Abstract
Background:
The use of proton pump inhibitors (PPIs) has been implicated as a potential contributor to the development of Clostridium difficile–associated disease (CDAD) because of the ability of these drugs to substantially reduce the bactericidal effect of gastric acid. This study focused on the impact of PPIs, among other known risk factors, during an outbreak of CDAD in a hospital setting.
Objectives:
The primary objective was to determine whether there was an association between current use of a PPI and the CDAD outbreak. Secondary objectives were to evaluate any correlations between the CDAD outbreak and past use of PPIs, use of antibiotics, diabetes mellitus, enteral feeding, cancer, gastrointestinal surgery, inflammatory bowel disease, and previous care or residence in an institutional setting.
Methods:
A retrospective case–control study was conducted. One hundred and fifty cases of hospital-acquired Clostridium difficile were identified. Patients were individually matched to controls for age, sex, date of admission to hospital, and hospital unit. The groups were compared with respect to each exposure.
Results:
Eight case patients could not be matched with suitable controls. Therefore, data from 142 cases and 142 controls were analyzed. There was no association between current use of a PPI and the CDAD outbreak (odds ratio [OR] 1.0, 95% confidence interval [CI] 0.99–1.01). Similarly, there was no correlation between the CDAD outbreak and diabetes, enteral feeding, cancer, gastrointestinal surgery, inflammatory bowel disease, or previous care or residence in an institution. However, the development of CDAD was positively associated with use of antibiotics within the 30 days preceding the infection (OR 12.0, 95% CI 4.0–35.7) and with past use of a PPI (OR 2.4, 95% CI 1.4–4.3).
Conclusions:
The development of CDAD during a hospital outbreak was associated with use of antibiotics and with past, not current, use of PPIs.
Keywords: antibiotic, Clostridium difficile, outbreak, proton pump inhibitor, risk factor
RÉSUMÉ
Contexte :
L’emploi des inhibiteurs de la pompe à protons (IPP) jouerait un rôle dans l’apparition des maladies associées au Clostridium difficile (MACD) à cause de leur capacité à réduire considérablement l’effet bactéricide de l’acide gastrique. Cette étude s’est penchée sur le rôle des IPP, parmi d’autres facteurs de risque connus, durant une éclosion de MACD dans un hôpital.
Objectifs :
Le principal objectif était de déterminer s’il existait un lien entre l’utilisation actuelle d’un IPP et l’éclosion de MACD. Les objectifs secondaires étaient d’évaluer les corrélations entre l’éclosion de MACD et l’utilisation antérieure d’IPP, l’utilisation d’antibiotiques, le diabète sucré, l’alimentation entérale, le cancer, les interventions chirurgicales gastro-intestinales, la maladie inflammatoire de l’intestin et le fait d’avoir séjourné ou de vivre dans un établissement.
Méthodes :
On a effectué une étude cas-témoins rétrospective. On a répertorié 150 cas de maladies nosocomiales associées au Clostridium difficile. Les patients ont été appariés individuellement à des témoins en fonction de l’âge, du sexe, de la date d’hospitalisation et de l’unité de soins. Les groupes ont été comparés pour ce qui est de chaque exposition.
Résultats :
Huit cas n’ont pu être appariés à des témoins convenables. Les données de 142 cas et de 142 témoins ont donc été analysées. Aucune association n’a été établie entre l’utilisation actuelle d’IPP et l’éclosion de MACD (risque relatif approché [RRA] 1,0, intervalle de confiance à 95 % [IC] 0,99–1,01). De même, on n’a observé aucune corrélation entre l’éclosion de MACD et le diabète, l’alimentation entérale, le cancer, une intervention chirurgicale gastro-intestinale, la maladie inflammatoire de l’intestin ou le fait de vivre dans un établissement. Cependant, on a établi une corrélation entre l’apparition de MACD et l’utilisation d’antibiotiques dans les 30 jours précédant l’infection (RRA 12,0, IC à 95 % 4,0–35,7) et l’utilisation antérieure d’IPP (RRA 2,4, IC à 95 % 1,4–4,3).
Conclusions :
On a établi un lien entre l’apparition de MACD pendant une éclosion à l’hôpital et l’utilisation d’antibiotiques ainsi que l’utilisation antérieure, mais pas actuelle, d’IPP.
[Traduction par l’éditeur]
Keywords: antibiotique, éclosion de Clostridium difficile, inhibiteur de la pompe à protons, facteur de risque
INTRODUCTION
Clostridium difficile is an anaerobic, gram-positive, spore-forming, rod-shaped bacterium commonly responsible for hospital-acquired enteric infections.1,2 It is associated with substantial morbidity and mortality.1,3 Clostridium difficile–associated disease (CDAD) is defined as 3 or more loose, watery stools within a 24-h period that are unusual or different for the patient, without any other identifiable cause.2 The diagnosis may be confirmed either by a positive result on a toxin assay or by visualization of the pseudomembranes on colonoscopy.2 Older age, previous residence or care in an institution, debilitation, enteral tube feeding, severe underlying comorbidities, pre-existing bowel syndromes, and bowel surgery have all been reported to contribute to the development of CDAD.4 Certain drugs can also predispose patients to infection. Antibiotics constitute one of the most important known risk factors for CDAD because of their ability to alter the normal intestinal flora.4–7 Specifically, cephalosporins, fluoroquinolones, and clindamycin are known to increase this risk.5,6 As well, cancer chemotherapy agents can alter the normal flora, allowing overgrowth of C. difficile.4,7,8
At present, there is controversy regarding the potential association between the use of proton pump inhibitors (PPIs) and enteric infections. In theory, PPIs increase gastric pH, thereby reducing gastric acidity, which in turn leads to diminished bactericidal effect. The bactericidal effect of gastric acid is most apparent at pH less than or equal to 3.0; however, some bactericidal effect is maintained at pH 4.0.9,10 There exist many reports about the bacterial overgrowth resulting from acid suppression9,11; specifically, many observational studies reported in the past decade have focused on C. difficile infection.12–19 Organisms such as Salmonella, Campylobacter, and Shigella are established causes of infection secondary to hypochlorhydria.9,10
Clostridium difficile exists in both vegetative and spore forms. The vegetative cells are acid-labile; however, the spores are resistant to gastric acid. The reduction in bactericidal effect with reduced stomach acidity allows for a greater quantity of both vegetative and spore forms of C. difficile to travel along the small bowel and settle in the colon, where the organism’s effects are pronounced.1
The controversy regarding PPIs and CDAD continues, despite numerous recent studies.12–18 Dial and her colleagues, who have retrospectively studied patients in both hospital and community settings, suggested a positive correlation between acid suppression and CDAD.12–14 Both case–control12–15 and cohort studies13 have retrospectively identified PPI use in patients with confirmed C. difficile infection. Cunningham and others15 have also demonstrated this positive association in a hospital environment. In contrast, other trials have contested this association.17–19 In a recent meta-analysis, the data from 12 studies were pooled in an attempt to determine the relationship between enteric infections and acid suppression.20 The authors concluded that PPIs were associated with an increase in CDAD, but there was significant heterogeneity among the studies.20 A cohort study17 and a nested case–control study19 not included in the meta-analysis also challenged this correlation.
The health care costs, morbidity, and mortality associated with CDAD are immense.3 As the incidence and severity of CDAD outbreaks rise,2,3 there is a need to identify new measures to prevent and treat C. difficile infection. Despite the controversy in the current literature, it was thought that any correlation between CDAD and PPI therapy could be clinically significant, given the lengthy duration of the outbreak at the study institution. When this study was initiated, the authors were aware of no published studies specifically investigating the use of PPIs during an outbreak of CDAD. Thus, a retrospective case–control study was planned to determine the relationship between the use of PPIs and the development of CDAD during an outbreak.
The primary objective was to determine whether there was an association between current use of PPIs and the outbreak of CDAD at the study institution. The secondary objectives were to determine any associations between other known risk factors—specifically past use of PPIs, use of antibiotics, diabetes mellitus, enteral feeding, cancer, history of gastrointestinal surgery, inflammatory bowel disease, and previous residence or care in an institution—and the CDAD outbreak.
METHODS
The Trillium Health Centre, located in Mississauga, Ontario, serves more than 1 million residents in the Peel Region. At the time of the study reported here, this hospital had 794 acute, rehabilitation, and chronic care beds. An outbreak of CDAD was declared on February 28, 2007, after evidence of transmission among patients and an increase in the incidence of disease above the institution’s baseline rate. For this study, data were collected for patients with confirmed infection identified from February 28, 2007, until January 22, 2008, when the predefined number of case participants was reached. The outbreak continued beyond the date of study completion.
Case patients with hospital-acquired infection were identified from reports generated by the hospital’s Infection Prevention and Control department, which listed all positive results on assays for C. difficile toxin. The diagnosis of C. difficile infection in any patient was based on the occurrence of at least one positive result on the toxin assay during the outbreak period. Potential case patients were excluded if they had a negative result on the toxin assay despite colonoscopic evidence of pseudomembranous disease, if the C. difficile infection had been acquired in the community, if they had cancer or malignancy that had been treated with cancer chemotherapy in the preceding 8 weeks, or if the CDAD was a relapse of an earlier infection. A relapse was defined as an occurrence of the infection within 2 months of the initial infection.2 Therefore, for patients with a relapse within 2 months, data were recorded from only the initial infection, whereas for patients with a recurrent infection occurring more than 2 months after the initial infection, the initial infection and the recurrent infection were considered as 2 separate cases.
A total of 150 consecutive cases of C. difficile were included in the review of electronic medication administration records and charts. When further clarification was required, the original paper charts were retrieved and reviewed.
Case patients met the definition of the primary outcome if the electronic medication profile or medication administration record indicated that a PPI was being taken on the date of the CDAD diagnosis. This definition of “current use” was chosen to ensure accurate data collection from the electronic records. Past use of a PPI at any time before the date of the CDAD diagnosis was also recorded. A lenient definition of “past use” was used, as there is a lack of evidence to support a specific timeframe within which patients’ risk increases as a result of altered acid secretion. PPIs can substantially reduce stomach acidity after only a single dose.21,22 Past use was identified through the hospital’s electronic medication records or from the patient’s acknowledgement of use before admission to hospital, as noted in admission records.
Potential controls were identified using a patient discharge report from the hospital’s medication information system (Meditech, version 5.62; Medical Information Technology, Inc, Westwood, Massachusetts). Controls were matched 1:1 to case patients for sex, age within 5 years, date of discharge within 1 month of the diagnosis of CDAD for the matched case, and hospital unit. Multiple potential controls were available for each case, and the matched control for any given case was selected at random from those available. Any control patient with microbiological evidence of a previous C. difficile infection or receipt of cancer chemotherapy within the preceding 8 weeks was excluded as a control. The electronic hospital records for all control patients were then reviewed. Paper charts were retrieved if required to clarify any information in the electronic file. The 1:1 ratio of cases to controls was chosen to ensure accuracy of data collection, appropriate matching of patients within the 2 groups, and feasibility of completing the study within the allotted time period.
Dichotomous data were collected for both groups regarding use of PPIs, use of antibiotics within the 30 days preceding diagnosis of the CDAD infection, diagnosis of diabetes mellitus, use of enteral feeding within the 30 days preceding the infection, diagnosis of cancer, previous gastrointestinal surgery, diagnosis of inflammatory bowel disease, and previous residence or care in an institution. Oral lansoprazole and IV pantoprazole were the formulary PPIs at the study institution. However, patients might also have been taking omeprazole, esomeprazole, or rabeprazole during or before admission. The term “antibiotic” was chosen to represent all antimicrobials, classified as follows: cephalosporins, clindamycin, fluoroquinolones, penicillins, and other. Previous residence or care in an institution included admission from another hospital or health care centre, long-term care centre, or nursing home.
The sample size was calculated a priori by estimating the statistical power for the primary objective. Assuming 80% power with 5% error, 139 patients were required to fulfill the estimated rate of exposure of 20%. One hundred and fifty patients with CDAD were included to account for potential mismatching of cases and controls. Each case patient had to be matched to a control for all 4 matching criteria, to minimize potential confounding and selection bias. Approval was received from the Research and Ethics Board at Trillium Health Centre before study commencement.
Selection bias was minimized by choosing control patients who had been admitted to the study institution, regardless of exposure to PPIs. Statisticians who were otherwise not involved with the study performed the statistical analysis on the matched pairs. A main effects forward stepwise logistic regression was performed, using SAS version 9.1 (SAS Institute, Cary, North Carolina) to determine odds ratios (ORs) and Wald confidence intervals (CIs) for each of the risk factors. Only the first-order terms for current and past use of PPIs, use of antibiotics, diabetes, enteral feeding, cancer, gastrointestinal surgery, inflammatory bowel disease, and previous residence or care in an institution were included in the analyses to ensure at least 10 observations per model variable, as suggested by Stokes and others23 (there was a maximum of 14 [142/10] variables that could be fitted in the model). For the forward stepwise regression, a p value ≤ 0.10 for Wald’s test was used for entry of a variable, whereas a p value ≤ 0.15 for Wald’s test was used for retention of variables in the model. The p values for inclusion or exclusion of variables were derived from Hosmer and Lemeshow,24 who stated that a p value of 0.15 to 0.20 is appropriate to ensure entry of variables with coefficients that are different from zero. In addition, a post hoc forward stepwise regression was conducted on the interactions between variables in the model that had the smallest p values in the model described above. We were also interested in determining if there were any interactions among the variables contributing to the model, to determine if one risk factor might have had an effect on another.
RESULTS
Cases were selected from reports of the Infection Prevention and Control department until a total of 150 patients meeting the inclusion criteria were identified. Eight potentially eligible patients identified from the Infection Prevention and Control reports were not included because they had undergone cancer chemotherapy in the preceding 8 weeks. The case patients were then matched to controls according to the previously stated criteria. Eight case patients were excluded at this stage because matched controls could not be found: 4 cases were unmatched for hospital unit, 3 for age, and 1 for month of hospital admission. Therefore, 142 cases and 142 controls were included in the analysis (Table 1). One case patient had a recurrent infection with an interval longer than 2 months between the original and recurrent infections; this patient was included as 2 separate cases, with different matched controls.
Table 1.
Baseline Characteristics of Cases and Controls
| Characteristic |
No. (%) of Patients* |
|
|---|---|---|
| Cases (n = 142) | Controls (n = 142) | |
| Age (years) (mean ± SD) | 75.4 ± 13 | 75.9 ± 12 |
| Sex (males) | 67 (47) | 67 (47) |
| Hospital unit | ||
| Cardiac | 26 (18) | 26 (18) |
| Medical and emergency | 59 (42) | 59 (42) |
| Mental health and continuing care | 2 (1) | 2 (1) |
| Neurology or musculoskeletal | 37 (26) | 37 (26) |
| Surgical | 18 (13) | 18 (13) |
| Women and children | 0 | 0 |
SD = standard deviation.
Unless indicated otherwise.
In the statistical analysis, the number of potential variables in the model increased from 9 to 15; however, we were still within the limit of 5 to 10 observations per model parameter recommended by Stokes and others.23 Nonetheless, all of the Wald test p values for the parameters of these variables were nonsignificant, the smallest being 0.29.
There was no correlation between current use of PPIs and development of CDAD (OR 1.0, 95% CI 0.99–1.01). Diabetes, use of enteral feeding, gastrointestinal surgery, and previous residence or care in an institution did not meet the initial significance criteria (p ≤ 0.10) for inclusion in the statistical analysis. Cancer and inflammatory bowel disease did meet the initial significance criteria but did not demonstrate a positive correlation with development of CDAD. Past use of PPIs and use of antibiotics were associated with the development of CDAD (Table 2). The past use of PPIs was associated with CDAD (OR 2.4, 95% CI 1.4–4.3), whereas the use of antibiotics was highly associated with the CDAD outbreak (OR 12.0, 95% CI 4.0–35.7). Specifically, fluoroquinolones and cephalosporins were most widely used among case patients (Table 3).
Table 2.
Evaluation of Risk Factors for Clostridium difficile–Associated Disease
| Outcome |
No. (%) of Patients* |
OR (95% CI)† | p value | |
|---|---|---|---|---|
| Cases (n = 142) | Controls (n = 142) | |||
| Primary | ||||
| Current use of PPI | 69 (49) | 69 (49) | 1.0 (0.9–1.01) | > 0.99 |
| Secondary | ||||
| Past use of PPI | 51 (36) | 34 (24) | 2.4 (1.4–4.3) | 0.03 |
| Use of antibiotics | 135 (95) | 110 (77) | 12.0 (4.0–35.7) | < 0.001 |
| Diabetes mellitus | 53 (37) | 43 (30) | NS | 0.21 |
| Enteral feeding | 23 (16) | 15 (11) | NS | 0.16 |
| Cancer | 23 (16) | 34 (24) | 0.5 (0.3–1.0) | 0.10 |
| Gastrointestinal surgery | 53 (37) | 50 (35) NS | 0.71 | |
| Inflammatory bowel disease | 8 (6) | 16 (11) | 0.4 (0.2–1.0) | 0.09 |
| Previous residence or care in institution | 26 (18) | 28 (20) NS | 0.76 | |
| Length of stay (days) (mean ± SD) | 48.9 ± 47 | 25.5 ± 56 | — | < 0.001 |
CI = confidence interval, NS = not significant, OR = odds ratio, PPI = proton pump inhibitor, SD = standard deviation.
Except where indicated otherwise.
Forward stepwise logistic regression.
Table 3.
Analysis of Antibiotic Use within 30 Days Preceding Diagnosis of Clostridium difficile—Associated Disease
| Antibiotic | Cases (n = 135) | Controls (n = 110) | p value* |
|---|---|---|---|
| Cephalosporin | 91 (67) | 63 (57) | 0.13 |
| Clindamycin | 4 (3) | 4 (4) | 0.95 |
| Fluoroquinolones | 87 (64) | 61 (55) | 0.19 |
| Penicillins | 36 (27) | 34 (31) | 0.56 |
| Other | 34 (25) | 28 (25) | 0.92 |
| No. of antibiotics per patient (mean ± SD) | 1.0 ± 1.1 | 1.4 ± 1.8 | – |
χ2 test with Yates correction.
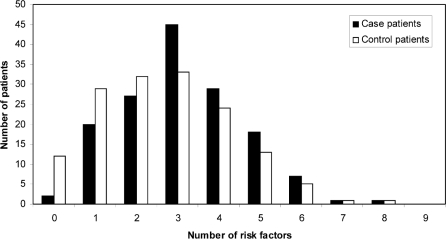
As discussed above, multiple risk factors have been established in the development of CDAD. Therefore, potential associations between risk factors were analyzed to determine if they influenced one another. Case patients seemed more likely than controls to have had 3 contributing risk factors (Figure 1). However, the statistical analysis for interactions, as described in the Methods section, suggested no interactions between risk factors.
Figure 1.
Distribution of patients according to number of risk factors.
DISCUSSION
In this outbreak study, current use of PPIs was not associated with the development of hospital-acquired CDAD. In both the case and control groups, a total of 69 patients had received a PPI during the study period. All patients in the case group who were receiving a PPI were also receiving an antibiotic at the time of CDAD diagnosis. This suggests that the use of antibiotics is associated with more substantial alteration of normal flora than the use of PPIs.
Furthermore, the past use of PPIs was correlated with the development of CDAD. In the presence of reduced gastric acidity, there is an abundance of both vegetative toxin-releasing forms and germinating spore forms contributing to colonization of C. difficile and development of clinical disease.5 It is unknown whether there is a specific incubation time for the development of clinical symptoms. However, the disparity between current and past use of PPIs for the patients in this study suggests that there is an incubation period that may leave patients at risk of infection. This disparity also suggests that long-term acid suppression puts patients at greater risk of C. difficile infection. Unfortunately, it was not possible to determine the existence of an incubation period from this study. The use of H2 receptor antagonists in both case and control patients was not determined, as it has been previously demonstrated that the acid-suppressing effects of these agents are less potent than those of PPIs.20
The use of antibiotics was also correlated with the development of CDAD. Theoretically, all antibiotics can alter normal flora, allowing pathogenic overgrowth. Generally, broad-spectrum and antianaerobic antibiotics pose the greatest risk because of their ability to alter normal flora in the large colon, where C. difficile damage occurs. As seen in Table 3, broad-spectrum fluoroquinolones and cephalosporins were most commonly used by the patients in this study. Current antibiotic stewardship practices should be continued to minimize unnecessary exposure to antibiotics.
The association between use of PPI and antibiotics and development of CDAD observed in this study resembles results from previously published non-outbreak studies. Dial and others12–14 studied the impact of PPIs in both hospital and community-acquired CDAD. Their cohort and case–control studies identified PPIs as a risk factor for the development of CDAD, as demonstrated by their results of OR 2.1 (95% CI 1.2–3.5) and OR 2.7 (95% CI 1.4–5.2), respectively.13 Furthermore, in their study of hospital-acquired CDAD, Cunningham and others15 also found that PPI use was associated with development of disease (OR 2.5, 95% CI 1.5–4.2). Finally, in a systematic review to determine the overall risk of PPIs, Leonard and others20 pooled data from 12 studies representing a total of 2948 patients with CDAD. Although there was significant heterogeneity among the studies, these authors determined a pooled OR of 1.94 (95% CI 1.37–2.75). This association was greater for the use of PPI than for H2 receptor antagonists. These data are congruent with the results of our study, suggesting that there is a potential risk of C. difficile infection in association with use of PPIs, although the risk has not yet been fully quantified.
The length of hospital stay was significantly longer among case patients. Although it cannot be inferred that length of stay was directly proportional to CDAD, as this variable was not included in the statistical analysis, it is possible that these patients required a longer stay because of their infection. This hypothesis is supported by data from a Canadian surveillance program,2 which indicated that patients with CDAD required an additional 3.6 days in hospital as a result of their infection. On average, case patients in that study had been in hospital for 21 days before development of the C. difficile infection.
Diabetes, use of enteral feeding, cancer, inflammatory bowel disease, gastrointestinal surgery, and previous residence or care in an institution were not associated with the development of CDAD. Furthermore, an analysis to determine if the total number of risk factors per patient affected development of CDAD revealed no such influence (see Figure 1).
This study had several limitations. Observational case–control studies are inherently limited by random sampling error of control patients. Selection bias was minimized by defining strict matching criteria and by choosing patients from the same patient population. Although a single investigator (S.L.) collected all of the data and therefore was not blinded, the matching and statistical analyses were conducted by individuals external to the study. Furthermore, the data were limited to records at the study institution and relied heavily both on information provided by patients at the time of admission and on physician notes. It is possible that there was some discordance between the electronic patient record from which the data were gathered and the actual time of administration of the PPI. Paper charts were used when required to confirm the information in the electronic records. Also, the study did not capture information about the strain of C. difficile in each case. The most important study limitation was the strict definition of the term “current use”, which might have limited the study findings. To ensure feasibility and accuracy of data collection from the computer system, current use of PPIs was defined as use on the date of CDAD diagnosis. As such, it is possible that the number of case patients whose CDAD developed as a result of PPI use was underreported in this study.
Given the increase in both severity and incidence of CDAD, this study attempted to further our understanding of the risk factors involved. The risk of CDAD during the outbreak was enhanced by the use of antibiotics and the past use of PPIs, but not the current use of PPIs.
This case–control study has undoubtedly contributed to the controversial evidence surrounding this issue. As other studies have suggested,12–19 it is imperative to determine approaches to minimize both the incidence and the severity of such life-threatening infections. Further research, involving larger populations of patients with C. difficile, is needed to quantify the significance of PPI as a risk factor in the development of CDAD.
Acknowledgments
The first author conducted this study as a component of a hospital pharmacy residency program accredited by the Canadian Society of Hospital Pharmacists at Trillium Health Centre. We gratefully acknowledge the assistance received from many employees of the study institution.
References
- 1.Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51–58. doi: 10.1503/cmaj.1031189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provincial Infectious Diseases Advisory Committee Best practices document for the management of Clostridium difficile in all health care settings Toronto (ON)Ministry of Health and Long Term Care; 2004[revised 2007 Jun; cited 2007 Jun 4]. Available from: www.health.gov.on.ca/english/providers/program/infectious/diseases/ic_cdiff.html [Google Scholar]
- 3.Gravel D, Miller M, Simor A, Taylor G, Gardam M, McGeer A, et al. Canadian Nosocomial Infection Surveillance Program. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48(5):568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 4.LaMont JT.Pathophysiology and epidemiology of Clostridium difficile infection Waltham (MA)UpToDate Online; 2007January28[cited 2007 Aug 10]. Available from: www.utdol.com Content password-protected. [Google Scholar]
- 5.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 6.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr Clostridium difficile associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459–74. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 7.Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1–15. doi: 10.1016/s0195-6701(98)90019-6. [DOI] [PubMed] [Google Scholar]
- 8.Blot E, Escande MC, Besson D, Barbut F, Granpeix C, Asselain B, et al. Outbreak of Clostridium difficile–related diarrhoea in an adult oncology unit: risk factors and microbiological characteristics. J Hosp Infect. 2003;53(3):187–192. doi: 10.1053/jhin.2002.1356. [DOI] [PubMed] [Google Scholar]
- 9.Williams C, McColl KEL. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 10.Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28(1):96–107. doi: 10.1136/gut.28.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39(1):54–9. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 13.Dial S, Alradasi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case–control studies. CMAJ. 2004;171(1):33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dial S, Delaney JAC, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175(7):745–748. doi: 10.1503/cmaj.060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54(3):243–245. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 16.Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613–619. doi: 10.1111/j.1365-2036.2006.03015.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272–1276. doi: 10.1086/508453. [DOI] [PubMed] [Google Scholar]
- 18.Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175–81. doi: 10.1093/qjmed/93.3.175. [DOI] [PubMed] [Google Scholar]
- 19.Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 20.Leonard J, Marshall JK, Moayyedi P. Systemtic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102(9):2047–2056. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 21.Pantoprazole Drugdex evaluation Micromedex Healthcare Series Thomson Healthcare; 2007[cited 2007 Sep 9]. Available from: www.thomsonhc.com Content password-protected. [Google Scholar]
- 22.RxFiles pocketbook. 6th ed. Saskatoon (SK): Saskatoon Health Region; 2007. Oral acid suppression comparison chart; p. 35. Also available from: wwwrxfilesca Content password-protected. [Google Scholar]
- 23.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS® system. 2nd ed. Cary (NC): SAS Institute Inc; 2000. [Google Scholar]
- 24.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York (NY): John Wiley & Sons; 2000. [Google Scholar]