Abstract
The ability of guanine-rich sequences to form quadruplex structures in telomeres for example is important in a number of biological processes such as aging, carcinogenesis and gene regulation. Ionizing radiation can cause damage to guanine moieties that can affect the stability or formation of the guanine quadruplex structures. In addition, the mechanisms of formation of these radiation damages in quadruplex structures may be different from those that occur in single- or double-stranded conformations. We have studied the quantitative aspects of the radiation induced formation of 8-hydroxy-2′-guanine base modifications and unaltered guanine base release in single-, double- and four-stranded conformations of polyriboguanylic acid as a model of guanine-rich sequences in telomere-like structures. The results show that the strandedness of guanine-rich sequences is an important variable in the observed yields of these base damages and suggests that telomere-like structures with G-quadruplexes will be relatively more radiosensitive than the other regions of duplex DNA. Hydroxyl radicals are the major reactive species that produce the DNA damage, although the presence of oxygen significantly reduces their radiation yields for all conformations of polyriboguanylic acid and changes the proportions of the yields of the various damages among the polymer conformations.
INTRODUCTION
Eukaryotic and prokaryotic genomes contain guanine-rich sequences in a number of important regions (1), such as minisatellites, ribosomal DNA, the promoter region of the c-myc gene (2), and telomeres (3).
An important characteristic of G-rich sequences is their ability to form Hoogsteen base pairs that can associate to induce the formation of cyclic tetrads wherein four guanine bases in a square planar array are arranged in a cyclic hydrogen-bonding pattern and each guanine is both the donor and acceptor of two hydrogen bonds (3). Such folding can lead to the formation of four-stranded structures (G-quadruplexes, tetraplexes or G4 structures) (4). The data obtained to date suggest that G-quadruplexes can play an important role in a number of biological processes, including the regulation of telomerase activity (5), regulation of gene expression (2), recombination (6) and meiosis (7).
The telomere is one of the most interesting and important guanine-rich motifs (3). Oxidatively generated damages in telomere DNA can lead to telomere shortening and premature senescence (8). Moreover, existing data indicate that telomeres are more sensitive to oxidative stress compared with random DNA sequences. Thus oxidizing and alkylating agents induce a higher density of single-strand breaks (SSBs) in telomeric DNA compared with minisatellites and the bulk genome (9) and provoke shortening of the 3′ telomeric single-strand tail (10). Further, the repair of oxidatively induced damages is reduced in telomeric DNA compared with that in the rest of the chromosomal DNA (11). The formation of 8-hydroxyguanine (8-OHG) base damage induced by oxidative stress in the GGG triplet in telomere sequences could also be a factor in the acceleration of telomere shortening because 8-hydroxyguanine DNA glycosylase introduces a strand break specifically at an 8-OHG residue (12). All of these effects can result from the specificity of telomere sequences and particularly from the presence of guanine repeats. Indeed, a number of studies indicate that the formation of 8-OHG is sequence-specific. It has been shown that UV radiation plus riboflavin as well as exposure to H2O2 plus Cu(II) induced approximately five times more 8-OHG residues in the DNA fragments containing the telomere sequence than in the DNA fragments that did not contain this sequence (13, 14). The relative yields of 8-OHG constituted approximately 70% of the total guanine modification in the DNA fragment containing the telomere sequence (15). The formation of 8-OHG alterations in telomeric sequences also can significantly reduce or even totally inhibit binding of human telomere repeat binding factors TRF1 and TRF2, which are important for regulation of telomere length (16).
In summary, the previous studies on G-quadruplex structures in telomeres and the formation of 8-OHG lesions in the G-rich regions of these structures by oxidative mechanisms have shown the importance of these lesions in the disruptive mechanisms that compromise the normal functions of telomeres. Since ionizing radiation also damages DNA in part by oxidative mechanisms, it is important to determine the radiation sensitivity of G-quadruplexes. But there are additional important unanswered questions related to the radiosensitivity of these structures. For example, do quadruplex G-structures have different relative radiosensitivities in terms of DNA damage than double- or single-stranded regions in telomeres? If the quadruplex structure were more radiosensitive than double- or single-stranded regions, this would suggest that quadruplexes might serve as focal points for radiation damage in telomeres and this could have implications for the mechanisms by which radiation disrupts telomere function. In this study, we used polyriboguanylic acid [poly(G)] as a model system to search for answers to these questions. We have quantified the relative yields of radiation-induced damages (8-OHG lesions and release of unaltered G bases) in single-, double- and quadruplex-stranded poly(G) solutions, and we suggest possible mechanisms to explain our observed results.
MATERIALS AND METHODS
Chemicals
Polyriboguanylic acid potassium salt, nuclease P1 (from penicillium citrinum) and acid phosphatase (type II: from potato) were purchased from Sigma (St Louis, MO) and were used without further purification. The nucleic acid damage product 8-hydroxyguanosine (8-OHGuo) was purchased from Oxis (Portland, OR). Water was purified with the Modulab Analytical Water System (MAU 158) and had an electrical resistance of at least 18 MΩ/cm. All chemicals were AR grade.
Sample Preparation and Irradiation
Samples were prepared by dissolving measured masses of poly(G) in 10 mM sodium acetate aqueous solution (NaAc), pH 6.5. The concentrations of these poly(G) solutions were calculated by measuring their optical absorption at 253 nm using the following extinction coefficient: ε = 9400 M−1 cm−1 at wavelength 253 nm (17). Concentrations were found to be 0.5 mM. To obtain entirely single-stranded poly(G), freshly prepared solutions were given a single heating cycle from room temperature to 85°C for 10 min followed by cooling to room temperature. These solutions of the single-stranded form (pG1) of poly(G) were irradiated without any additional treatment (18). To obtain the four-stranded form (pG4) of poly (G), the solutions were stored for 7 days at 4°C (19). The double-stranded form (pG2) of poly(G) was formed by heating the solution of four-stranded poly(G) to 95°C for 10 min followed by rapid cooling on ice (20). During heating, the four-stranded poly(G) units dissociate into two double-stranded units that then undergo a conformational change and a shift in hydrogen bonding.
To verify the strandedness of the poly(G) in solution, the UV-absorption temperature dependences for the three forms of poly(G) were obtained at the maximum absorbance of 253 nm with a Cary 3 Bio UV-Visible Spectrophotometer equipped with a temperature-controlled optical cell system. The heating rate was 0.4°C/min. A solution of 10 mM sodium acetate was used as an optical blank.
The samples were exposed to γ rays under simple air, nitrous oxide (N2O) and nitrogen (N2) gassing conditions using a 60Co γ-ray source. The dose rate was 19 Gy/min, and the dose range was from 50 Gy up to 2 kGy. To establish N2O and N2 gassing conditions, the solutions were bubbled with these purified gases for 20 min before irradiation and were then constantly bubbled during irradiation.
After irradiation the samples of poly(G) were heated to 95°C for 10 min and were cooled to room temperature. Then the samples (0.4 ml) were incubated with 40 μl of nuclease P1 buffer (200 mM sodium acetate, 1 mM ZnCl2, pH 5.3) and 37 units of nuclease P1. The samples were incubated at 50°C for 2 h (21). Dephosphorylation of the resulting nucleotides was achieved by the addition of 40 μl of acid phosphatase buffer (200 mM sodium acetate, 1 mM ZnCl2, pH 5.3) and 2 units of acid phosphatase. After incubation for 8 h at 37°C, the samples were analyzed by HPLC-UV.
HPLC-UV
Chromatography was performed with an HPLC-UV “Alliance” system consisting of a Waters model 2690 separation module and Waters model 2487 dual absorbance UV detector. This system was equipped with an Xterra RP18 5-μm (3.9 × 150 mm) column (Waters). A linear gradient (2.5% per min) of sodium acetate (0.5 M, pH 7.9) containing 20% acetonitrile in a solution of sodium acetate (0.5 M, pH 7.9) was used. The flow rate was 0.5 ml/min. The detection wavelength was set at 254 nm. Experimental yields of 8-OHGuo were determined by co-chromatography with known quantities of standard 8-OHGuo. Yields of unaltered guanine (G) base release were determined directly by comparison with the standard retention time. The quantification of 8-OHGuo and released unaltered bases was accomplished by determining their UV absorbance at 254 nm and integrating the area under the peaks for comparison with standard values. 8-OHGuo and G standards were used for identification and peak calibration. Although there are more sensitive detection methodologies available for quantifying these molecules in solution (e.g., HPLC coupled with electrochemical detection), we found that our UV detection techniques provided strong, stable and reproducible spectra with excellent signal-to-noise ratios at the concentrations of poly(G) used in these studies.
RESULTS
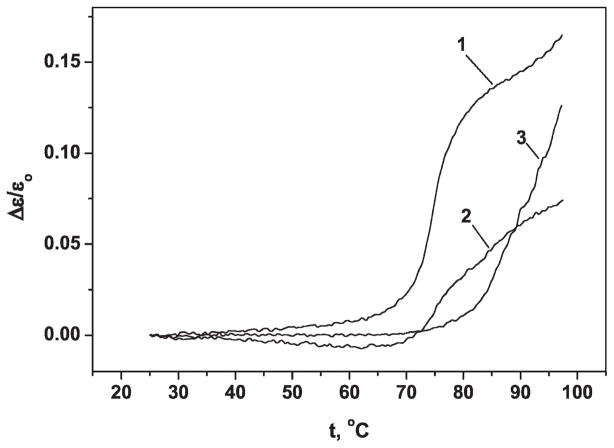
Three different conformations of poly(G) were obtained as described above. Each conformation of poly(G) yields a characteristic UV absorbance-temperature profile (22). These profiles were obtained for each conformation of poly(G) in this study and are shown in Fig. 1 in which ε0 is the extinction coefficient (ε = A/c; A = UV absorption; c = polymer concentration) at the initial temperature t0 and Δε is the change in the extinction coefficient during heating from t0 to t.
FIG. 1.
The UV-absorption temperature dependence of poly(G). 1, double-stranded poly(G); 2, single-stranded poly(G); 3, four-stranded poly(G) ε0, extinction at beginning temperature t0; Δε, change of the extinction coefficient under heating from t0 to t.
Curve 1 [double-stranded poly(G)] exhibits an S-shape form with sharply increasing UV absorption in a narrow temperature interval (Δt ~ 8°C). This type of profile is typical of a cooperative transition from helical to random coil conformation as is found for double-helical conformations of nucleic acid polymers. Curve 2 [single-stranded poly(G)] exhibits half the increase in UV absorption found in curve 1, and this occurs over a broader temperature range of the transition (Δt ~ 25°C) that is characteristic of single-stranded nucleic acid polymers (18, 23). The four-stranded poly(G) (curve 3) also exhibits a sharp increase in UV absorption in a narrow temperature interval, but the transition temperature is greater than 95°C. Such high thermostability of the biopolymer is caused by the large number of hydrogen bonds involved in the tetraplex structure (19, 23).
Radiolytic Product Analysis
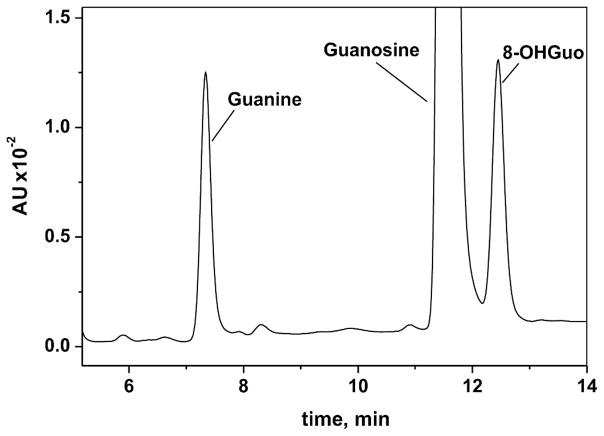
The products of radiolysis of dilute solutions of poly(G) were analyzed by the HPLC methodology described earlier. Figure 2 shows a typical chromatogram of a digest of ribonucleosides obtained from irradiated poly(G). The HPLC retention times of 8-OHGuo and guanine are 12.4 min and 7.3 min, respectively.
FIG. 2.
Section of reverse-phase HPLC elution profile of the products generated by the enzymatic digestion of the irradiated (2 kGy) poly(G). HPLC elution conditions (detection at 254 nm): A linear gradient (2.5% per min) of sodium acetate (0.5 M, pH 7.9) containing 20% of acetonitrile in solution of sodium acetate (0.5 M, pH 7.9) was used. Flow rate was 0.5 ml/min. The retention times of 8-OHGuo and guanine are 12.4 min and 7.3 min, respectively. 8-OHGuo and guanine amounts were calculated according area of the peaks. 8-OHGuo, guanine and guanosine standards were used for identification and calibration.
To determine the influence of G-rich sequence conformation on the radiation yields of 8-OHG and unaltered guanine base release, we obtained and analyzed radiation dose–response dependences for single-, double- and four-stranded conformations of poly(G). To separate out the contribution of oxygen (O2), hydroxyl radicals (•OH) and hydrated electrons (eaq−) to the formation of these lesions, the dependences were obtained under three different gassing conditions: N2, N2O and simple air. In deaerated solution (N2), the two major radiolytic species are eaq− and •OH, with nearly equivalent yields. In aerated solutions, the eaq− quickly reacts with O2 to form superoxide anion radical (O2•−). In solutions saturated with nitrous oxide, the eaq− reacts with N2O to form •OH, which effectively doubles the yield of •OH (24).
Formation of 8-OHG under Different Gassing Conditions
Dose–response curves for the formation of 8-OHGuo and unaltered guanine release in irradiated solutions of poly(G) are presented in Figs. 3–5. Figure 3 shows the dose response for the formation of 8-OHG in irradiated solutions of single-stranded, double-stranded and four-stranded poly(G) saturated with air, N2 and N2O.
FIG. 3.
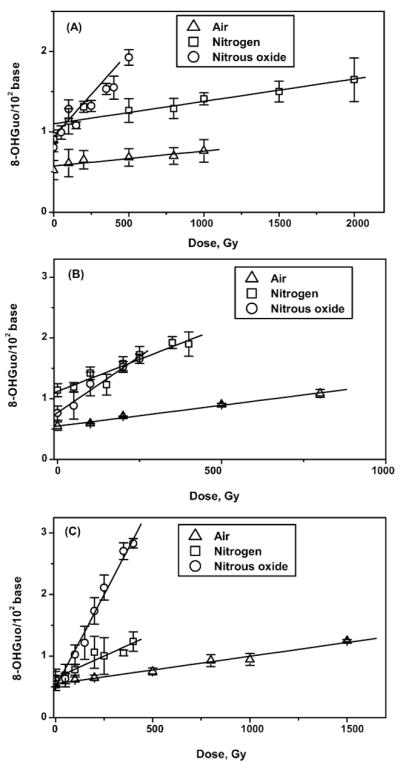
Yield of 8-hydroxyguanosine from γ-irradiated solutions of poly(G) saturated with air, N2 and N2O. Panel A: Single-stranded poly(G); panel B: double-stranded poly(G); panel C: four-stranded poly(G).
FIG. 5.
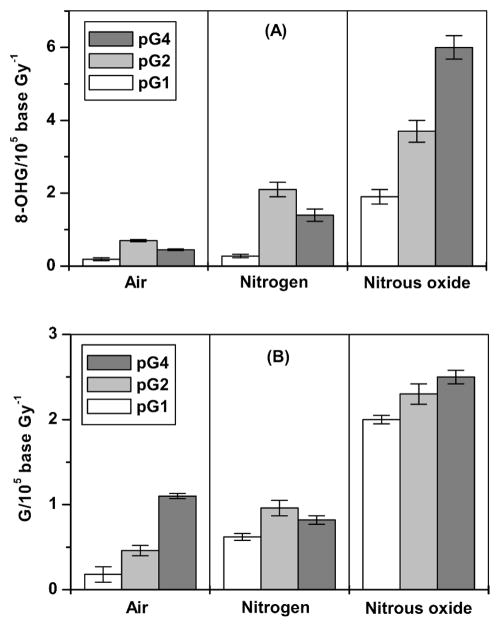
Radiation yields of 8-hydroxyguanine (panel A) and unaltered guanine bases (panel B). Yields were calculated from the slopes of the linear regression analysis of the dose-yield dependence. pG1, single-stranded poly(G); pG2, double-stranded poly(G); pG4, four-stranded poly(G).
The formation of 8-OHG, expressed as 8-OHGuo/102 of total guanine, was linear for single-stranded poly(G) (Fig. 3A) up to 1 kGy under air, up to 2 kGy under N2, and up to 500 Gy under N2O conditions. For double-stranded poly(G) (Fig. 3B) linearity was observed up to 800, 450 and 250 Gy under air, nitrogen and N2O, respectively; for the quadruplex poly(G), the formation of 8-OHG was linear up to 1.5 kGy under aerated conditions and up to 450 Gy under nitrogen and N2O (Fig. 3C). The radiation yield of 8-OHG was determined as the slope of a dose–response dependence by use of the linear regression method. The yields are presented in Table 1.
TABLE 1.
Radiation Yields of 8-OHG and Unaltered Guanine Release in Solutions of poly(G)
| pG1 |
pG2 |
pG4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Gassing condition | Air | N2 | N2O | Air | N2 | N2O | Air | N2 | N2O |
| 8-hydroxyguanine (8-OHG/105 base Gy−1) (mean ± SD) | 0.19 ± 0.04 | 0.28 ± 0.05 | 1.89 ± 0.20 | 0.69 ± 0.03 | 2.11 ± 0.24 | 3.71 ± 0.28 | 0.45 ± 0.02 | 1.42 ± 0.17 | 6.02 ± 0.32 |
| Guanine (G/105 base Gy−1) (mean ± SD) | 0.18 ± 0.09 | 0.62 ± 0.04 | 1.99 ± 0.05 | 0.46 ± 0.06 | 0.96 ± 0.09 | 2.32 ± 0.13 | 1.13 ± 0.03 | 0.82 ± 0.05 | 2.50 ± 0.08 |
Note. pG1, single-stranded conformation of poly(G); pG2, double-stranded conformation of poly(G); pG4, four-stranded conformation of poly(G).
Under aerated conditions, the yield of 8-OHG was significantly larger (P < 0.001) for double- and four-stranded polymers (0.69 and 0.45 8-OHG/105 G Gy−1, respectively) than for single-stranded poly(G) (0.19 8-OHG/105 G Gy−1) (Table 1). In addition, the yield of 8-OHG in double-stranded poly(G) was found to be 1.6-fold higher than in the four-stranded polymer (P < 0.001).
In nitrogen-saturated solutions, an increase in the 8-OHG yield was found for all three polymer conformations. As found in aerated solutions, the yield of 8-OHG was significantly larger (P < 0.001) for double-stranded poly(G) (2.11 8-OHG/105 G Gy−1) than for single-stranded (0.28 8-OHG/105 G Gy−1) and four-stranded poly(G) (1.42 8-OHG/105 G Gy−1).
Under N2O gassing, the yield of 8-OHG was greatest for four-stranded polymers and lowest for single-stranded polymers (6.02 and 1.9 8-OHG/105 G Gy−1), respectively. Thus the distribution of 8-OHG radiation yields among single-, double- and four-stranded conformations of poly(G) can be described as pG1 < pG4 < pG2 for air and nitrogen conditions and pG1 < pG2 < pG4 for N2O-saturated solutions.
Unaltered Guanine Release under Various Gassing Conditions
Figure 4 shows dose responses for unaltered guanine release in irradiated solutions of single-, double- and four-stranded poly(G) saturated with air, nitrogen and nitrous oxide.
FIG. 4.
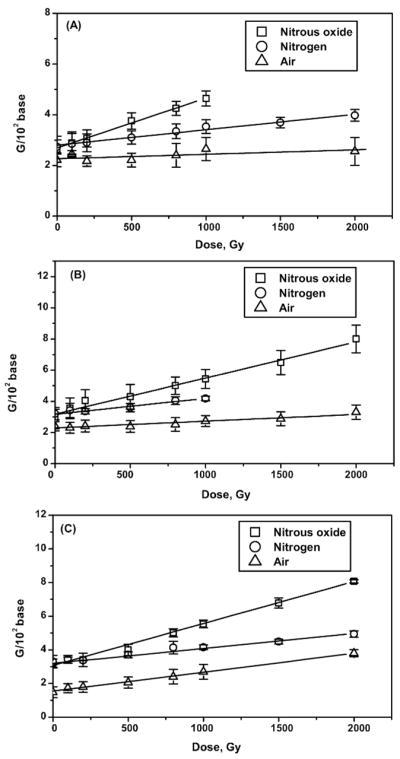
Unaltered bases released from γ-irradiated solutions of poly(G) saturated with air, nitrogen and N2O. Panel A: Single-stranded poly(G); panel B: double-stranded poly(G); panel C: four-stranded poly(G).
The unaltered guanine release, expressed as G/102 of total guanine, was linear for all conformations of poly(G) (Fig. 4) up to 2 kGy under air and nitrogen gassing conditions except for double-stranded polymer in nitrogen-saturated solution. In this case, the dependence was linear up to 1 kGy. In N2O-saturated solutions, the yield of unaltered guanine was linear up to 1 kGy for single-stranded polymer and up to 2 kGy for double- and four-stranded polymers. The yield of unaltered G was lowest (P < 0.001) for single-stranded polymer conformations under all gassing conditions (Table. 1). Under aerated conditions, the yield in four-stranded poly(G) was 2.4- and sixfold higher than in double-stranded and single-stranded poly(G), respectively (P < 0.001). In nitrogen-saturated solutions, the yield in four-stranded poly(G) was higher than in single-stranded poly(G) but was not significantly different compared with that in the double-stranded structure.
There was no statistically significant difference between the yields of unaltered G in double-stranded and four-stranded poly(G) in N2O-saturated solutions (P > 0.01). The yield in single-stranded polymer was lower compared with double- and four-stranded polymers (P < 0.001).
DISCUSSION
The Effect of poly(G) Conformation on Radiation Yield of 8-OHG
The mechanisms of 8-OHG formation in the presence of oxygen have been widely studied in DNA, small oligonucleotides and nucleotides (25–28). Under anaerobic conditions, the major water radiolysis products are the •OH, eaq− and the hydrogen atom (H•). In the presence of oxygen, the eaq− is converted to the superoxide anion radical O2•−. A fraction of the •OH radicals that are formed near poly(G) molecules during water radiolysis react with guanine base and result in the formation of 8-hydroxy-7,8-dihydroguanyl radicals, (G8OH)•, at the C8 position or a radical at the C4 position, (G4OH)•, that can dehydrate, yielding a neutral guanine radical G(-H)•. Additionally, (G8OH)• can be oxidized by oxygen to 8-OHG or reduced to 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) through the opening of the imidazole ring at C8-N9 (25, 26, 29). The relative radiation-induced yields of 8-OHG and FapyG obtained from double-stranded DNA depend on gassing conditions. Thus, in aerated solutions, the yield of 8-OHG is higher than that of FapyG (30), whereas in deaerated solutions, the yields of 8-OHG and FapyG are not significantly different (30)
In the presence of oxygen, the reaction of G(-H)• with O2•− leads to formation of 2,2,4-triamino-5(2H)-oxazolone through the intermediate species 2,5-diamino-4H-imidazol-4-one (29, 31). Under deoxygenated conditions, G(-H)• hydration leads to 8-OHG formation (27, 31). Differences between the relative yields of 8-OHG in solutions of guanine nucleotide, single- and double-stranded DNA have been reported in several studies. For example, it was reported that in the presence of oxygen, the G values (molecule/100 eV) of 8-OHG for 2′-deoxyguanosine 5′-monophosphate (5′-dGMP) and double-stranded DNA were 0.4 and 0.3 and for N2O-saturated solution, the G values were 0.55 and 0.17, respectively (32). As was shown by Fuciarelli et al., under aerobic conditions, the yield of 8-OHG was not significantly different between single- and double-stranded DNA conformations. However, in solutions saturated with N2O, the lesion yield was higher for the double-stranded DNA conformation (33). Previous work showed that the radiation-induced yields of 8-OHG in double-stranded DNA were 20- and twofold higher in DNA compared with those in deoxyguanosine in the presence and absence of oxygen, respectively (34). On the other hand, the formation of 8-OHG after UV irradiation in the presence of various photosensitizers was significantly lower for the single-stranded DNA conformation compared with the double-stranded conformation (35). Such disagreements between existing data can be caused by differences in experimental conditions (e.g., ionizing or UV radiation) that can lead to variations in the relative yields of the stable products from guanine radiolysis.
In the case of poly(G), our results show that the relative magnitude of the 8-OHG radiation yields among single-, double- and four-stranded conformations of poly(G) in the presence of oxygen varies as pG1 < pG4 < pG2 (Table 1, Fig. 5A).
Taking into account the increased yield of 8-OHG for all the polymer conformations under deoxygenated conditions (Table 1, Fig. 5A) and the fact that 8-OHG is oxidized more easily than guanine (28, 36, 37), we suggest that in the presence of oxygen, a fraction of 8-OHG formed in poly(G) can undergo further oxidation (36, 38, 39). Thus one-electron oxidation of 8-OHG by G(-H)• (28, 36) followed by reaction with O2•− can lead to the formation of secondary products such as dehydroguanidinohydantoin (27, 37). Also, the reaction of •OH with 8-OHG can lead to the formation of hydroxylated neutral radicals, (5-OH-8-oxo-G)• (39, 40), which can be further oxidized (27). Assuming that the reactivity of •OH with guanine and 8-OHG is similar and that the concentration of G is significantly greater than the 8-OHG concentration, an approximate calculation of the reaction probability of 8-OHG with •OH shows that up to ~20% of 8-OHG can react with hydroxyl radicals to form (5-OH-8-oxo-G)•. Thus, if the reaction rate constant of G and 8-OHG with •OH is 4.7 × 109 M−1 s−1, the rate constant of ribose is 1.3 × 109 M−1 s−1 (24), the concentration of G base is 0.5 mM, the concentration of 8-OHG is ~ 2.5 μM (taking to account background level), and the radiation yield of •OH is 0.27 μM Gy−1 (24), then ~ 1 nM of hydroxyl radicals generated per 1 Gy can react with 8-OHG. This indicates that from ~10% to 20% of 8-OHG can be converted to secondary products per 1 Gy depending on poly(G) conformation. However, the degradation of 8-OHG cannot completely explain the greater yields of 8-OHG observed under deoxygenated conditions compared with those found in aerated solutions. It can be speculated that the G(-H)• hydration pathway in poly(G) under deoxygenated conditions is more efficient than that in the presence of oxygen. However, an elaboration of the mechanisms underlying this effect will require a separate detailed study of the distribution of degradation products.
In the presence of oxygen, the radiation yields of 8-OHG for the three conformations of poly(G) exhibited a negative correlation with the average solvent accessible surface areas for guanines in single-, double- and four-stranded conformations (253, 148, and 164 Å2, respectively) (41). This correlation suggests that the average solvent-accessible surface area has an influence on the amount of decomposition of 8-OHG by oxidative mechanisms in aerated solutions. Another factor that can affect the 8-OHG yield distribution is the lower charge density of four-stranded poly(G) compared with that in double-stranded poly(G). There are several experimental findings that support this possibility. For example, it was shown that the negatively charged ligand Tel12 exhibits increased selectivity for the G-quadruplex structure over double-stranded DNA due to charge-charge repulsion (42). Further, the melting temperature of the G-quadruplex structure exhibits a reduced sensitivity to changes in cation concentration, probably due to the presence of site-bound counter ions that lower the overall charge density of the structure (43).
Under nitrogen gassing conditions, the distribution of radiation yields of 8-OHG among single-, double- and four-stranded conformations of poly(G) were similar to those obtained in aerated solutions (Table 1, Fig. 5A). Under these deaerated conditions, such a distribution suggests that eaq− reacts via pathways that reduce the 8-OHG yield. Indeed, under N2O conditions wherein eaq− is converted to •OH (24), a significant increase in the 8-OHG yield was found for all poly(G) conformations. Conventional radiation chemistry of dilute aqueous solutions predicts that the conversion of eaq− to •OH via the N2O reaction mechanism should lead to an increase in the 8-OHG yield by a factor of ~2 if eaq− does not affect the product formation and the •OH is the principal reactive species producing the 8-OHG. Such an increase was detected for double-stranded poly(G) but not for single- and four-stranded poly(G). In fact, increases of ~7 and ~4 times the deaerated yield of 8-OHG were observed for single- and four-stranded conformations, respectively. This finding also supports our proposed mechanism involving the reducing effect of eaq− on 8-OHG formation. As in the case of oxygenated solutions, the radiation yield of 8-OHG under N2 conditions exhibited a negative correlation with the average solvent-accessible surface areas. However in irradiated N2O-saturated solutions the distribution of 8-OHG yields changed and followed the sequence pG1 < pG2 < pG4. This reordering of the 8-OHG yields compared with the relative order of those found for air and nitrogen conditions [where O2, O2•− and eaq− likely define the yields ratio among poly(G) conformations] supports the hypothesis that the average solvent-accessible surface area and/or the magnitude of the polymer charge density plays a more important role under these conditions than in the cases where the yield of 8-OHG may depend largely on the probability of •OH interaction with the guanine moiety or on electron transfer from neutral guanine radicals. Such probability apparently increases as strandedness increases from single- to four-stranded poly(G).
It should be noted that for DNA the decrease in 8-OHG yield under deoxygenated conditions has been observed in several previous studies (30, 32–34). Thus, in ref. (34), the yield of 8-OHG in solutions of DNA irradiated under aerobic condition was ~8 times higher than under anaerobic conditions. In another study, the level of 8-OHG in irradiated solutions of DNA was found to be higher under aerated conditions than under nitrogen conditions but lower than under N2O or N2O/O2 (4:1) conditions (30). In ref. (32), the yields of 8-OHG in irradiated DNA samples were found to be 3.0 × 10−2 μmol J−1 and 1.7 × 10−2 μmol J−1 in the presence of oxygen and N2O, respectively. Fuciarelli et al. found that the yields of 8-OHG in solutions of both double-and single-stranded DNA were lower under N2O and N2 conditions than under aerated conditions (33).
The mechanism(s) responsible for the increased yields of 8-OHG found under deoxygenated conditions for guanine-rich sequences such as poly(G) compared with those found in DNA under the same conditions is unknown. As stated above, it can be speculated that the difference could result from variations in the mechanistic pathways that predominate in poly(G) compared to DNA, i.e., the G(-H)• hydration pathway in poly(G) compared to the pathways that involve oxygen in DNA.
Effect of poly(G) Conformation on the Radiation Yield of Unaltered Guanine Bases
The release of unaltered guanine bases caused by ionizing irradiation of nucleotides, polynucleotides and DNA in aqueous solutions is related to the formation of sugar radicals with subsequent ruptures of carbon-carbon bonds (44, 45). The reaction of •OH with sugar moieties occurs via hydrogen abstraction and sugar radical formation. It has been shown that under aerated conditions, the fast reaction of oxygen with sugar radicals leads to the formation of peroxyl radicals and results in the cleavage of carbon-carbon bonds, producing alkali-labile sites (45) or strand breakage followed by release of an altered sugar and an intact base (44). Under deoxygenated conditions, the sugar radicals also undergo cleavage with formation of strand breaks followed by release of unaltered bases and formation of altered sugars (44).
Our results show that in the presence of oxygen the distribution of the radiation yields of unaltered bases among the three polymer conformations increases in the order pG1 < pG2 < pG4 (Table 1, Fig. 5B). A similar increased yield of unaltered base release caused by •OH for double-stranded and single-stranded DNA has been reported previously (46). These variations in the yields of released bases for different polymer conformations can be attributed to two factors: (a) the difference in accessibility of the hydrogen atoms on the carbons of sugar moieties depending on their position and (b) by variations in end-product species formed.
As has been shown for the B-form of DNA, hydroxyl radicals preferentially abstract hydrogen from the 5′-and 4′-carbon positions of sugar moieties (47). This chemistry is observed because the 4′- and 5′-hydrogen atoms are significantly more accessible to the solvent than those at the other carbon positions and thus have a greater probability of being abstracted (48). The accessibility of hydrogen atoms at the 5′-position is greater than that at the 4′-position (47). While both hydrogen atoms at the 5′-position are accessible from the minor groove, one atom points away from the groove toward the solvent (48). Abstraction of either hydrogen at the 5′-carbon position leads to the release of an unaltered G base. On the other hand, if hydrogen abstraction occurs at the 4′-position, the subsequent fragmentation of the 4′-sugar radical in the presence of oxygen can lead to formation of base propenals (49) that cannot be detected as unaltered free bases. It seems reasonable to postulate that for the single-stranded polymer conformation the difference in accessibility of the hydrogen atoms at the 4′- and 5′-positions is the smallest of the three conformations followed by that for double-stranded and then that for four-stranded polymers. These variations in the accessibility could account for the dissimilarity in the yields of unaltered bases for different polymer conformations. Thus, for double- and four-stranded polymer conformations, the yields of sugar decomposition products associated with the release of intact bases would be enhanced relative to those for the single-stranded polymer.
This suggestion is supported by the results obtained for polymers in solutions saturated with N2O, in which the yields of the hydroxyl radical are increased and oxygen concentrations are greatly reduced (Table 1, Fig. 5B). In deaerated, N2O-saturated solutions, the radiation yields of intact base release are significantly increased for all poly(G) conformations and the differences between the yields for the three conformations are greatly reduced. Thus the differences in yields are not statistically significant for double- and four-stranded conformation and are slightly lower for the single-stranded conformation.
Increased yields of unaltered base release for single-and double-stranded polymer conformations were also observed in deaerated, N2-saturated solutions. Under these conditions the increases in yields were smaller than those obtained with N2O gassing, and only a small decrease was detected for four-stranded polymer. Similar to the yields of 8-OHG, the distribution of yields of unaltered base release in deaerated solutions followed the sequence pG1 < pG4 < pG2 and were more than two times higher under N2O gassing than under deaerated conditions. These results implicate the hydrated electron in the mechanism(s) involved in the release of unaltered guanine bases in deaerated solutions. The mechanism of such involvement is currently unknown.
In conclusion, the experimental results presented here support the hypothesis that the conformation of G-rich sequences in polymer structures such as telomeres plays an important role in the relative radiation sensitivity of these structures in terms of the formation of 8-OHG base lesions as well as in the release of unaltered bases. More specifically this suggests that telomere-like structures with G-quadruplexes will be relatively more radiosensitive than the other regions of duplex DNA. The hydroxyl radical is the major reactive species involved in the formation of the damage products studied here. The presence of oxygen significantly reduces the radiation yields of these damages for all conformations of poly(G) and changes the distribution of the yields among the polymer conformations. The formation of four-stranded G-rich structures appears to change the average solvent-accessible surface area and the polymer charge density that can result in the changes in damage product yields we have observed.
Acknowledgments
This work was supported in part by NIH Grant no. CA80211.
References
- 1.Todd AK, Johnston M, Neidle S. Highly prevalent putative quadruplex sequence motifs in human DNA. Nucleic Acids Res. 2005;33:2901–2907. doi: 10.1093/nar/gki553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc Natl Acad Sci USA. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 4.Phan AT, Mergny JL. Human telomeric DNA: G-quadruplex, i-motif and Watson-Crick double helix. Nucleic Acids Res. 2002;30:4618–4625. doi: 10.1093/nar/gkf597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher TM, Sun D, Salazar M, Hurley LH. Effect of DNA secondary structure on human telomerase activity. Biochemistry. 1998;37:5536–5541. doi: 10.1021/bi972681p. [DOI] [PubMed] [Google Scholar]
- 6.Han H, Hurley LH. G-quadruplex DNA: a potential target for anti-cancer drug design. Trends Pharmacol Sci. 2000;21:136–142. doi: 10.1016/s0165-6147(00)01457-7. [DOI] [PubMed] [Google Scholar]
- 7.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334:364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 8.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 9.Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 10.Stewart SA, Ben-Porath I, Carey VJ, O’Connor BF, Hahn WC, Weinberg RA. Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet. 2003;33:492–496. doi: 10.1038/ng1127. [DOI] [PubMed] [Google Scholar]
- 11.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 12.Roldan-Arjona T, Wei YF, Carter KC, Klungland A, Anselmino C, Wang RP, Augustus M, Lindahl T. Molecular cloning and functional expression of a human cDNA encoding the antimutator enzyme 8-hydroxyguanine-DNA glycosylase. Proc Natl Acad Sci USA. 1997;94:8016–8020. doi: 10.1073/pnas.94.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oikawa S, Tada-Oikawa S, Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40:4763–4768. doi: 10.1021/bi002721g. [DOI] [PubMed] [Google Scholar]
- 14.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann NY Acad Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 15.Oikawa S, Kawanishi S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453:365–368. doi: 10.1016/s0014-5793(99)00748-6. [DOI] [PubMed] [Google Scholar]
- 16.Opresko PL, Fan J, Danzy S, Wilson DM, III, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savintsev IV, Vekshin NL. Nonstacking binding of 7-aminoactinomycin D and actinomycin D to DNA and model nucleotide systems in solutions. Mol Biol. 2002;36:575–580. [PubMed] [Google Scholar]
- 18.Klump H. A calorimetric study of polyguanylic acid at neutral pH. Biophys Chem. 1976;5:359–361. doi: 10.1016/0301-4622(76)80047-6. [DOI] [PubMed] [Google Scholar]
- 19.Lesnik EA, Kochkina IM, Tikhonenko AS, Varshavskii IaM. Structure of polyriboguanylic acid in solution. Mol Biol (Mosk) 1980;14:820–829. [PubMed] [Google Scholar]
- 20.Petrovic AG, Polavarapu PL. The quadruplex-duplex structural transition of polyriboguanylic acid. J Phys Chem B. 2008;112:2245–2254. doi: 10.1021/jp0758723. [DOI] [PubMed] [Google Scholar]
- 21.Kasai H, Tanooka H, Nishimura S. Formation of 8-hydroxyguanine residues in DNA by X-irradiation. Gann. 1984;75:1037–1039. [PubMed] [Google Scholar]
- 22.Mergny JL, Phan AT, Lacroix L. Following G-quartet formation by UV-spectroscopy. FEBS Lett. 1998;435:74–78. doi: 10.1016/s0014-5793(98)01043-6. [DOI] [PubMed] [Google Scholar]
- 23.Blagoi YP, Galkin VL, Gladchenko GO, Kornilova SV, Sorokin VA, Shkorbatov AG. Metallocomplexes of Nucleic Acids in Solution. Naukova Dumka; Kiev: 1991. [Google Scholar]
- 24.Buxton GV, Greenstock CL, Helman WP, Ross AB, Tsang W. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data. 1988;17:513–886. [Google Scholar]
- 25.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 26.Steenken S. Purine bases, nucleosides, and nucleotides: aqueous solution redox chemistry and transformation reactions of their radical cations and e− and OH adducts. Chem Rev. 1989;89:503–520. [Google Scholar]
- 27.Pratviel G, Meunier B. Guanine oxidation: one- and two-electron reactions. Chemistry. 2006;12:6018–6030. doi: 10.1002/chem.200600539. [DOI] [PubMed] [Google Scholar]
- 28.Steenken S, Jovanovic SV, Bietti M, Bernhard K. The trap depth (in DNA) of 8-oxo-7,8-dihydro-2′deoxyguanosine as derived from electron-transfer equilibria in aqueous solution. J Am Chem Soc. 2000;122:2373–2374. [Google Scholar]
- 29.Cadet J, Berger M, Buchko GW, Joshi PC, Raoul S, Ravanat JL. 2,2-Diamino-4-[(3,5-di-O-acetyl-2-deoxy-β-D-erythro-pentofuranosyl)amino]-5-(2H)-oxazolone: a novel and predominant radical oxidation product of 3′,5′-di-O-acetyl-2′-deoxyguanosine. J Am Chem Soc. 1994;116:7403–7404. [Google Scholar]
- 30.Douki T, Martini R, Ravanat JL, Turesky RJ, Cadet J. Measurement of 2,6-diamino-4-hydroxy-5-formamidopyrimidine and 8-oxo-7,8-dihydroguanine in isolated DNA exposed to gamma radiation in aqueous solution. Carcinogenesis. 1997;18:2385–2391. doi: 10.1093/carcin/18.12.2385. [DOI] [PubMed] [Google Scholar]
- 31.Misiaszek R, Crean C, Joffe A, Geacintov NE, Shafirovich V. Oxidative DNA damage associated with combination of guanine and superoxide radicals and repair mechanisms via radical trapping. J Biol Chem. 2004;279:32106–32115. doi: 10.1074/jbc.M313904200. [DOI] [PubMed] [Google Scholar]
- 32.Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985;24:4476–4481. doi: 10.1021/bi00337a032. [DOI] [PubMed] [Google Scholar]
- 33.Fuciarelli AF, Wegher BJ, Blakely WF, Dizdaroglu M. Yields of radiation-induced base products in DNA: effects of DNA conformation and gassing conditions. Int J Radiat Biol. 1990;58:397–415. doi: 10.1080/09553009014551761. [DOI] [PubMed] [Google Scholar]
- 34.Svoboda P, Harms-Ringdahl M. Protection or sensitization by thiols or ascorbate in irradiated solutions of DNA or deoxyguanosine. Radiat Res. 1999;151:605–616. [PubMed] [Google Scholar]
- 35.Hirakawa K, Yoshida M, Oikawa S, Kawanishi S. Base oxidation at 5′ site of GG sequence in double-stranded DNA induced by UVA in the presence of xanthone analogues: relationship between the DNA-damaging abilities of photosensitizers and their HOMO energies. Photochem Photobiol. 2003;77:349–355. doi: 10.1562/0031-8655(2003)077<0349:boasog>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Ravanat JL, Saint-Pierre C, Cadet J. One-electron oxidation of the guanine moiety of 2′-deoxyguanosine: influence of 8-oxo-7,8-dihydro-2′-deoxyguanosine. J Am Chem Soc. 2003;125:2030–2031. doi: 10.1021/ja028608q. [DOI] [PubMed] [Google Scholar]
- 37.Misiaszek R, Uvaydov Y, Crean C, Geacintov NE, Shafirovich V. Combination reactions of superoxide with 8-oxo-7,8-dihydroguanine radicals in DNA: kinetics and end products. J Biol Chem. 2005;280:6293–6300. doi: 10.1074/jbc.M412253200. [DOI] [PubMed] [Google Scholar]
- 38.Kim JE, Choi S, Yoo JA, Chung MH. 8-Oxoguanine induces intramolecular DNA damage but free 8-oxoguanine protects intermolecular DNA from oxidative stress. FEBS Lett. 2004;556:104–110. doi: 10.1016/s0014-5793(03)01385-1. [DOI] [PubMed] [Google Scholar]
- 39.Grey CE, Adlercreutz P. Time and concentration dependence of Fenton-induced oxidation of dG. Nucleosides Nucleotides Nucleic Acids. 2006;25:259–278. doi: 10.1080/15257770500446956. [DOI] [PubMed] [Google Scholar]
- 40.Munk BH, Burrows CJ, Schlegel HB. An exploration of mechanisms for the transformation of 8-oxoguanine to guanidinohydantoin and spiroiminodihydantoin by density functional theory. J Am Chem Soc. 2008;130:5245–5256. doi: 10.1021/ja7104448. [DOI] [PubMed] [Google Scholar]
- 41.Szalai VA, Thorp HH. Electron transfer in tetrads: adjacent guanines are not hole traps in G quartets. J Am Chem Soc. 2000;122:4524–4525. [Google Scholar]
- 42.Kern JT, Kerwin SM. The aggregation and G-quadruplex DNA selectivity of charged 3,4,9,10-perylenetetracarboxylic acid diimides. Bioorg Med Chem Lett. 2002;12:3395–3398. doi: 10.1016/s0960-894x(02)00763-1. [DOI] [PubMed] [Google Scholar]
- 43.Jin R, Gaffney BL, Wang C, Jones RA, Breslauer KJ. Thermodynamics and structure of a DNA tetraplex: a spectroscopic and calorimetric study of the tetramolecular complexes of d(TG3T) and d(TG3T2G3T) Proc Natl Acad Sci USA. 1992;89:8832–8836. doi: 10.1073/pnas.89.18.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dizdaroglu M, Schulte-Frohlinde D, von Sonntag C. Radiation chemistry of DNA, II. Strand breaks and sugar release by gamma-irradiation of DNA in aqueous solution. The effect of oxygen. Z Naturforsch C. 1975;30:826–828. doi: 10.1515/znc-1975-11-1225. [DOI] [PubMed] [Google Scholar]
- 45.Dizdaroglu M, Schulte-Frohlinde D, von Sonntag C. γ-Radiolysis of DNA in oxygenated aqueous solution. Structure of an alkali-labile site. Z Naturforsch C. 1977;32:1021–1022. doi: 10.1515/znc-1977-11-1226. [DOI] [PubMed] [Google Scholar]
- 46.Sugden KD, Wetterhahn KE. Direct and hydrogen peroxide-induced chromium(V) oxidation of deoxyribose in single-stranded and double-stranded calf thymus DNA. Chem Res Toxicol. 1997;10:1397–1406. doi: 10.1021/tx970135r. [DOI] [PubMed] [Google Scholar]
- 47.Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci USA. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drew HR, Wing RM, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci USA. 1981;78:2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burger RM, Peisach J, Horwitz SB. Effects of O2 on the reactions of activated bleomycin. J Biol Chem. 1982;257:3372–3375. [PubMed] [Google Scholar]