, including PPARγ-mediated signaling in addition to POX. Instead, POX knockdown interfered with the autophagic pathway. Whether oxidized lipids use POX as a switch for autophagy is an attractive possibility. Clearly, POX plays a role in regulating autophagy in cancer cells. The fact that decreased cellular viability in oxLDL-treated cells was further accentuated by POX knockdown emphasizes the prosurvival role of POX upregulation. Together with the finding that POX knockdown interfered with autophagy activation led us to conclude that POX activates protective autophagy and serves as a survival response to the cytotoxic effect of oxLDL. This finding becomes even more intriguing because the POX metabolic system was recently defined as part of the ‘ecophagy’ machinery. Ecophagy literally means ‘eating its environment’ and was proposed as a mechanism (38) among others, such as autophagy (26) and entosis (47) for survival of cancer cells. The temporal sequence of these processes in the tumor microenvironment remains unclear, but our current work shows that activation of the central player of ecophagy, POX, may precede and activate autophagy. As we mentioned previously, this sequence may be advantageous for the cancer cell in that it has a ‘sparing’ effect (48). Moreover, oxLDL-related carcinogenic effects may arise when oxLDL damage the cells; repair mechanism and POX-dependent autophagy are activated, which altogether may allow selection of cancer stem cells (49). Although POX was initially found to be involved in the apoptotic process downstream of p53 (50), HT29 has mutant and PC3 has null p53; therefore, all POX-dependent regulation of autophagy described here is p53 independent.
POX is a multifunctional enzyme and its structure underlines its multiple functions (51). The pair of electrons transferred from proline to the flavine adenine dinucleotide of POX may be donated to the electron transport chain to generate ATP (23). Alternatively, electrons may directly reduce solvent oxygen to produce superoxide and its derivatives (18,51). These POX-dependent products may also serve as direct signaling molecules. Strikingly, we found that nearly two-thirds of oxLDL-mediated ROS generation is POX dependent. Therefore, POX knockdown decreased autophagy and simultaneously decreased POX-dependent ROS generation. Using a POX-overexpressing cell model, DLD-1 Tet-off POX, we found that beclin-1 gene expression is directly dependent on POX catalytic activity and POX-dependent superoxide. Superoxide is a specific ROS generated by POX (20). Recently, autophagy was found to be regulated by mitochondrial superoxide (52), but to our knowledge, this is the first report of a specific metabolic enzyme responsible for this regulation. Thus, we describe here for the first time that POX, acting as a stress response mechanism, regulates protective autophagy in cancer cells by generating ROS.
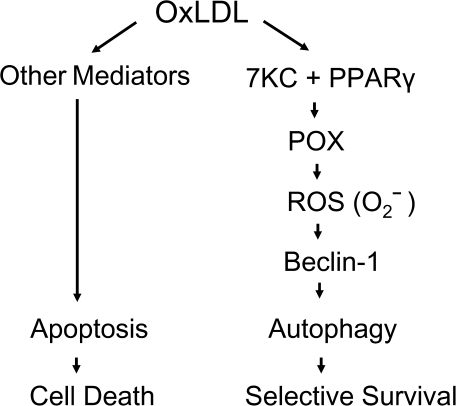
To summarize, we have established that oxLDL can initiate cell death in cancer cells. The key metabolic enzyme of proline degradation, POX, is regulated by oxLDL and it plays a regulatory role in oxLDL-mediated prosurvival autophagy. This mechanism is based on one of the chemical components of oxLDL, 7KC, through PPARγ. We also showed that the POX effect on autophagic machinery is executed through the generation of superoxide and subsequent regulation of beclin-1 (Figure 5).
Fig. 5.
Dual role of oxLDL in cancer cells. OxLDL activate both apoptosis and autophagy in cancer cells. While apoptosis definitely leads to cellular death, autophagy is activated as a prosurvival mechanism. Autophagy, specifically beclin-1 transcription, is being regulated by POX-dependent superoxide through oxLDL-derived 7KC involving PPARγ. This mechanism provides sustained cellular survival under the conditions of oxLDL-mediated stress.
Funding
Intramural Research Program of the National Institutes of Health; National Cancer Institute; Center for Cancer Research.
Acknowledgments
The content of this publication does not necessarily reflect the views and policies of the Department of Health and Human Services nor does the mention of trade names, commercial products or organizations imply endorsement by the US government.
Authors thank Dr Anna Ilinskaya and Dr Vladimir Pak, HIV Drug Resistance Program, National Cancer Institute-Frederick, for the help in cloning; Dr Michael Waalkes and Dr Wei Qu, Inorganic Carcinogenesis Section, LCC, NIEHS, for measuring the levels of residual copper in lipoproteins.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ATP
adenosine triphosphate
- HUVEC
human umbilical vein endothelial cell
- 7KC
7-ketocholesterol
- LC3
light-chain 3 protein
- LDL
low-density lipoprotein
- mRNA
messenger RNA
- mTOR
mammalian target of rapamycin
- nLDL
non-oxidized low-density lipoprotein
- oxLDL
oxidized low-density lipoprotein
- PCR
polymerase chain reaction
- POX
proline oxidase
- PPARγ
peroxisome proliferator-activated receptor gamma
- ROS
reactive oxygen species
- RT
reverse transcription
References
- 1.Suzuki K, et al. Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: a case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol. Biomarkers Prev. 2004;13:1781–1787. [PubMed] [Google Scholar]
- 2.Ross JS, et al. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann. N. Y. Acad. Sci. 2001;947:271–292. discussion 292–293. [PubMed] [Google Scholar]
- 3.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couillard C, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J. Clin. Endocrinol. Metab. 2005;90:6454–6459. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 5.Delimaris I, et al. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007;40:1129–1134. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Ishigaki Y, et al. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118:75–83. doi: 10.1161/CIRCULATIONAHA.107.745174. [DOI] [PubMed] [Google Scholar]
- 7.Zettler ME, et al. OxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H644–H653. doi: 10.1152/ajpheart.00494.2001. [DOI] [PubMed] [Google Scholar]
- 8.Heinloth A, et al. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J. Am. Soc. Nephrol. 2000;11:1819–1825. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 9.Esterbauer H, et al. Possible mutagens derived from lipids and lipid precursors. Mutat. Res. 1990;238:223–233. doi: 10.1016/0165-1110(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 10.Zettler ME, et al. Oxidized low-density lipoprotein retards the growth of proliferating cells by inhibiting nuclear translocation of cell cycle proteins. Arterioscler. Thromb. Vasc. Biol. 2004;24:727–732. doi: 10.1161/01.ATV.0000120373.95552.aa. [DOI] [PubMed] [Google Scholar]
- 11.Maziere C, et al. Oxidized LDL induces an oxidative stress and activates the tumor suppressor p53 in MRC5 human fibroblasts. Biochem. Biophys. Res. Commun. 2000;276:718–723. doi: 10.1006/bbrc.2000.3528. [DOI] [PubMed] [Google Scholar]
- 12.Cheng J, et al. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology. 2007;148:2085–2094. doi: 10.1210/en.2006-1709. [DOI] [PubMed] [Google Scholar]
- 13.Duerrschmidt N, et al. Lectin-like oxidized low-density lipoprotein receptor-1-mediated autophagy in human granulosa cells as an alternative of programmed cell death. Endocrinology. 2006;147:3851–3860. doi: 10.1210/en.2006-0088. [DOI] [PubMed] [Google Scholar]
- 14.Martinet W, et al. 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:2296–2301. doi: 10.1161/01.ATV.0000146266.65820.a1. [DOI] [PubMed] [Google Scholar]
- 15.Phang JM, et al. PPARgamma and proline oxidase in cancer. PPAR Res. 2008;2008:542694. doi: 10.1155/2008/542694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy L, et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 17.Pandhare J, et al. Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J. Biol. Chem. 2006;281:2044–2052. doi: 10.1074/jbc.M507867200. [DOI] [PubMed] [Google Scholar]
- 18.Donald SP, et al. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–1815. [PubMed] [Google Scholar]
- 19.Maxwell SA, et al. Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J. Biol. Chem. 2003;278:9784–9789. doi: 10.1074/jbc.M210012200. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis. 2005;26:1335–1342. doi: 10.1093/carcin/bgi083. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, et al. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 2006;25:5640–5647. doi: 10.1038/sj.onc.1209564. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, et al. Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Res. 2009;69:6414–6422. doi: 10.1158/0008-5472.CAN-09-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandhare J, et al. Regulation and function of proline oxidase under nutrient stress. J. Cell Biochem. 2009;107:759–768. doi: 10.1002/jcb.22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell. Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 25.Levine B, et al. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew R, et al. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HW, et al. Oxidized low-density lipoproteins, autoantibodies against oxidized low-density lipoproteins and carotid intima media thickness in a clinically healthy population. Cardiology. 2008;110:252–259. doi: 10.1159/000112409. [DOI] [PubMed] [Google Scholar]
- 29.Raijmakers MT, et al. Low plasma levels of oxidized low density lipoprotein in preeclampsia. Acta Obstet. Gynecol. Scand. 2004;83:1173–1177. doi: 10.1111/j.0001-6349.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 30.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabirnyk O, et al. Nanoparticles as a novel class of autophagy activators. Autophagy. 2007;3:278–281. doi: 10.4161/auto.3916. [DOI] [PubMed] [Google Scholar]
- 32.Mueller E, et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc. Natl Acad. Sci. USA. 2000;97:10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brito PM, et al. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2008;205:126–134. doi: 10.1016/j.atherosclerosis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Nicklin P, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, et al. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 36.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 37.Karantza-Wadsworth V, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phang JM, et al. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids. 2008;35:681–690. doi: 10.1007/s00726-008-0063-4. [DOI] [PubMed] [Google Scholar]
- 39.Sukhanov S, et al. Global analysis of differentially expressed genes in oxidized LDL-treated human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2003;306:443–449. doi: 10.1016/s0006-291x(03)00990-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen CY, et al. Proteomic approach to study the effects of various oxidatively modified low-density lipoprotein on regulation of protein expression in human umbilical vein endothelial cell. Life Sci. 2007;80:2469–2480. doi: 10.1016/j.lfs.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Szweda LI, et al. Inactivation of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Selective modification of an active-site lysine. J. Biol. Chem. 1993;268:3342–3347. [PubMed] [Google Scholar]
- 42.Phang JM, et al. Inhibition of proline oxidase by long-chain acyl coenzyme-A. Fed. Proc. 1978;37:1480. [Google Scholar]
- 43.Brown AJ, et al. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki H, et al. Vascular smooth muscle cell apoptosis induced by 7-ketocholesterol was mediated via Ca2+ and inhibited by the calcium channel blocker nifedipine. Metabolism. 2007;56:357–362. doi: 10.1016/j.metabol.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Rao ML, et al. Induction of apoptosis and necrosis in human neuroblastoma cells by cholesterol oxides. Ann. N. Y. Acad. Sci. 1999;893:379–381. doi: 10.1111/j.1749-6632.1999.tb07860.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim KY, et al. Apoptotic action of peroxisome proliferator-activated receptor-gamma activation in human non small-cell lung cancer is mediated via proline oxidase-induced reactive oxygen species formation. Mol. Pharmacol. 2007;72:674–685. doi: 10.1124/mol.107.035584. [DOI] [PubMed] [Google Scholar]
- 47.Overholtzer M, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 48.Seleverstov O, et al. Semiconductor nanocrystals in autophagy research: methodology improvement at nanosized scale. Methods Enzymol. 2009;452:277–296. doi: 10.1016/S0076-6879(08)03618-5. [DOI] [PubMed] [Google Scholar]
- 49.Beachy PA, et al. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 50.Polyak K, et al. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 51.White TA, et al. Structure and kinetics of monofunctional proline dehydrogenase from Thermus thermophilus. J. Biol. Chem. 2007;282:14316–14327. doi: 10.1074/jbc.M700912200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, et al. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]