Abstract
Hypoxia-inducible factors (HIFs), in particular HIF-1α, have been implicated in tumor biology. However, HIF target genes in the esophageal tumor microenvironment remain elusive. Gene expression profiling was performed upon hypoxia-exposed non-transformed immortalized human esophageal epithelial cells, EPC2-hTERT, and comparing with a gene signature of esophageal squamous cell carcinoma (ESCC). In addition to known HIF-1α target genes such as carbonic anhydrase 9, insulin-like growth factor binding protein-3 (IGFBP3) and cyclooxygenase (COX)-2, prostaglandin E synthase (PTGES) was identified as a novel target gene among the commonly upregulated genes in ESCC as well as the cells exposed to hypoxia. The PTGES induction was augmented upon stabilization of HIF-1α by hypoxia or cobalt chloride under normoxic conditions and suppressed by dominant-negative HIF-1α. Whereas PTGES messenger RNA (mRNA) was negatively regulated by normoxia, PTGES protein remained stable upon reoxygenation. Prostaglandin E2 (PGE2) biosynthesis was documented in transformed human esophageal cells by ectopic expression of PTGES as well as RNA interference directed against PTGES. Moreover, hypoxia stimulated PGE2 production in a HIF-1α-dependent manner. In ESCC, PTGES was overexpressed frequently at the mRNA and protein levels. Finally, COX-2 and PTGES were colocalized in primary tumors along with HIF-1α and IGFBP3. Activation of the COX-2–PTGES axis in primary tumors was further corroborated by concomitant upregulation of interleukin-1β and downregulation of hydroxylprostaglandin dehydrogenase. Thus, PTGES is a novel HIF-1α target gene, involved in prostaglandin E biosynthesis in the esophageal tumor hypoxic microenvironment, and this has implications in diverse tumors types, especially of squamous origin.
Introduction
Oxygen tension is among the key elements in the tumor microenvironment that influences cancer development and progression. Hypoxia-inducible factors (HIFs), comprising an oxygen-sensitive α-subunit and a constitutively expressed β-subunit, facilitate global cellular adaptation to hypoxia and oxygen delivery by transcriptionally activating genes essential in various processes such as glucose transport, glycolysis, angiogenesis and erythropoiesis (1,2).
The normal esophageal epithelium expresses very little HIF-α protein (3). In contrast, HIF-1α is expressed highly in 30–70% of primary esophageal squamous cell carcinomas (ESCCs) and associated with induction of vascular endothelial growth factors, tumor invasion, lymphatic invasion or lymph node metastasis and a lower post-operative survival rate (4–7). Interestingly, the normal esophageal epithelium adjacent directly to the tumor expresses HIF-1α to a variable extent, implying changes in the esophageal tumor microenvironment (8). Moreover, HIF-1α expression has been detected in ∼50% of early-stage esophageal cancers (8). HIF-1α protein expression is highly inducible by hypoxia treatment in cultured esophageal cancer cell lines (5), suggesting that overexpression of HIF-1α in primary esophageal tumors is mainly accounted for by the lack of oxygen availability. However, information is limited regarding the expression and function of HIF target genes in the hypoxic esophageal tumor microenvironment.
Prostaglandin E2 (PGE2)-mediated signaling and the enzymes regulating its biosynthesis play a pivotal role in cancer development (9). In particular, cyclooxygenase (COX)-2 has been studied extensively as a key rate-limiting enzyme for prostanoid biosynthesis, implicated in the pathogenesis, disease progression and poor survival rates in various tumor types, including ESCC (10–12). Prostaglandin E synthase (PTGES) has emerged as another essential enzyme not only functioning downstream of COX-2 but also being activated by proinflammatory stimuli such as interleukin-1β (IL-1β) and lipopolysaccharide (13). PTGES is upregulated in gastrointestinal cancers and premalignant lesions such as colonic adenomatous polyps (14–18). Although PTGES has been shown to be expressed in esophageal adenocarcinoma (19), its expression and regulatory mechanisms in ESCC remain to be elucidated.
In this study, we carried out gene array experiments using an immortalized human esophageal epithelial cell line, EPC2-hTERT (20), exposed to hypoxia. Comparison of hypoxic gene signature in EPC2-hTERT with gene expression profiling data in primary esophageal tumors revealed the regulation of prostanoid biosynthesis by the COX-2–PTGES enzyme axis as a novel hypoxia target pathway in esophageal cancer.
Materials and methods
Tissue samples
Esophageal tissues were procured via surgery at the Okayama University Hospital (M.T., Y.S. and Y.N.), Kitano Hospital (MK) and the Hospital of the University of Pennsylvania through the Cooperative Human Tissue Network. All were pathologically diagnosed as ESCC. Forty paired tumors and adjacent normal tissues, including 35 pairs on tissue microarray, were available as paraffin blocks. Frozen tissues were available for RNA (13 cases) and protein (13 cases) analyses. All the clinical materials were obtained from informed consent patients in accordance with Institutional Review Board standards and guidelines.
Cell cultures
Primary normal human esophageal cells EPC1 and EPC2 and non-transformed immortalized diploid human esophageal cell line, EPC2-hTERT, and its derivative transformed by epidermal growth factor receptor, p53R175H and cyclin D1, were grown under normoxic conditions (21% O2, 5% CO2 and 95% humidity) at 37°C in Keratinocyte-SFM serum-free medium (Invitrogen, Carlsbad, CA) as described previously (20–23). Subconfluent cells were exposed to hypoxia (0.2 or 1% O2) in an Invivo2 hypoxia chamber (Ruskinn Technologies, Bridgend, UK). Cobalt chloride (Sigma Aldrich, St. Louis, MO) was used at a concentration of 250 μM.
Constructs and retrovirus- and lentivirus-mediated gene transduction
Wild-type and dominant-negative HIF-1α complementary DNAs (cDNAs) in pcDNA3.1(+), provided by Drs Chen and Kobayashi (Hokkaido University, Sapporo, Japan) (24), were isolated by restriction digestion with BamHI for wild-type HIF-1α and polymerase chain reaction (PCR) with restriction site-tagged sense 5′-(BglII)-gccagatctgccgccaccatggggggttctcatcatcatcatcatcatcatggtatggc-3′ and antisense 5′-(XhoI)-ggcctcgagtcatttgtcatttgtcaaagaggctact-3′ primers for dominant-negative HIF-1α, respectively, and subcloned into pBABE-puro-loxP at its BamHI site for the former and BamHI and SalI sites for the latter, resulting in creation of pBABE-puro-loxP-WT-HIF-1α and pBABE-puro-loxP-DN-HIF-1α, respectively. PTGES cDNA derived from TE7 esophageal cancer cell line (25) was subcloned into pBABE-bla (23), resulting in creation of pBABE-bla-PTGES. The constructs were verified by DNA sequencing. Replication-incompetent retrovirus was produced as described previously (23).
Lentiviral pGIPZ expressing short hairpin RNA directed against human PTGES (clone ID # V2LHS_67663 and V2LHS_67660) or a non-silencing-GIPZ lentiviral shRNAmir control vector (Open Biosystems, Huntsville, AL) was transfected into HEK-293T cells with Arrest-In Transfection Reagent (Open Biosystems) according to the manufacturer's instructions. The virus-containing medium was harvested 48 and 72 h after transfection, filtered (0.45 μm) (Pall Gelman Laboratory, Ann Arbor, MI) and stored at −80°C until use.
For virus infection, 0.5–1.5 × 105 cells were seeded per well in a six-well tissue culture plate and subjected to the spin infection method in the presence of 4 μg/ml polybrene (Sigma-Aldrich Corp., St Louis, MO) as described previously (23). Cells transduced with wild-type HIF-1α, dominant-negative HIF-1α or an empty vector (pBABE-puro-loxP) was selected with 5 μg/ml of puromycin (Invitrogen) for 5 days following retrovirus infection. To establish PTGES overexpressing cells and empty vector (pBABE-bla)-transduced control cells, 10 μg/ml of blasticidin S (Invitrogen) was added 36 h after infection and selected for 5 days. Cells transduced with short hairpin RNA were analyzed by fluorescence-activated cell sorter and sorted as the top 20% of the most highly green fluorescent protein-expressing cells in the Flow Cytometry and Cell Sorting Facility at the University of Pennsylvania.
Transient transfection and dual-luciferase assays
HIF-mediated transcriptional activity was determined using (eHRE)3-Luc, containing three copies of the hypoxia response element from the EPO gene enhancer in pGL3 (Promega, Madison, WI) (26). Transfection was carried out using the FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Briefly, 1 × 105 cells were seeded per well in 24-well plates 24 h before transfection. Four hundred nanograms of the (eHRE)3-Luc or pGL3-Promoter (Promega) control vector plasmid DNA was transfected along with 5 ng of phRL-SV40-renilla luciferase vector (Promega) to calibrate the variation of transfection efficiencies among wells. Cells were exposed to hypoxia or cobalt chloride 24 h after transfection and incubated for 24 h before cell lysis. Luciferase activities were determined using the Dual-Luciferase™ Reporter Assay system (Promega) and the ORION Microplate Luminometer (Berthold Detection Systems USA, Oak Ridge, TN). The mean of fire fly luciferase activity was normalized with the cotransfected renilla luciferase activity. Transfection was carried out at least three times, and variation between experiments was not >15%.
RNA isolation, cDNA synthesis and real-time reverse transcription–PCR
Total RNA was isolated from cultured cells and snap-frozen tissues with the RNeasy Mini Kit and the RNeasy Fibrous Tissue Midi Kit (Qiagen, Valencia, CA), respectively. Integrity of total RNA was determined by Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was synthesized with the Superscript™ First Strand Synthesis System (Invitrogen) as described previously (25).
Real-time PCR was done using the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) with TaqMan® Gene Expression Assays (Applied Biosystems) for PTGES (Hs00610420_m1), COX-2 (Hs00153133_m1), hydroxylprostaglandin dehydrogenase (HPGD) (Hs00168359_m1), IL-1β (Hs00174097_m1) and carbonic anhydrase 9 (Hs00154208_m1). SYBR green reagent (Applied Biosystems) was used to quantitate messenger RNA (mRNA) for insulin-like growth factor binding protein-3 (IGFBP3) and β-actin as described previously (25). All PCRs were performed in triplicate. The relative expression level of each mRNA was normalized to the β-actin mRNA level as an internal control.
Microarray analysis
Gene array experiments were performed using an Affymetrix gene chip (U133+v2.0) (Affymetrix, Santa Clara, CA) upon RNA representing EPC2-hTERT exposed to 0.2% O2 (severe hypoxia), 1% O2 (moderate hypoxia) or 21% O2 (normoxia) for 24 h. Preparation of complementary RNA, hybridization and scanning of the arrays were performed as described previously (25). Similarly, gene array experiments were done on paired ESCC tumor and normal tissues (n = 5). .CEL files (probe level data) were exported from Affymetrix® GeneChip® Operating Software that was also used to calculate detection flags, which were exported in .chp files. Probe level data (.CEL files) were imported with Partek Genomics Suite (v. 6.4; Partek, St Louis, MO). The data were normalized and summarized to the probe set level using GeneChip Robust Multiarray Average, yielding log2 intensities for all probe sets on the array. These intensity values were then filtered based on the detection flags, retaining only those probe sets flagged as present in at least one of four samples for each of the hypoxia-exposed cell experiments and those present in at least 4 of 10 for the tumor and normal tissue samples. Significance analysis of microarrays (Ver. 3.02) was done to identify differentially expressed transcripts and calculate Q-values, d-scores and fold change on all probe sets passing filter. The hypoxia datasets were treated as a two-class unpaired design, whereas the ESCC tumor dataset was treated as a two-class paired experiment. Further analysis was done to create gene lists and a Venn diagram based on cut-off values; fold change >2, Q <1% for 0.2% O2, Q <3% for 1% O2 and Q <10% for tissue samples. Microarray data (accession # GSE17353 for hypoxia experiments and GSE17351 for ESCC tumors) were deposited with Gene Expression Omnibus at the NCBI (http://www.ncbi.nlm.nih.gov/geo).
Western blotting
Western blotting was done as described previously (25). Twenty micrograms of cleared cell lysate or tissue homogenate was denatured and fractionated on a NuPAGE Bis–Tris 4–12% gel (Invitrogen) and transferred to Immobilon-P membranes (Millipore, Billerica, MA). The membranes were incubated with anti-HIF-1α (H1alpha67) (Novus Biologicals, Littleton, CO) at 1:500 dilution, anti-PTGES (Oxford Biomedical Research, Oxford, MI) at 1:1000 dilution, anti-IGFBP3 (DSL-Rc00536) (Diagnostic Systems Laboratories, Webster, TX) at 1:1000 dilution or anti-β-actin (AC-74) (Sigma Aldrich) at 1:3000 dilution. Following incubation with horseradish peroxidase-conjugated secondary antibodies at 1:5000 dilution for 1 h at room temperature, the signal was developed by enhanced chemiluminescence solution (ECL Plus; Amersham Biosciences, GE Healthcare, Little Chalfont, UK) and detected on Blue Lite Autorad Film (ISC BioExpress, Kaysville, UT). β-Actin was detected to confirm equal protein loading.
Enzyme-linked immunosorbent assay
PGE2 levels in culture supernatants were quantified by enzyme-linked immunosorbent assays (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. Briefly, 1.5 × 106 cells were seeded per 60 mm dish in 5 ml of fresh medium and incubated for 48 h. Absorbance was measured at 410 nm by BioTek Synergy™ HT Multi-Mode Microplate Reader. Since hypoxia reduced cell proliferation by 30–50% compared with normoxic controls (data not shown), the PGE2 concentrations were normalized by cell number at the time of harvesting culture supernatants.
Immunohistochemistry
Immunohistochemistry was performed with the VECTASTAIN® ABC kit (Vector Laboratories, Burlingame, CA). In brief, sections were incubated with anti-PTGES rabbit polyclonal antibody (Oxford Biomedical Research) at 1:150 dilution, anti-COX-2 mouse monoclonal antibody (Cayman Chemical) at 1:250 dilution, anti-HIF-1α rabbit polyclonal antibody (Novus Biologicals) at 1:5000 dilution or anti-human IGFBP-3 mouse monoclonal antibody (Clone 84728.111) (R&D Systems, Minneapolis, MN) at 1:250 dilution, followed by incubation with biotinylated secondary IgG and signal development using the DAB Peroxidase Substrate Kit (Vector Laboratories). Signal was intensified for HIF-1α using Catalyzed Signal Amplification system (Dako, Carpinteria, CA). The staining was assessed independently by two of the authors (H.N. and A.J.P.K.). The intensity was scored as negative (0), weakly positive (0.5), definitively positive (1), strongly positive (1.5) or very strongly positive (2).
Statistical analysis
Data from triplicate experiments are presented as mean ± standard error and were analyzed by two-tailed Student's t-test. P <0.05 was considered significant.
Results
Hypoxic gene signature reveals PTGES as a potential HIF-inducible gene essential in esophageal tumor biology
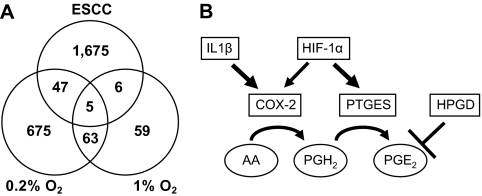
To delineate the role of hypoxia in esophageal epithelial and tumor biology, we first carried out gene array experiments using a non-transformed immortalized diploid human esophageal cell line, EPC2-hTERT (20). Unlike cancer cell lines, EPC2-hTERT has no genetic alterations at early passages that may affect the cellular response to hypoxia. When EPC2-hTERT was exposed to moderate (1% O2) or severe (0.2% O2) hypoxia, ≥2-fold of gene expression was detected by 133 and 790 probe sets on the Affymetrix U133+v2.0 chip, respectively (Figure 1A). Both oxygen tensions induced 68 probe sets commonly, although lower oxygen tension induced higher levels of mRNA in most of the genes induced by hypoxia (Figure 1A and supplementary Table 1 is available at Carcinogenesis Online). They included 52 HIF target genes such as carbonic anhydrase 9, glucose transporter SLC2A1 (a.k.a. GLUT1), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatases PFKFB3 and PFKFB4, phosphoglycerate kinase 1 (PKG1), proly hydroxylases Egln1 (PHD2) and Egln3 (PHD3), and IGFBP3. Severe and moderate hypoxia induced 31 (59.6%) and 16 (30.8%) of such HIF inducible genes, respectively (27,28) (supplementary Table 2 is available at Carcinogenesis Online).
Fig. 1.
Hypoxia may activate the COX-2–PTGES axis in ESCC. Gene array experiments imply the hypoxic activation of the central pathway regulating prostaglandin biosynthesis in the tumor microenvironment. (A) Venn diagram representing the overlap of genes that are significantly different in tumors relative to normal tissues (n = 5), severe hypoxia (0.2% O2) and moderate hypoxia (1% O2) relative to normoxia (21% O2). Fold change, >2; Q <10% for ESCC, Q <1% for 0.2% O2 and Q <3% for 1% O2. (B) PTGES, also known as mPGES-1, generates PGE2 from PGH2. COX-2, also known as PTGS2, produces PGH2 from arachidonic acids (AA). HPGD mediates PGE2 degradation. IL-1β is known to activate the COX-2–PTGES axis. Although HIF is known to induce COX-2, hypoxic regulation of PTGES remains to be elucidated.
To gain insight into the role of HIF in hypoxic tumor microenvironment, the above hypoxic gene array results were compared with a set of gene array data (GSE17351) representing five paired primary esophageal tumors (ESCC) and adjacent normal mucosa. Only five genes, PTGES (also known as mPGES-1), IGFBP3, GLUT1, Lysyl oxidase-like 2 (LOXL2) and proline 4-hydroxylase(P4HA1), appeared to be overexpressed commonly in primary tumors as well as hypoxia at a high filtering stringency (<3% of the false discovery rates, i.e. Q-values) for hypoxic conditions (Figure 1A), whereas 28 genes passed filtering at a low stringency without setting a Q-value cut-off (supplementary Table 3 is available at Carcinogenesis Online). Among these were HIF target genes regulating glucose metabolism (e.g. GLUT1 and PDK1) and cancer invasion and metastasis through stromal remodeling (e.g. LOXL2). Our previous studies implied IGFBP3 in the pathogenesis of ESCC (23,25). To explore novel HIF target genes in esophageal tumor biology, we first considered those induced as a function of the extent of hypoxia. Robust induction of PTGES at lower oxygen tension (151.1-fold, Q = 0.0%) (Figure 1A and supplementary Table 3 is available at Carcinogenesis Online) was appealing given the role of PGE2 in tumor biology, especially regulation of blood flow and inflammation. Interestingly, PTGS2 (COX-2) was also induced by 0.2% O2 (supplementary Tables 2 and 3 are available at Carcinogenesis Online). Thus, we hypothesized that tumor microenvironment is permissive for activation of the COX-2–PTGES axis leading to increased biosynthesis of PGE2 (Figure 1B).
Hypoxia induces PTGES in a HIF-1α-dependent manner in esophageal epithelial cells
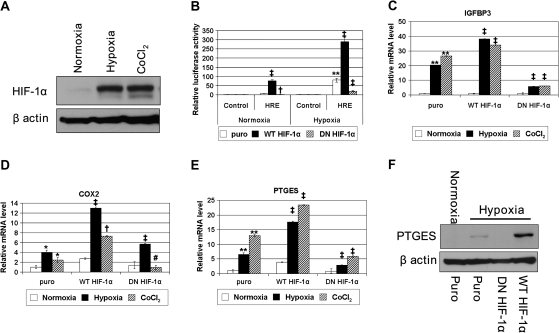
To functionally validate the role of HIF-1α in the expression of hypoxia-induced genes revealed by DNA microarray experiments, we established EPC2-hTERT cell derivatives that have been stably transduced with a retrovirus expressing wild-type HIF-1α and dominant-negative HIF-1α. The dominant-negative HIF1α, a deletion mutant (amino acids 30–389) lacking DNA binding and transactivation domains, suppresses transcription from reporter plasmids containing hypoxia response element-like enhancer elements, yet they maintain the ability to heterodimerize through the Per/ARNT/Sim (PAS) domains of HIF (29,30). Under hypoxic conditions, HIF-1α protein was stabilized in EPC2-hTERT cells (Figures 2A and 3B and data not shown). As expected, hypoxia stimulated HIF-mediated transcriptional activity, and that was enhanced by ectopically expressed wild-type HIF-1α or suppressed by dominant-negative HIF-1α (Figure 2B). Hypoxia induced the mRNA expression of HIF-1α target genes, including IGFBP-3, COX-2 and CA9 (27,28,31,32) (Figure 2C and D, and data not shown). In contrast, dominant-negative HIF-1α decreased the mRNA expression of the HIF-1α target genes (Figure 2C and D, and data not shown). It should be noted that hypoxic induction of COX-2 mRNA appeared to be relatively modest in EPC2-hTERT cells although it was stimulated by wild-type HIF-1α (Figure 2D and supplementary Figure 1 is available at Carcinogenesis Online) compared with IGFBP3 (Figure 2C) and CA9 (data not shown). Hypoxia induced PTGES mRNA and protein, which were further augmented by wild-type HIF-1α and suppressed by dominant-negative HIF-1α (Figure 2E and F). When cells were treated with cobalt chloride that stabilizes HIF in the presence of oxygen (Figure 2A), HIF-mediated transcription and mRNA induction occurred efficiently under normoxic conditions, which were further enhanced by wild-type HIF-1α or suppressed by dominant-negative HIF-1α (Figure 2C–E, and data not shown). These results indicate that the HIF-dependent transcriptional activity is not only necessary but also sufficient for PTGES induction under hypoxic conditions.
Fig. 2.
HIF regulates PTGES mRNA and protein expression. EPC2-hTERT cells expressing wild-type (WT) HIF-1α, dominant-negative (DN) mutant HIF-1α or empty control vector (puro) were exposed to normoxia (21% O2), hypoxia (1% O2) or 250 μM cobalt chloride for 24 h. (A) HIF-1α protein was detected in EPC2-hTERT-puro cells by western blotting. (B) HIF-mediated transcriptional activation through hypoxia response elements was determined following transfection of (eHRE)3-Luc (HRE) or pGL-3-Promoter (control). (C–E) Real-time reverse transcription–PCR determined the expression level of IGFBP3 (C), COX-2 (D) and PTGES (E). ‡P < 0.001 versus puro; †P < 0.01 versus puro; #P < 0.05 versus puro; **P < 0.001 versus normoxia; *P < 0.05 versus normoxia (n = 3). (F) Western blotting determined PTGES protein expression.
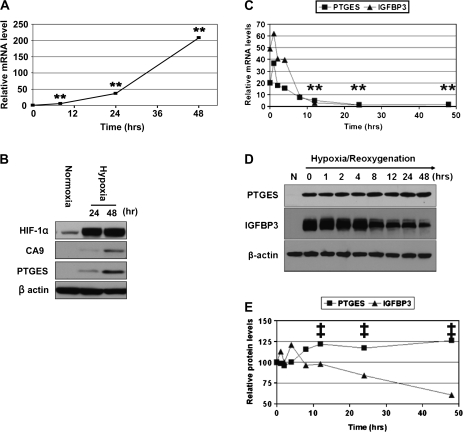
Fig. 3.
PTGES mRNA and protein are induced by hypoxia but regulated differentially upon reoxygenation. (A and B) EPC2-hTERT cells exposed to hypoxia (1% O2) for up to 48 h and PTGES mRNA (A) and protein (B) were determined at the indicated time point. Note that HIF-1α protein was stabilized within 24 h. CA9 was determined as a HIF-1α target gene in (B). (C–E) EPC2-hTERT cells were exposed to hypoxia (1% O2) for 48 h to induce PTGES and IGFBP3 serving as a control of hypoxia-inducible gene. Cells were then re-exposed to normoxia (21% O2) starting at time 0. Time course of PTGES mRNA (C) and protein (D and E) expression levels were determined following reoxygenation. Note that hypoxia increased the PTGES and IGFBP3 mRNA levels to 20 ± 0.6-fold and 49.2 ± 0.2-fold (n = 3), respectively, prior to reoxygenation (time 0) in (C). The half-life (t1/2) for PTGES and IGFBP3 mRNAs during their exponential decay was calculated using the following formula: t1/2 = t/log2 (N4/N12), where t = 8 h (elapsed time) and N4 and N12 represent the mRNA level at 4 and 12 h after reoxygenation. PTGES and IGFBP3 protein were also barely detectable without hypoxic exposure (N) in (D). (E) Densitometry of immunoblots in (D) setting the signal intensity at time 0 as 100%. **P < 0.001 versus time 0; ‡P < 0.001 versus IGFBP3 (n = 3). Note: error bars were not visible due to standard error <0.1 in (A), (C) and (E).
PTGES protein, but not mRNA, is stabilized under normoxic conditions
We next explored the kinetics of PTGES mRNA and protein expression as a novel HIF-inducible gene. Under moderate hypoxic conditions (1% O2), PTGES mRNA was typically induced within 12 h and increased further as a function of time in EPC2-hTERT cells (Figure 3A). PTGES protein was induced by hypoxia exhibiting kinetics similar to other HIF-1α target genes such as CA9 and IGFBP3 (Figure 3B and data not shown). Importantly, such hypoxic induction of PTGES was observed not only in EPC2-hTERT cells but also primary cultured non-immortalized normal human esophageal cells EPC1 and EPC2 (supplementary Figure 2 is available at Carcinogenesis Online).
When cells were re-exposed to normoxia following hypoxic exposure for 48 h to induce PTGES and IGFBP3 as a control, both mRNA species rapidly declined to their basal levels within 24 h (Figure 3C), indicating that transcripts for PTGES and IGFBP3 are unstable under normoxic conditions. In fact, the half-life for decaying PTGES and IGFBP3 mRNAs upon reoxygenation was estimated at 4.8 and 2.2 h, respectively (Figure 3C). In contrast, the PTGES protein level was increased modestly or remained unchanged for 48 h upon reoxygenation, whereas IGFBP3 protein level was decreased (Figure 3D and E). Thus, unlike its transcriptional regulation, PTGES protein stability did not appear to be subjected to regulation by oxygen tension.
PTGES contributes to biosynthesis of PGE2
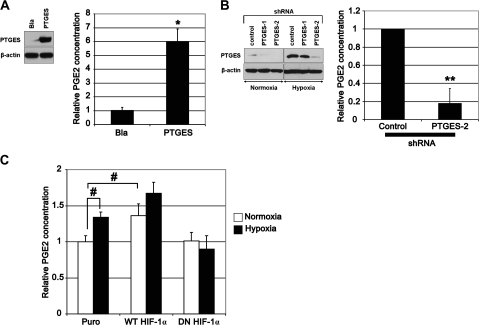
To elucidate the biological activity of PTGES in esophageal epithelial cells, we ectopically expressed PTGES by retrovirus-mediated stable transduction (Figure 4A) or knocked down PTGES by RNA interference (Figure 4B). When overexpressed, PTGES increased the PGE2 level in conditioned media (Figure 4A). RNA interference prevented hypoxic induction of PTGES and suppressed basal PGE2 production under normoxic conditions (Figure 4B). These data indicate that PTGES functionally contributes to production of PGE2. Moreover, hypoxia stimulated PGE2 production and that was enhanced by ectopically expressed wild-type HIF-1α and suppressed by dominant-negative HIF-1α (Figure 4C), in agreement with HIF-1α-mediated PTGES regulation. However, relatively modest (<2-fold) induction of PGE2 by hypoxia (Figure 4C) may imply a negative feedback mechanism limiting prostanoid biosynthesis. In line with such a notion was induction of HPGD, a chief enzyme catalyzing prostaglandin, under moderate hypoxic conditions (1% O2) (supplementary Figure 3A is available at Carcinogenesis Online). Interestingly enough, gene array data on primary tumors showed downregulation of HPGD as validated by quantitative reverse transcription–PCR showing sharp suppression in 10 of 13 (76.9%) primary ESCC samples (supplementary Figure 3B is available at Carcinogenesis Online), suggesting that loss of HPGD expression may lead to stabilization of PGE2 in tumor tissues.
Fig. 4.
PTGES and hypoxia regulate PGE2 production in human esophageal cells. Western blotting confirmed ectopic expression of PTGES (A) and the suppression of PTGES expression by RNA interference (B) in EPC2-hTERT-EGFR-p53R175H-cyclin D1 cells, an EPC2-hTERT cell derivative. Ectopically expressed PTGES was detectable under normoxic conditions in the PTGES but not empty control vector (Bla)-transduced cells in (A). Hypoxia (1% O2) induced PTGES in transformed EPC2-hTERT, although two independent short hairpin RNA (shRNA) sequences (PTGES1 and PTGES2) suppressed PTGES expression compared with scrambled short hairpin RNA sequence (control) in (B). Histograms represent the PGE2 levels in conditioned media of the PTGES-manipulated cells in (A and B). *P < 0.01 versus Bla in (A); **P < 0.01 versus control in (B) (n = 3). In (C), PGE2 levels were determined in conditioned media of the indicated EPC2-hTERT cell derivatives described in Figure 2. #P < 0.05 versus puro under normoxia (n = 3). Note that the PGE2 levels in basal conditions appeared to be 10–30 pg/ml per 106 cells in EPC2-hTERT and its derivatives.
The COX-2–PTGES enzyme axis is activated in ESCC
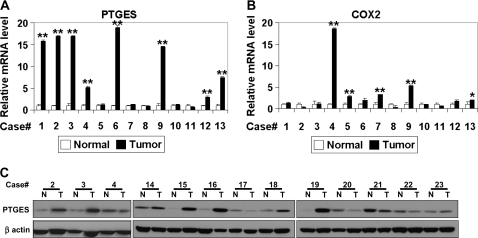
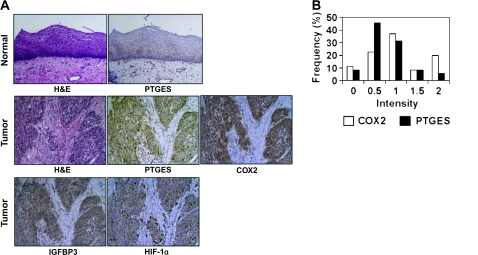
Our in vitro data indicate that PTGES is a novel HIF-inducible gene regulating PGE2 biosynthesis in concert with other factors such as COX-2 and HPGD. In addition, gene array data indicated upregulation of PTGES and COX-2 in primary ESCC tumors (supplementary Table 3 is available at Carcinogenesis Online). In fact, quantitative reverse transcription–PCR documented ≥2-fold overexpression of mRNA for PTGES and COX-2 in 8 of 13 (61.5%) and 5 of 13 (38.5%), respectively, in tumors compared with adjacent normal mucosa (Figure 5A and B), although there was no correlation at the mRNA levels between PTGES and COX-2 (Pearson correlation coefficient = −0.085; P = 0.783). Western blotting revealed PTGES upregulation in 7 of 13 (53.8%) tumor tissue samples evaluated (Figure 5C). When immunohistochemistry was done on an tissue microarray representing 35 paired ESCC and adjacent normal mucosa as well as independent seven primary tumor samples, including those from the identical cases (case # 1–5 in Figure 5) used for gene array experiments, PTGES was found negative or weakly positive in the normal esophageal epithelium (Figure 6A). In tumors, PTGES colocalized to the cytoplasm along with other HIF target genes such as IGFBP3 and COX-2 (Figure 6A and data not shown), although the expression levels were not correlated between PTGES and COX-2 (Figure 6B, Pearson correlation coefficient = 0.329; P = 0.054). In addition, the expression level of each protein tended to spatially vary within a single tumor (data not shown), suggesting the heterogeneous nature or gradient of the tumor hypoxic microenvironment. Nonetheless, PTGES was upregulated markedly in 5 of 35 (14.3%) and moderately in 11 of 35 (31.4%) in the tumor tissue microarray samples (Figure 6B), consistent with the frequency of PTGES mRNA overexpression in tumor tissues (Figure 5A). Lack of correlation in the protein and mRNA expression level between PTGES and COX-2 may be accounted for by differential regulation by independent factors other than hypoxia.
Fig. 5.
PTGES is overexpressed in ESCC. The mRNA levels for PTGES (A) and COX-2 (B) in ESCC tissues were determined by real-time reverse transcription–PCR. **P < 0.001 versus normal; *P < 0.05 versus normal; ns, not significant versus normal (n = 3). (C) PTGES protein was detected in ESCC tissues. N, normal; T, tumor.
Fig. 6.
PTGES is colocalized with HIF target genes in ESCC. (A) Representative immunohistochemistry done for indicated molecules along with hematoxylin and eosin (H&E) staining on ESCC tissues. Magnification, ×100. (B) Immunohistochemistry on a tissue microarray representing 35 paired ESCC tumors and adjacent normal mucosa were stained for PTGES and COX-2 to score their intensities. Expression was judged upregulated when its intensity was stronger than matched normal tissue whose intensity was typically scored <1.
One such regulatory factor may be the inflammatory cytokine IL-1β, known to induce COX-2 (33), which appeared to be frequently overexpressed in 8 of 13 (61.5%) ESCC tissue samples (supplementary Figure 4A is available at Carcinogenesis Online). Interestingly, the expression level of IL-1β was found to be correlated with that of COX-2 (supplementary Figure 4B is available at Carcinogenesis Online) but not that of PTGES (Pearson correlation coefficient = −0.276; P = 0.361). In culture, IL-1β stimulated PGE2 production (supplementary Figure 5A is available at Carcinogenesis Online), albeit to a lesser extent compared with ectopically expressed PTGES (Figure 4A). Interestingly, IL-1β failed to enhance PGE2 production when PTGES was overexpressed (supplementary Figure 5A is available at Carcinogenesis Online). Moreover, IL-1β did not affect the hypoxic induction of HIF-1α or PTGES in EPC2-hTERT cell derivatives (supplementary Figure 5B is available at Carcinogenesis Online and data not shown), suggesting that IL-1β may not regulate PTGES directly in esophageal epithelial cells, whereas HIF-1α can regulate both COX2 and PTGES.
Discussion
This is the first demonstration of PTGES as a novel target of hypoxia in esophageal epithelial cells (Figures 1–3), regulating PGE2 production in culture (Figure 4) in addition to its overexpression in a subset of primary ESCC (Figures 5 and 6). In agreement with its potential role in carcinogenesis, PTGES has been shown to transform HEK293 cells in concert with COX-2 (13). When COX-2 and PTGES were simultaneously targeted in the gastric glandular epithelium in transgenic mice, development of hyperplastic tumors was observed along with induction of inflammatory cytokines, chemokines and growth factors, increased PGE2 levels and recruitment of macrophages (34). Genetic deletion of PTGES in ApcMin mice suppressed the size and number of preneoplastic aberrant crypt foci as well as tumor growth (35). These mouse models imply a complex cross talk between the COX-2–PTGES axis and tumor microenvironment. Thus, future in vivo studies should determine the interplay among PTGES, COX-2 and HPGD as well as the role of influential factors such as hypoxia and inflammation in tumor tissues.
HIF-dependent PTGES induction in this study is a novel finding. Grimmer et al. (36) have recently demonstrated that hypoxia induced PTGES along with HIF-1α in chondrocytes. They implied HIF by antagonizing hypoxic induction of PTGES with 2-methoxyestradiol. However, 2-methoxyestradiol affects HIF-1α (37) as well as other transcription factors such as nuclear factor-kappaB and p53 (38,39), which can be activated by cellular stress under hypoxic conditions. PTGES is expressed in KYSE-170 and KYSE-270 ESCC lines and augmented by exposure to acidified cell culture medium supplemented with chenodeoxycholic acid and trypsin (40), implying a role for PTGES in gastroesophageal reflux disease and Barrett's esophagus. Using a rat esophagoduodenal anastomosis model, Jang et al. (41) showed activation of the COX-2–PTGES axis in the reflux-induced esophageal squamous dysplasia and Barrett's metaplasia. These data indicate cellular stress may induce PTGES in esophageal cells. Consistent with this premise, glutathione peroxidase 2, an antioxidant enzyme, appeared to inhibit PGE2 production through downregulation of COX-2 and PTGES in HT-29 colon cancer cells (42). Thus, hypoxic induction of PTGES may involve reactive oxygen species that may serve as signals from a cellular oxygen sensor leading to stabilization of HIF (43).
The primary role of HIF in PTGES mRNA expression under hypoxic conditions was reinforced by our experiments using cobalt chloride under normoxic conditions (Figure 2E), excluding the direct transactivation of PTGES by hypoxia-inducible transcription factors other than HIF. In addition, dominant-negative HIF antagonized PTGES induction (Figure 2E and F), indicating the requirement of HIF. The mechanisms underlying transcriptional regulation of PTGES remain to be explored. Within 5 kb from the predicted transcription start site, there are three potential HIF-responsive elements (A/G)CGT(G/C)C (−976 to −971, −466 to −461 and −317 to −312). In addition, three other potential sites exist within the first intron of PTGES. However, our data do not exclude the possibility that transcription factors activated by HIF, but not HIF per se may transactivate PTGES. Thus, future studies should determine HIF-responsive elements in the promoter and adjacent regulatory regions of PTGES.
Gene expression under hypoxic conditions is subjected to unique regulation through transcriptional as well as translational mechanisms. Under hypoxic stress, global translation is reduced to conserve energy (44). However, hypoxia is permissive for selective translation of the mRNAs encoding proteins essential in hypoxic adaptation (45). Corroborating the premise for PTGES as a hypoxia-inducible gene, more pronounced upregulation of PTGES as well as CA9 proteins was observed at 24 h or later upon hypoxic exposure (Figure 3B).
In addition to COX-2 and PTGES, the other upstream and downstream molecules are thought to influence the PGE2 level and its signaling pathway. PGE2 receptor EP2 is overexpressed in a subset of ESCC and correlate with poor prognosis (46), although our quantitative reverse transcription–PCR data showed EP2 upregulation in only 1 of 13 ESCC samples (data not shown). IL-1β, a COX2 inducing cytokine (33), may cooperate with PTGES in PGE2 biosynthesis without affecting PTGES expression (supplementary Figure 5 is available at Carcinogenesis Online). However, lack of IL-1β-mediated PGE2 stimulatory effect in the presence of ectopically expressed PTGES (supplementary Figure 5A is available at Carcinogenesis Online) or relatively modest PGE2 induction under hypoxic conditions (Figure 4C) may imply negative feedback mechanisms. Such an idea may be supported by hypoxic induction of HPGD mRNA in EPC2-hTERT cells (supplementary Figure 3A is available at Carcinogenesis Online); HPGD is downregulated in several gastric and colorectal cancers (47–49). HPGD inactivation may serve as a mechanism of resistance to celecoxib chemoprevention of colon tumors (50). Interestingly, HPGD mRNA appeared to be severely downregulated in all our ESCC samples tested (supplementary Figure 3B is available at Carcinogenesis Online), which has not been reported previously. These data reinforce the critical role of the COX-2–PTGES–PGE2 axis in esophageal carcinogenesis.
In summary, our data suggest that PTGES may play an essential role in biosynthesis of PGE2 in esophageal epithelial cells in response to hypoxia and other factors in tissue microenvironment such as inflammatory cytokines. Whereas PTGES mRNA expression is regulated by HIF-dependent transcriptional activation, PTGES protein is stabilized upon reoxygenation. Thus, the regulation of PTGES may be subject to complex levels of regulation involving other intrinsic enzymes such as COX-2 and HPGD.
Supplementary material
Supplementary Tables 1–3 and Figures 1–5 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (NIH) (K01-DK-066205 to H.N., R01DK077005 to M.N., S.O., V.H. and H.N., P01-CA-098101 [Mechanisms of Esophageal Carcinogenesis] to H.N., M.N., S.O., G.W., M.T., C.Z.M., D.B., A.J.P.K.); Nihon Trim, Co. Ltd (to M.K.); NIH Center for Molecular Studies in Digestive and Liver Diseases and its core facilities.
Supplementary Material
Acknowledgments
We thank Dr Don A.Baldwin (Microarray Core Facility), Dr Charles H. (Hank) Pletcher (Flow Cytometry and Cell Sorting Facility), Dr Gary P.Swain (Morphology Core) and Ross Kalman for technical assistance; Jian Chen and Masanobu Kobayashi (Hokkaido University) for reagents and Charles Miller and Drs Doug B.Stairs, Katharine D.Grugan and Jiri Kalabis (Rustgi Laboratory) for helpful discussions. Finally, we are grateful to Meenhard M.Herlyn and Anil K.Rustgi for their support and advice and to Dr Rustgi for the critical review of the manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- cDNA
complementary DNA
- COX
cyclooxygenase
- ESCC
esophageal squamous cell carcinoma
- HIF
hypoxia-inducible factor
- HPGD
hydroxylprostaglandin dehydrogenase
- IGFBP3
insulin-like growth factor binding protein-3
- IL
interleukin
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- PGE2
prostaglandin E2
- PTGES
prostaglandin E synthase
References
- 1.Pugh CW, et al. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 2.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 3.Talks KL, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsuta M, et al. Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp. Mol. Pathol. 2005;78:123–130. doi: 10.1016/j.yexmp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Kimura S, et al. Expression of hypoxia-inducible factor (HIF)-1 alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur. J. Cancer. 2004;40:1904–1912. doi: 10.1016/j.ejca.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Kurokawa T, et al. Overexpression of hypoxia-inducible-factor 1 alpha(HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br. J. Cancer. 2003;89:1042–1047. doi: 10.1038/sj.bjc.6601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuyama T, et al. Expression of hypoxia-inducible factor-1alpha in esophageal squamous cell carcinoma. Cancer Sci. 2005;96:176–182. doi: 10.1111/j.1349-7006.2005.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koukourakis MI, et al. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001;61:1830–1832. [PubMed] [Google Scholar]
- 9.Muller-Decker K, et al. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol. Carcinog. 2007;46:705–710. doi: 10.1002/mc.20326. [DOI] [PubMed] [Google Scholar]
- 10.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit. Rev. Clin. Lab. Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 11.Li L, et al. Meta-analysis: clinicopathological and prognostic significance of cyclooxygenase-2 expression on esophageal squamous cell carcinoma. Aliment. Pharmacol. Ther. 2009;30:589–596. doi: 10.1111/j.1365-2036.2009.04069.x. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann KC, et al. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 13.Kamei D, et al. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J. Biol. Chem. 2003;278:19396–19405. doi: 10.1074/jbc.M213290200. [DOI] [PubMed] [Google Scholar]
- 14.Hasan S, et al. Expression analysis of the prostaglandin E2 production pathway in human pancreatic cancers. Pancreas. 2008;37:121–127. doi: 10.1097/MPA.0b013e31816618ba. [DOI] [PubMed] [Google Scholar]
- 15.Seo T, et al. Microsomal prostaglandin E synthase protein levels correlate with prognosis in colorectal cancer patients. Virchows Arch. 2009;454:667–676. doi: 10.1007/s00428-009-0777-z. [DOI] [PubMed] [Google Scholar]
- 16.Takii Y, et al. Expression of microsomal prostaglandin E synthase-1 in human hepatocelluar carcinoma. Liver Int. 2007;27:989–996. doi: 10.1111/j.1478-3231.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 17.van Rees BP, et al. Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int. J. Cancer. 2003;107:551–556. doi: 10.1002/ijc.11422. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimatsu K, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin. Cancer Res. 2001;7:3971–3976. [PubMed] [Google Scholar]
- 19.von Rahden BH, et al. Expression of prostaglandin E synthase in Barrett's cancer. Dis. Esophagus. 2008;21:304–308. doi: 10.1111/j.1442-2050.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 20.Harada H, et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol. Cancer Res. 2003;1:729–738. [PubMed] [Google Scholar]
- 21.Andl CD, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J. Biol. Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 22.Okawa T, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaoka M, et al. IGFBP-3 regulates esophageal tumor growth through IGF-dependent and independent mechanisms. Cancer Biol. Ther. 2007;6 doi: 10.4161/cbt.6.4.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, et al. Dominant-negative hypoxia-inducible factor-1 alpha reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am. J. Pathol. 2003;162:1283–1291. doi: 10.1016/s0002-9440(10)63924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaoka M, et al. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64:7711–7723. doi: 10.1158/0008-5472.CAN-04-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu F, et al. Dynamic, site-specific interaction of hypoxia-inducible factor-1alpha with the von Hippel-Lindau tumor suppressor protein. Cancer Res. 2001;61:4136–4142. [PubMed] [Google Scholar]
- 27.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 28.Wenger RH, et al. Integration of oxygen signaling at the consensus HRE. Sci. STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 29.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindebro MC, et al. Protein-protein interaction via PAS domains: role of the PAS domain in positive and negative regulation of the bHLH/PAS dioxin receptor-Arnt transcription factor complex. EMBO J. 1995;14:3528–3539. doi: 10.1002/j.1460-2075.1995.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldser D, et al. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 32.Kaidi A, et al. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 33.Abdalla SI, et al. Effect of inflammation on cyclooxygenase (COX)-2 expression in benign and malignant oesophageal cells. Carcinogenesis. 2005;26:1627–1633. doi: 10.1093/carcin/bgi114. [DOI] [PubMed] [Google Scholar]
- 34.Oshima H, et al. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi M, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 36.Grimmer C, et al. Hypoxia-inducible factor 1alpha is involved in the prostaglandin metabolism of osteoarthritic cartilage through up-regulation of microsomal prostaglandin E synthase 1 in articular chondrocytes. Arthritis Rheum. 2007;56:4084–4094. doi: 10.1002/art.23136. [DOI] [PubMed] [Google Scholar]
- 37.Mabjeesh NJ, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 38.Kumar AP, et al. 2-Methoxyestradiol interferes with NF kappa B transcriptional activity in primitive neuroectodermal brain tumors: implications for management. Carcinogenesis. 2003;24:209–216. doi: 10.1093/carcin/24.2.209. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay T, et al. Induction of apoptosis in human lung cancer cells after wild-type p53 activation by methoxyestradiol. Oncogene. 1997;14:379–384. doi: 10.1038/sj.onc.1200835. [DOI] [PubMed] [Google Scholar]
- 40.Soma T, et al. Induction of prostaglandin E synthase by gastroesophageal reflux contents in normal esophageal epithelial cells and esophageal cancer cells. Dis. Esophagus. 2007;20:123–129. doi: 10.1111/j.1442-2050.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- 41.Jang TJ, et al. Expression of cyclooxygenase 2, microsomal prostaglandin E synthase 1, and EP receptors is increased in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004;53:27–33. doi: 10.1136/gut.53.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banning A, et al. GPx2 counteracts PGE2 production by dampening COX-2 and mPGES-1 expression in human colon cancer cells. Antioxid. Redox Signal. 2008;10:1491–1500. doi: 10.1089/ars.2008.2047. [DOI] [PubMed] [Google Scholar]
- 43.Acker T, et al. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc. Res. 2006;71:195–207. doi: 10.1016/j.cardiores.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Holcik M, et al. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell. Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 45.Young RM, et al. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J. Biol. Chem. 2008;283:16309–16319. doi: 10.1074/jbc.M710079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo KT, et al. Prognostic role of PGE2 receptor EP2 in esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2009;16:352–360. doi: 10.1245/s10434-008-0242-2. [DOI] [PubMed] [Google Scholar]
- 47.Backlund MG, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J. Biol. Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Backlund MG, et al. Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer Res. 2008;68:9331–9337. doi: 10.1158/0008-5472.CAN-08-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thiel A, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin. Cancer Res. 2009;15:4572–4580. doi: 10.1158/1078-0432.CCR-08-2518. [DOI] [PubMed] [Google Scholar]
- 50.Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proc. Natl Acad. Sci. USA. 2009;106:9409–9413. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.