Abstract
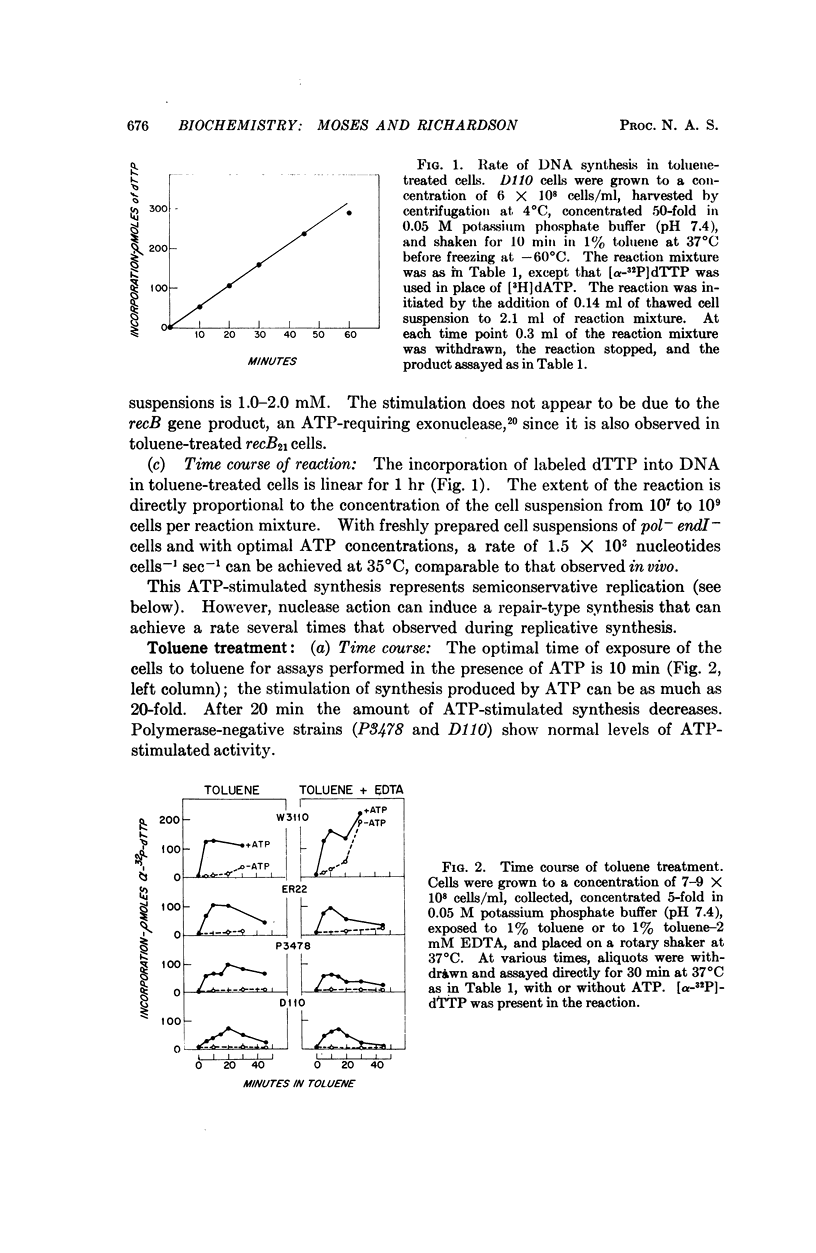
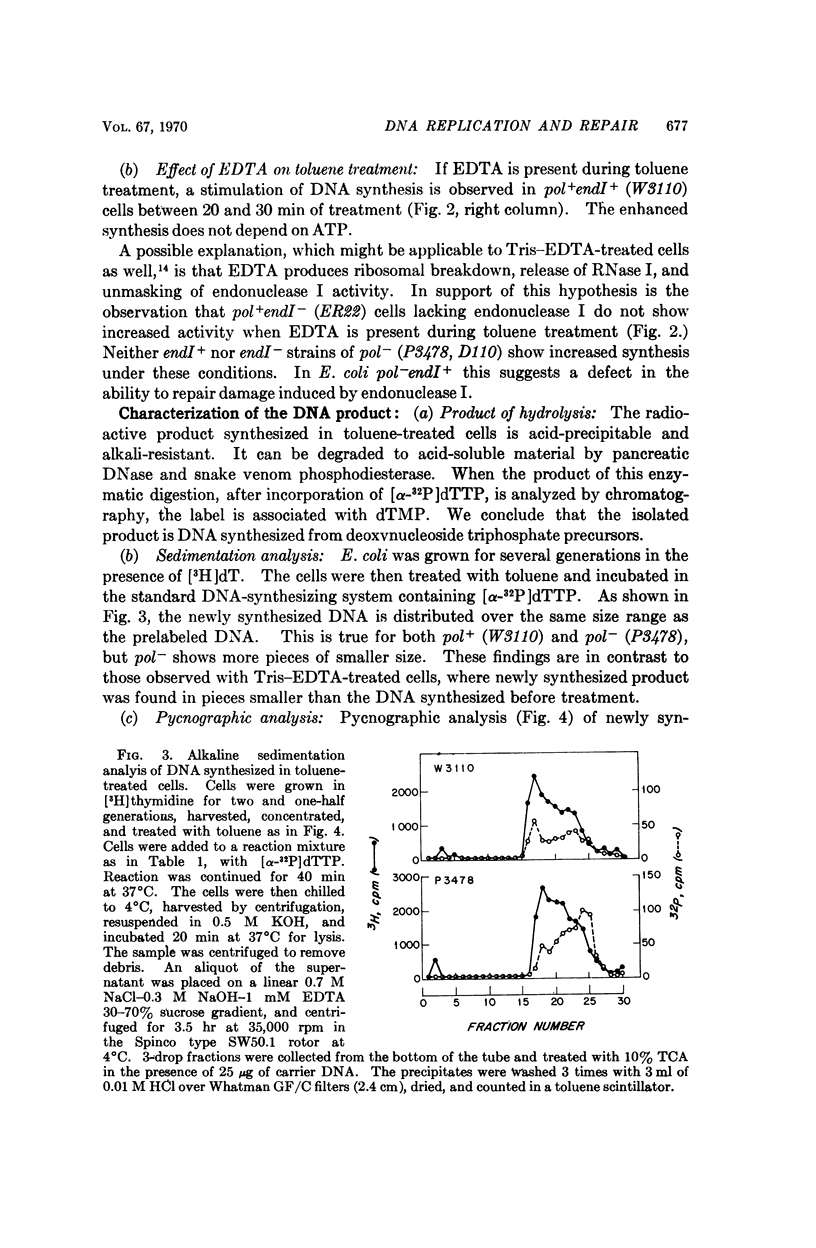
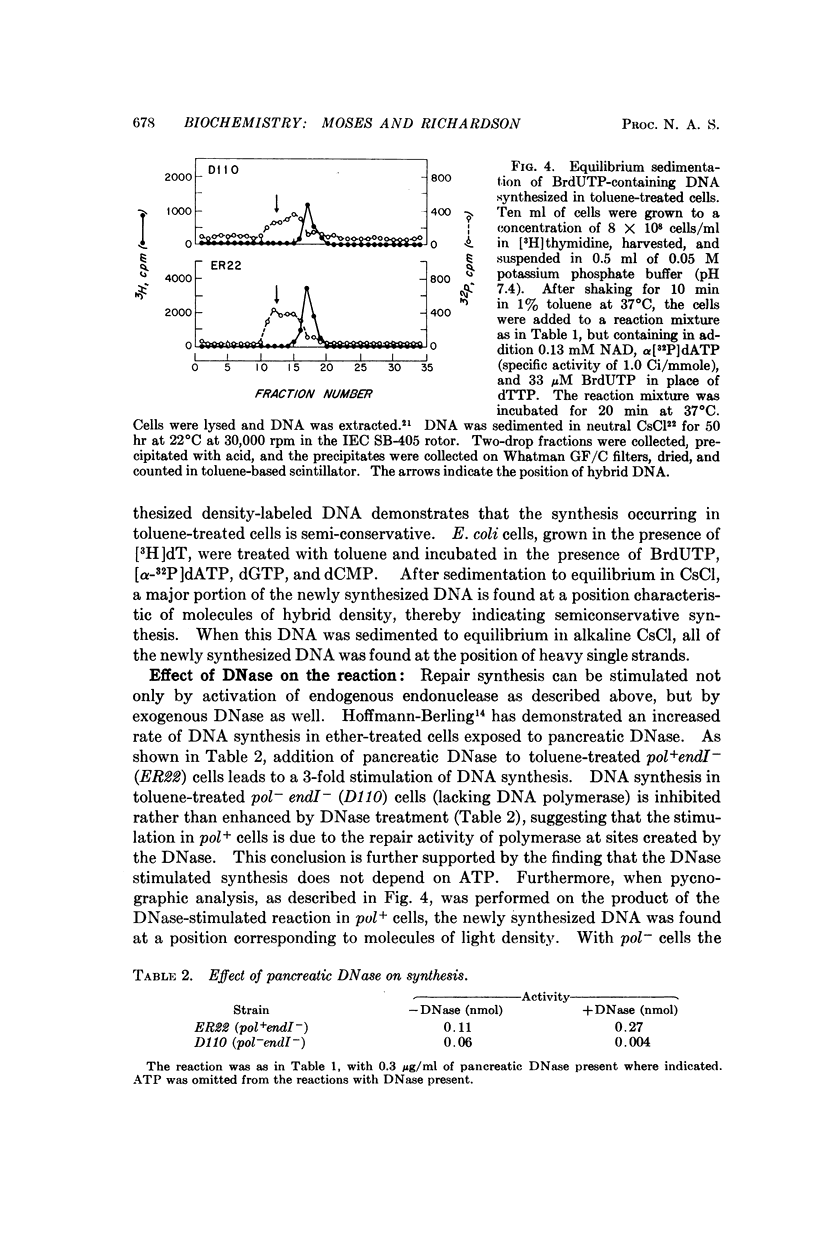
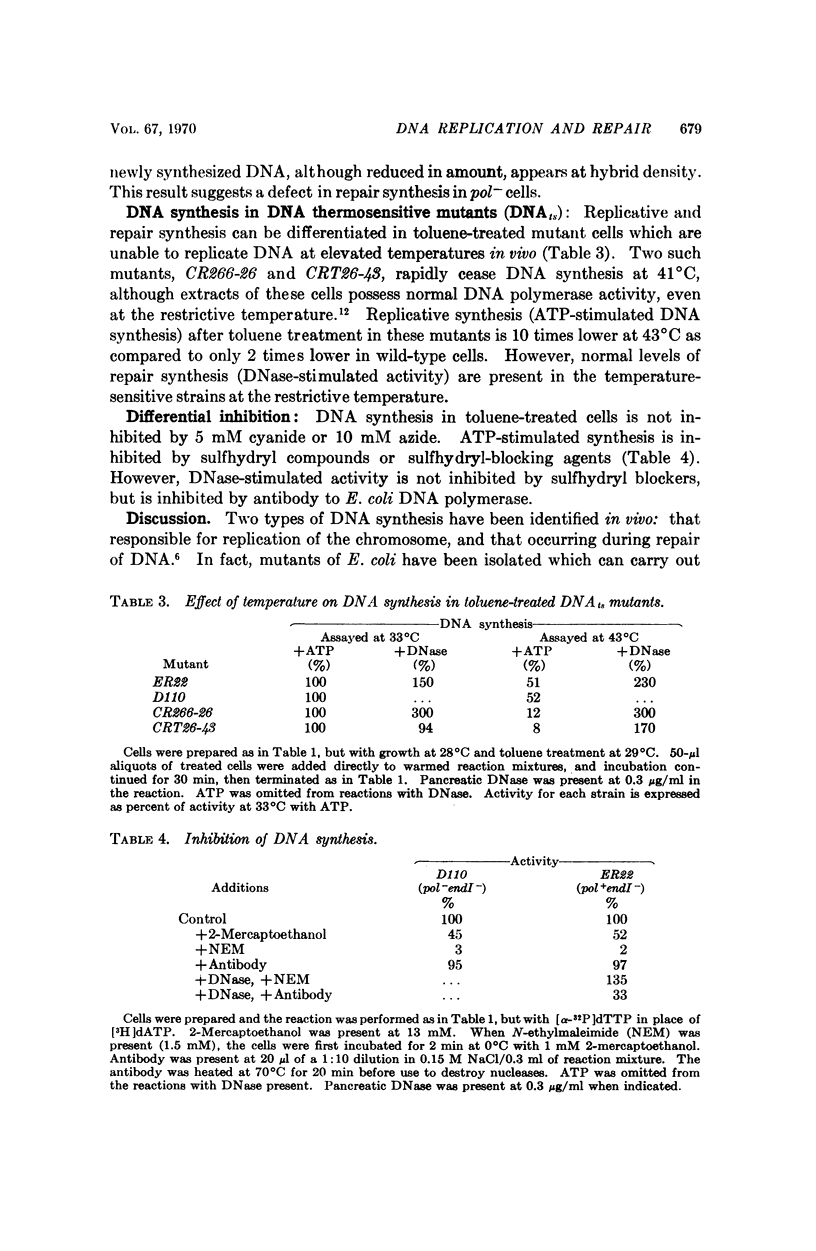
DNA synthesis has been studied in Escherichia coli cells made permeable to nucleotides by treatment with toluene. Replicative synthesis, as distinguished from repair synthesis, occurs at a rate comparable to that observed in vivo; it is dependent on the presence of all four deoxyribonucleoside triphosphates, but does not require exogenous DNA; and it is stimulated by ATP. Furthermore, replicative synthesis can be abolished at the restrictive temperature in DNA temperature-sensitive mutants. N-ethylmaleimide completely inhibits this type of synthesis, whereas it does not inhibit repair synthesis. Repair synthesis further differs from replicative synthesis in the following points: it does not require ATP; it persists at the restrictive temperature in DNA temperature-sensitive mutants; it can be induced by endogenous or exogenous nuclease activity; and its demonstration requires a Pol+ strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Frankel F. R., Majumdar C., Weintraub S., Frankel D. M. DNA polymerase and the cell membrane after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:495–500. doi: 10.1101/sqb.1968.033.01.057. [DOI] [PubMed] [Google Scholar]
- Ganesan A. T. Studies on in vitro replication of Bacillus subtilis DNA. Cold Spring Harb Symp Quant Biol. 1968;33:45–57. doi: 10.1101/sqb.1968.033.01.010. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Knippers R., Strätling W. The DNA replicating capacity of isolated E. coli cell wall-membrane complexes. Nature. 1970 May 23;226(5247):713–717. doi: 10.1038/226713a0. [DOI] [PubMed] [Google Scholar]
- LEVIN D. H., THANG M. N., GRUNBERG-MANAGO M. SYNTHESIS IN VIVO OF POLYNUCLEOTIDE PHOSPHORYLASE IN ESCHERICHIA COLI. I. EFFECT OF AMINO ACIDS ON POLYNUCLEOTIDE PHOSPHORYLASE ACTIVITY IN A CHLORAMPHENICOL-INHIBITED SYSTEM. Biochim Biophys Acta. 1963 Dec 20;76:558–571. doi: 10.1016/0006-3002(63)90082-9. [DOI] [PubMed] [Google Scholar]
- NAGATA T. The molecular synchrony and sequential replication of DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1963 Apr;49:551–559. doi: 10.1073/pnas.49.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., INMAN R. B., KORNBERG A. ENZYMIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. 18. THE REPAIR OF PARTIALLY SINGLE-STRANDED DNA TEMPLATES BY DNA POLYMERASE. J Mol Biol. 1964 Jul;9:46–69. doi: 10.1016/s0022-2836(64)80090-5. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Enzymes in DNA metabolism. Annu Rev Biochem. 1969;38:795–840. doi: 10.1146/annurev.bi.38.070169.004051. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., RICHARDSON C. C., KORNBERG A. ENZYMIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XVII. SOME UNUSUAL PHYSICAL PROPERTIES OF THE PRODUCT PRIMED BY NATIVE DNA TEMPLATES. J Mol Biol. 1964 Jul;9:24–45. doi: 10.1016/s0022-2836(64)80089-9. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Schaller H. E., Bonhoeffer F. J. DNA synthesis in vitro. Nature. 1970 May 23;226(5247):711–713. doi: 10.1038/226711a0. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]



