Abstract
Marijuana is the most widely used illicit substance among teenagers, yet little is known about the possible neural influence of heavy marijuana use during adolescence. We previously demonstrated an altered functional magnetic resonance imaging (fMRI) activity related to spatial working memory (SWM) among adolescents who were heavy users of after an average of 8 days of abstinence, but the persisting neural effects remain unclear. To characterize the potentially persisting neurocognitive effects of heavy marijuana use in adolescence, we examined fMRI response during SWM among abstinent marijuana-using teens. Participants were 15 MJ teens and 17 demographically similar non-using controls, ages 16–18. Teens underwent biweekly urine toxicology screens to ensure abstinence for 28 days before fMRI acquisition. Groups performed similarly on the SWM task, but MJ teens demonstrated lower activity in right dorsolateral prefrontal and occipital cortices, yet significantly more activation in right posterior parietal cortex. MJ teens showed abnormalities in brain response during a SWM task compared with controls, even after 1 month of abstinence. The activation pattern among MJ teens may reflect different patterns of utilization of spatial rehearsal and attention strategies, and could indicate altered neurodevelopment or persisting abnormalities associated with heavy marijuana use in adolescence.
Keywords: Drugs, Adolescence, Neuroimaging, Cognition
1. Introduction
Marijuana is the most commonly used illicit drug among teenagers: almost half of 12th graders have used cannabinoids, 20% report past-month use, and 5% disclose daily use (Johnston et al., 2006). During this period of increasing marijuana use, continued neuromaturation includes synaptic refinement, myelination, and improved cognitive and functional efficiency (Huttenlocher and Dabholkar, 1997; Giedd et al., 1999; Casey et al., 2000; Paus et al., 2001; Gogtay et al., 2004). The potential long-term consequences of marijuana use on the developing adolescent brain have not been well delineated, but they could have major implications for academic, occupational and social achievement.
Neuropsychological studies in adults have indicated that within a few days of abstinence, heavy users demonstrate impairments in learning and memory, attention, visuospatial skills, processing speed, and executive functioning (Varma et al., 1988; Pope and Yurgelun-Todd, 1996; Pope et al., 1997; Croft et al., 2001; Bolla et al., 2002; Solowij et al., 2002; Lyons et al., 2004).Event-related potential studies suggest slowed information processing and difficulty focusing attention (Solowij et al., 1991, 1995). Heavy marijuana users have demonstrated reduced cerebellar and frontal blood flow both at rest and during verbal learning and memory, while also showing poorer verbal learning abilities (Loeber and Yurgelun-Todd, 1999; Block et al., 2000; Lundqvist et al., 2001; Block et al., 2002). Functional magnetic resonance imaging (fMRI) evidence suggests that marijuana users show increased and widespread spatial working memory (SWM) activation after 6–36 h of abstinence, both in anterior cingulate and prefrontal regions normally associated with SWM, as well as in additionally recruited brain areas not activated among controls (Kanayama et al., 2004). During verbal working memory, marijuana users had similar fMRI response patterns as controls, yet failed to show practice-related decreases in parietal activation (Jager et al., 2006). However, it is unclear whether these neurocognitive findings only represent effects of recent use.
Pope et al. (2001) demonstrated deficits on verbal learning up to 7 days after use among current heavy marijuana users compared with former users and non-using controls. However, after 28 days of abstinence, current users performed similarly to former users and controls on all tests, suggesting that neurocognitive decrements may resolve within a month of abstinence (Pope et al., 2001). Importantly, fMRI evidence indicates that both abstinent users and active users show brain response abnormalities relative to controls during visual attention (Chang et al., 2006), suggesting lasting changes in patterns of neural activity. Together, these studies indicate that neuropsychological decrements observed after 1 week of use may not persist, and highlight the importance of examining neural responding after several weeks of abstinence.
Few studies have examined neurocognitive functioning among adolescent marijuana users. Among polysubstance using youths, marijuana use has been linked to poorer learning and memory (Millsaps et al., 1994) and attention (Tapert et al., 2002). In a longitudinal study, Fried and colleagues (Fried et al., 2005) assessed cognitive functioning in 9- to 12-year-olds before the initiation of marijuana use, and again when youths were ages 17–21. After controlling for baseline performance and demographics, they found that current heavy marijuana users showed deficits in immediate and delayed memory, processing speed, and overall IQ. Further, a longitudinal study of 10 cannabis-dependent adolescents demonstrated incomplete recovery of learning and memory impairments after 6 weeks of monitored abstinence (Schwartz et al., 1989), indicating that adolescents may be more susceptible to long-term changes than adults (Pope et al., 2001). Together, these studies point to dysfunctional working memory and attention abilities among adolescents who are heavy marijuana users that may persist after several weeks of abstinence.
We previously investigated fMRI response to a SWM task among adolescents with comorbid marijuana and alcohol use disorders compared with teens with alcohol use disorder alone and non-abusing teens. After an average of 8 days of abstinence, adolescents with comorbid marijuana and alcohol use disorders showed brain response abnormalities not evidenced by those with alcohol use disorders alone, including increased dorsolateral prefrontal activation and reduced inferior frontal response, suggesting compensatory working memory and attention activity associated with heavy marijuana use during youth (Schweinsburg et al., 2005b). Yet it is unclear whether these abnormalities are solely a function of recent use or would be present after a longer period of abstinence, suggesting persistent effects. A preliminary fMRI study explored verbal working memory among seven adolescent marijuana, seven demographically similar tobacco smokers, and seven non-users after a month of abstinence (Jacobsen et al., 2004). Compared with other groups, marijuana users demonstrated increased right hippocampal activity and poorer attention and verbal working memory performance. Recently, these researchers evaluated verbal working memory among abstinent adolescent marijuana users and non-users during nicotine withdrawal (Jacobsen et al., 2007). After at least 2 weeks of abstinence, marijuana users showed increased parietal activation during nicotine withdrawal and poorer verbal delayed recall, while non-marijuana users did not (Jacobsen et al., 2007). Together, these studies suggest persisting brain response abnormalities during working memory among adolescent marijuana users.
To investigate the potentially enduring neurocognitive effects of chronic marijuana use during adolescence, we examined fMRI response during an SWM task among marijuana-using teens and non-abusing controls after 28 days of monitored abstinence. Blood-oxygen-level-dependent (BOLD) fMRI was obtained during an SWM task that typically activates bilateral prefrontal and posterior parietal networks in adolescents (Schweinsburg et al., 2005a), and has been associated with neural dysfunction among youths with alcohol use disorders (Tapert et al., 2004) as well as comorbid alcohol and marijuana use disorders (Schweinsburg et al., 2005b). We predicted that after 28 days of monitored abstinence, marijuana-using teens would demonstrate intact performance on the SWM task yet increased brain response in frontal and parietal regions.
2. Methods
2.1. Participants
Flyers were distributed at high schools in San Diego County to recruit adolescent participants ages 16–18. Interested teens and a parent provided informed assent and consent (for 18-year-olds, the youth provided consent and the parent consented to a collateral informant interview), approved by the University of California San Diego Human Research Protection Program. Each adolescent and a parent were separately administered detailed screening interviews (Tapert et al., 2003; Schweinsburg et al., 2005b). The computerized NIMH Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b) (Shaffer et al., 2000; Lucas et al., 2001) excluded adolescents with a psychiatric disorder (including conduct disorder, attention deficit-hyperactivity disorder, and other drug use disorders) based on youth or parent report. Teens in the marijuana group who met DSM-IV criteria for alcohol use disorder (two cases of abuse and two cases of dependence) were included due to high comorbidity with marijuana use disorder (Agosti et al., 2002). Additional exclusionary criteria included prenatal substance exposure, psychotropic medication use, neurological dysfunction, head injury, family history of bipolar I or psychotic disorder as ascertained by the Family History Assessment Module screener (Rice et al., 1995), left-handedness, learning disorder, MRI contraindications, or substance use in the 28 days before scanning.
Eligible teens were 15 heavy marijuana users (MJ) and 17 demographically similar non-using controls (see Table 1 for participant characteristics). While most MJ teens were current users, five reported no use in the month before the monitored abstinence period. Groups were similar in gender and ethnic composition. Importantly, both MJ and control teens demonstrated similar levels of estimated premorbid IQ, as assessed by the Wechsler Abbreviated Scale of Intelligence Vocabulary subtest (Wechsler, 1999) and socioeconomic status (Hollingshead, 1965). MJ teens showed higher levels of depressive symptoms on the Hamilton Depression Rating Scale (Hamilton, 1960) (P < 0.05) and the Beck Depression Inventory (Beck, 1978) (P = 0.07), as well as higher levels of anxiety on the Hamilton Anxiety Rating Scale (Hamilton, 1959) (P < 0.05). MJ teens had more lifetime and recent experience with alcohol than controls (P < 0.05), yet five MJ teens reported no alcohol use in the month before the abstinence period. Among current alcohol users, most were weekend binge drinkers. Both groups had low rates of nicotine use, but MJ teens had used cigarettes more recently than controls, and four MJ teens smoked cigarettes on the day of the scan. Although MJ teens divulged more use of other drugs than controls ( P < 0.05), such use was limited to 25 lifetime experiences, most commonly narcotic pain pills or hallucinogens.
Table 1.
Participant characteristics
| MJ (n = 15) M (S.D.) or % |
Controls (n = 17) M (S.D.) or % |
|
|---|---|---|
| Age | 18.1 (0.7) | 17.9 (1.0) |
| % Female | 26.7 | 29.4 |
| % Caucasian | 73.3 | 58.8 |
| % Family history negative a | 66.7 | 76.5 |
| CBCL Externalizing T-score | 48.0 (6.2) | 44.9 (7.3) |
| CBCL Internalizing T-score | 49.0 (6.7) | 46.1 (9.2) |
| Beck Depression Inventory total | 4.9 (7.2) | 1.2 (2.0) |
| Hamilton Depression Score * | 4.5 (6.0) | 0.9 (2.0) |
| Spielberger State Anxiety T-score | 58.1 (2.8) | 57.4 (2.8) |
| Hamilton Anxiety Score * | 2.7 (3.7) | 0.1 (0.5) |
| Parent annual salary (thousands) | 138.9 (86.7) | 131.7 (65.3) |
| WASI Vocabulary scaled score | 54.8 (9.4) | 55.8 (7.6) |
| Years since first marijuana use b | 4.0 (1.6) | 3.6 (0.8) |
| Years since first weekly marijuana use ** |
2.7 (1.8) | 0.0 (0.0) |
| Lifetime marijuana use episodes ** |
480.7 (277.2) | 0.5 (1.3) |
| Marijuana use/month, past 3 months ** |
13.5 (11.6) | 0.0 (0.0) |
| Days since last marijuana use b, * | 60.4 (54.1) | 608.3 (210.7) |
| Lifetime drinks ** | 184.1 (135.0) | 15.1 (38.8) |
| Drinks/month, past 3 months ** | 34.2 (22.1) | 2.7 (9.4) |
| Days since last alcohol use c, * | 44.0 (63.4) | 167.8 (146.4) |
| Tobacco cigarettes per day | 1.3 (2.7) | 0.8 (2.6) |
| Days since last cigarette use d, * | 36.1 (67.8) | 475.5 (426.5) |
| FTND total (max = 10) | 0.3 (0.8) | 0.1 (0.2) |
| Lifetime other drug use episodes * | 6.7 (8.8) | 0.0 (0.0) |
| Narcotic pain pills * | 3.7 (6.8) | 0.0 (0.0) |
| Hallucinogens * | 1.9 (2.7) | 0.0 (0.0) |
| Amphetamines | 0.4 (1.3) | 0.0 (0.0) |
| Inhalants | 0.2 (0.8) | 0.0 (0.0) |
| Ketamine | 0.1 (0.3) | 0.0 (0.0) |
| Benzodiazepines | 0.1 (0.3) | 0.0 (0.0) |
| Cocaine | 0.1 (0.4) | 0.0 (0.0) |
| MDMA | 0.1 (0.3) | 0.0 (0.0) |
| PCP | 0.1 (0.3) | 0.0 (0.0) |
| Barbiturates | 0.0 (0.0) | 0.0 (0.0) |
MJ: 28-day-abstinent marijuana-using teens; CBCL: Child Behavior Checklist; WASI: Wechsler Abbreviated Scale of Intelligence; FTND: Fagerstrom Test for Nicotine Dependence.
No first-degree biological relative with alcohol or drug abuse or dependence.
Figure includes only those who reported history of use (n = 3 controls).
Figure includes only those who reported history of use (n = 9 controls).
Figures include only those who reported history of use (n = 12 MJ teens and n = 4 controls).
P < 0.05.
P < 0.001.
2.2. Measures
2.2.1. Substance involvement
Substance use was characterized with the Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998), which collected lifetime and past 3-month information on marijuana, alcohol, nicotine and other drug use, withdrawal symptoms, and DSM-IV abuse and dependence criteria. Based on CDDR reports, typical blood-alcohol concentration achieved during drinking episodes was calculated using the Widmark method, based on amount and duration of drinking, height, weight, and gender (Fitzgerald, 1995). The Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991) assessed degree of nicotine dependence on a scale of 0–10. The Timeline Followback (Sobell and Sobell, 1992) assessed substance use for 28 days before starting monitored abstinence, and for the 28 days of the abstinence period. Teens were asked to indicate for each day whether they used or drank, and if so, how many hits of marijuana, standard drinks of alcohol, or amounts of other substances were used.
2.2.2. State and behavioral measures
The Hamilton Depression Rating Scale (Hamilton, 1960), the Beck Depression Inventory (Beck, 1978), the Hamilton Anxiety Rating Scale (Hamilton, 1959), and the State scale of the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970) assessed mood at the time of scanning. The NEO Five-Factor Inventory (NEO-FFI) (Costa and McCrae, 1992) ascertained the following characteristics according to the five-factor model of personality: degree of neuroticism, extraversion, openness, agreeableness, and conscientiousness. Parents were given the Child Behavior Checklist (Achenbach and Rescorla, 2001) to assess level of psychopathological syndromes.
2.2.3. SWM task
The SWM task (Tapert et al., 2001; Kindermann et al., 2004) consisted of 18 21-s blocks that alternated between baseline vigilance and working memory conditions, and three blocks of resting fixation (21 s at the beginning and end of scanning, and 42 s in the middle of the task). The task began with 6 s of blank screen to allow the scanner to reach steady state; total scanning time was 7 min 48 s. Each block began with a 1-s word cue that indicated the type of upcoming block. In the SWM condition, the word “WHERE” cued subjects to remember the locations of abstract line drawings that were individually presented in one of eight spatial locations on a screen. Subjects were instructed to press a button every time a figure appeared in the same location as a previous design within that block, regardless of the shape. Unbeknownst to subjects, repeat location stimuli were two-back, and three of 10 trials in each block were targets. The baseline vigilance condition began with the word “DOTS,” followed by presentation of the same abstract stimuli shown in the same possible spatial locations as in the SWM condition; subjects were to press a button every time a figure appeared with a dot above it (approximately 30% of trials). Resting blocks displayed the word “LOOK” followed by presentation of a fixation cross in the center of the screen. For both the vigilance and working memory conditions, stimuli were presented for 1000 ms with an interstimulus interval of 1000 ms. All teens were trained with a 4-min version of the task and monitored to ensure comprehension of task instructions before scanning. Responses were collected with a fiber optic button box.
2.3. Procedures
2.3.1. Toxicology screening
The toxicology procedure was designed to minimize the possibility that participants used substances in the 28 days before fMRI assessment. Cannabinoid metabolites remain detectable in urine for at least 4 days (Fraser et al., 2002) and 27 days on average in heavy users (Ellis et al., 1985). Urine samples were collected 2–3 times per week during the 28 days preceding the fMRI session to detect metabolites indicating recent use of cannabis, amphetamines, methamphetamines, benzodiazepines, cocaine, barbiturates, codeine, morphine, phencyclidine, and ethanol. Samples were analyzed in the VA Medical Center laboratory using cloned enzyme donor immunoassay (CEDIA) assay kits (Fremont, CA). Observed sample collection reduced the possibility of participant tampering. Quantitative indices from samples were tracked to determine if cannabinoid metabolite levels decreased over the 28 days. Youths with initial samples positive for cannabis remained eligible if the values continued to decrease. If levels increased, the participant was given one chance to restart the 28-day toxicology screening process. Three quarters of marijuana users successfully completed this toxicology screening indicating abstinence for 28 days before scanning, and only these 15 subjects were included in analyses. Participants who were unable to complete 28 days of abstinence were not scanned.
2.3.2. Imaging protocol
Anatomical and functional imaging data were acquired using a 1.5 Tesla General Electric Signa LX scanner. The high-resolution structural scan was sagittally collected using an inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (Wong et al., 2000) (TR = 2000 ms, TE = 16 ms, FOV = 240 mm, resolution = 0.9375 × 0.9375 × 1.328 mm, 128 continuous slices, acquisition time = 8:36). The functional scan was axially acquired using T2*-weighted spiral gradient recall echo imaging (TR = 3000 ms, TE = 40 ms, flip angle = 90°, FOV = 240 mm, 19–21 slices covering the whole brain, slice thickness = 7 mm, reconstructed in-plane resolution = 1.875 × 1.875 mm, 156 repetitions).
2.4. Data analyses
Imaging data from each teen were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). Before statistical analysis, the time series data were corrected for motion by registering each acquisition to a selected repetition with an iterated least squares algorithm (Cox and Jesmanowicz, 1999), creating an output file specifying adjustments made for three rotational and three displacement parameters for each participant. Two independent raters inspected time series data to remove any repetitions on which the algorithm did not adequately adjust for motion (Schweinsburg et al., 2005a). On average, only 5% of repetitions were removed for excessive motion; thus, on average, subjects retained 95% of repetitions, the minimum was 74%, and groups did not differ in the number of repetitions removed [F(1, 31) = 0.33, P < 0.10]. Using deconvolution processing (Ward, 2002), the time series data were correlated with a reference function coding the hypothesized BOLD signal across the task and modeling anticipated delays in hemodynamic response (Cohen, 1997). This multiple linear regression approach yielded a fit coefficient for each subject in each voxel, representing the relationship between the observed and hypothesized signal change while controlling for linear trends and degree of motion correction applied. Fit coefficients were obtained for contrasts between SWM and vigilance, SWM and fixation, and vigilance and fixation conditions. Anatomical and functional data sets were warped into standard space (Talairach and Tournoux, 1988), and functional data were resampled into 3.0-mm3 voxels and smoothed with a 5.0-mm full-width/half-maximum Gaussian filter.
Group differences in the fit coefficient representing the BOLD response contrast to SWM relative to vigilance were evaluated using independent samples t-tests in each brain voxel. To control for Type I error in these analyses, significant group difference clusters consisted of contiguous significant voxels (P < 0.05) that exceeded 1328 µl in volume, yielding an overall clusterwise α = 0.05. To understand the nature of group differences between SWM and vigilance brain response, we performed follow-up t-test analyses in SPSS 14.0 examining each group’s average response to SWM relative to fixation, and vigilance relative to fixation, within each significant group difference cluster from the main analysis (α = 0.025).
To describe general activation patterns in each group, single sample t-tests identified clusters of task-related brain response in each group separately (clusters ≥ 1328 µl, P < 0.05). Exploratory follow-up regression analyses among MJ teens determined the relationships between substance use or behavioral characteristics and BOLD response in regions demonstrating significant group differences. For any variable that was related to brain response, we determined whether the group difference in brain response remained after controlling for the substance use of behavior characteristic.
3. Results
3.1. Task performance
Task accuracy was similar between groups, with MJ teens performing correctly on 96.43 ± 1.74% trials of vigilance and 93.43 ± 5.64% trials of SWM, and controls performing correctly on 95.73 ± 2.53% trials of vigilance and 93.20 ± 5.49% trials of SWM. A repeated measures ANOVA revealed a main effect for better accuracy on vigilance than on SWM[F(1,27) = 6.30,P = 0.018], but no main effect for group or a group × task condition interaction. Groups had similar reaction times, with 625.36 ± 41.01ms for vigilance and 537.55 ± 92.79ms for SWM on average among MJ teens, and 638.09 ± 68.71 ms for vigilance and 554.98 ± 88.65 ms for SWM among controls. A main effect of condition indicated that subjects responded faster on SWM than on vigilance [F(1,27) = 29.38, P < 0.001]; no main effect of group or group × task condition interaction was found for reaction time.
3.2. Motion
Groups did not significantly differ on bulk motion for any of the six motion parameters (x, y, z, roll, pitch, yaw), but there was a trend in the anterior-to-posterior direction, although such movements were quite small, with MJ teens moving 0.15 ± 0.10 mm and controls moving 0.10 ± 0.08 mm (P = 0.08). No motion parameter was related to brain response in any cluster exhibiting a group difference in fMRI response.
3.3. fMRI response
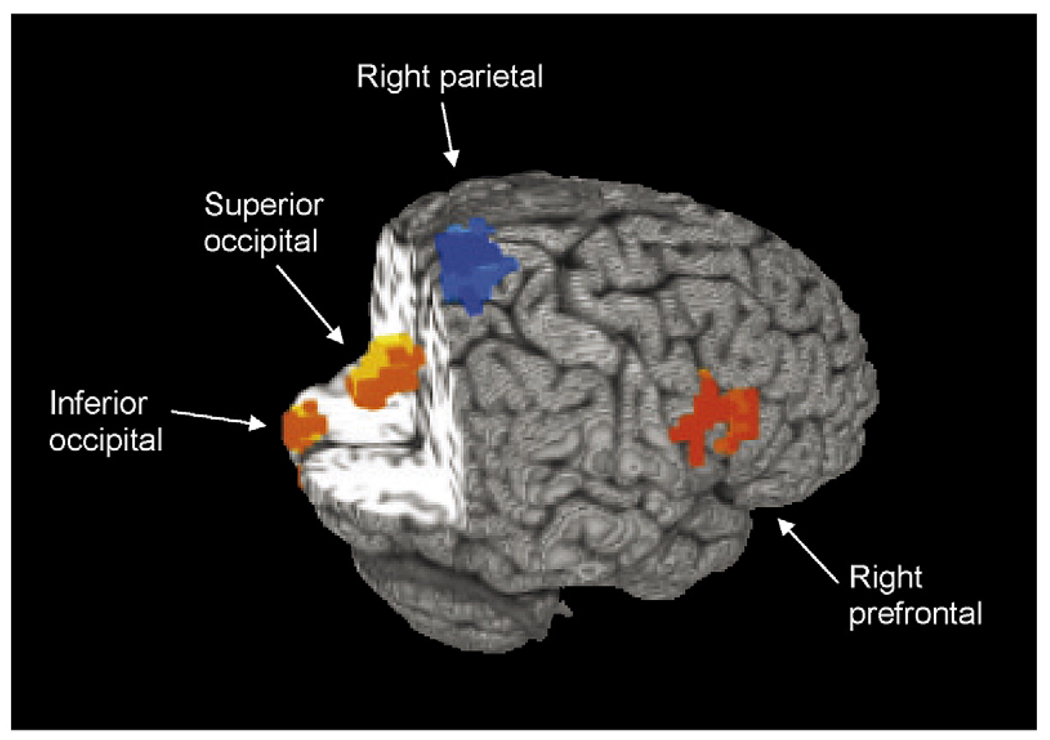
Overall, teens demonstrated greater activation to SWM than vigilance in bilateral superior parietal regions and dorsolateral and superior medial prefrontal cortices, and greater response to vigilance than SWM in medial anterior prefrontal and anterior cingulate regions, as well as inferior parietal, posterior cingulate, and occipital cortices (clusters > 1328 µl, P < 0.05). Significant group differences in BOLD response to SWM relative to vigilance were found in four clusters: right superior parietal lobule [Brodmann’s area (BA) 7], right dorsolateral prefrontal cortex (middle frontal gyrus, BA 9), medial right superior cuneus (BA 19), and left lingual gyrus/inferior cuneus (BA 17/18) (all clusters > 1328 µl, P < 0.05; see Table 2 and Fig. 1). MJ teens demonstrated significantly more activity during SWM relative to vigilance than controls in the right parietal cluster, but less activity during SWM relative to vigilance than controls in right dorsolateral prefrontal cortex. In both of these regions, MJ and control teens demonstrated greater activation during SWM relative to fixation, suggesting that group differences in parietal and prefrontal BOLD response were not due to SWM deactivation or group differences in vigilance response. Follow-up tests revealed that in the superior cuneus cluster, MJ teens demonstrated decreased BOLD response to SWM relative to fixation [t(14) = −5.25, P < 0.001], while controls showed no significant activation or deactivation during SWM or vigilance. In the inferior cuneus, MJ teens showed greater response during vigilance than SWM [ t(14) = −5.93, P < 0.001], while controls did not. These results remained significant even after excluding the four MJ teens with alcohol use disorders.
Table 2.
BOLD response differences to the spatial working memory task between abstinent marijuana users and control adolescents
| Anatomic region | Brodmann’s area |
Volume (µl) |
Talairach coordinates a |
Effect size |
||
|---|---|---|---|---|---|---|
| x | y | z | Cohen’s db |
|||
| SWM > Vigilance | ||||||
| Controls > MJ | ||||||
| R middle frontal gyrus |
9 | 2376 | 47 | 8 | 36 | 1.77 |
| MJ > Controls | ||||||
| R superior parietal lobule |
7 | 2997 | 8 | −53 | 69 | 1.57 |
| Vigilance > SWM | ||||||
| MJ > Controls | ||||||
| R cuneus | 19 | 2511 | 2 | −77 | 36 | 1.29 |
| L lingual gyrus and cuneus |
17, 18 | 1782 | −14 | −89 | −13 | 1.52 |
MJ: 28-day-abstinent marijuana-using teens; SWM: spatial working memory.
Talairach coordinates refer to maximum signal intensity group difference within the cluster; R, right; L, left.
Cohen’s d calculated from average t-value of all voxels within the cluster.
Fig. 1.
Significant clusters of group difference in spatial working memory fMRI BOLD response in marijuana-using (MJ) teens compared with controls. Orange indicates clusters in which MJ teens showed less response than controls during spatial working memory relative to vigilance; blue indicates cluster in which MJ teens showed greater response than controls during spatial working memory relative to vigilance; cluster P < 0.05, volume > 1328 µl.
Exploratory regression analyses within the MJ group examined the relationship between marijuana use characteristics and BOLD response in each cluster demonstrating a group difference. An earlier age of onset of regular marijuana use was associated with lower activity during SWM in the superior occipital cluster (F(1,13) = 7.57, P < 0.025, R2 = 0.37, β = 61) and inferior occipital cluster (F(1,13) = 8.55, P < 0.025, R2 = 0.40, β = 0.63), and more years of regular marijuana use was associated with lower activity during SWM in the superior occipital cluster [F(1,13) = 12.95, P < 0.005; R2 = 0.50, β = −0.71]. Number of lifetime uses and recency of use were not associated with brain response in any region showing a group difference.
MJ teens in this study exhibited moderately heavy alcohol use, and our previous work has suggested alcohol-related neural dysfunction during this SWM task among heavy drinkers (Tapert et al., 2001, 2004). Thus, we performed regressions to examine whether BOLD response differences among MJ teens might be related to frequency, quantity, duration, or recency of alcohol use. Brain response in the superior parietal lobe was negatively associated with estimated typical blood-alcohol concentration among MJ teens [F(1,13) = 6.55, P < 0.025, R2 = 0.34, β = −0.58]. No other measure of alcohol use was significantly related to neural activation among MJ teens, and group differences remained significant after excluding the four MJ teens with alcohol use disorders. Use of cigarettes and other drugs was not related to BOLD response in any region showing a group difference, and results remained significant after excluding teens who smoked on the day of the scan.
Exploratory analyses examined the relationship between performance and fMRI response in brain regions demonstrating a group difference. In the right superior parietal lobe, increased brain response was linked to more accurate vigilance performance [F(1,27) = 9.27, P = 0.005, R2 = 0.26, β = 0.51]; response in other brain regions was not associated with task performance, and was not related to marijuana use characteristics (i.e., lifetime use, frequency, recency) among MJ teens.
Within each brain region demonstrating a group difference, we also performed exploratory analyses to examine fMRI response in relation to depressive symptoms, anxiety symptoms, externalizing and internalizing scores, and personality characteristics. NEO-FFI agreeableness scores were positively associated with SWM response in the right middle frontal gyrus [F(1,30) = 7.98, P < 0.01, R2 = 0.21, β = 0.46], superior occipital [F(1,30) = 15.97, P < 0.005, R2 = 0.35, β = 0.59], and inferior occipital cortex [F(1,30) = 10.19, P < 0.005, R2 = 0.25, β = 0.50], though groups did not significantly differ on overall agreeableness scores. In each of these regions, group differences remained significant after controlling for agreeableness, and agreeableness remained a significant predictor of brain response. No measure of mood, behavior, or personality accounted for group differences in brain response.
4. Discussion
This study examined fMRI brain activation during a spatial working memory task among marijuana-using teens and controls after 28 days of monitored abstinence, verified by biweekly urine toxicology screens. Despite similar overall patterns of brain response to SWM, group differences were observed in right dorsolateral prefrontal cortex, right posterior parietal cortex, medial superior occipital cortex, and medial inferior occipital cortex.
MJ teens displayed reduced SWM BOLD response relative to control teens in the right dorsolateral prefrontal cortex, which is consistently implicated in spatial working memory (Wager and Smith, 2003). In contrast, our previous work revealed increased SWM response in this region among heavy alcohol- and marijuana-using teens who had been abstinent an average of just 8 days (Schweinsburg et al., 2005b). Kanayama and colleagues (Kanayama et al., 2004) also observed greater dorsolateral prefrontal response among adult heavy marijuana users on a similar SWM task 6–36 h after marijuana use. However, after 25 days of abstinence, adult marijuana users showed decreased left dorsolateral prefrontal blood flow during a modified Stroop task (Eldreth et al., 2004). Moreover, during visual attention, active marijuana users with positive urine toxicology screens evidenced greater reductions in right prefrontal fMRI response than abstinent users (Chang et al., 2006). Considered together with the results of the current study, these findings suggest a change in neural recruitment throughout the course of abstinence. This could relate to residual drug effects or withdrawal symptoms during early abstinence, less need for neural compensation, or a change in neurocognitive strategy as the brain adapts to different stages of sobriety. We did not observe a correlation between brain response and recency of marijuana use in this sample, but most neuropsychological recovery appears to occur during the first week of abstinence (Pope et al., 2001). Thus, there may be little change in neurocognitive functioning after 28 days of abstinence, or such an effect may be too subtle to detect with a relatively small sample. Careful examination of neural response within the first month of abstinence may better clarify this relationship.
Compared with controls, MJ teens demonstrated increased SWM activation in right posterior parietal cortex, a region involved in SWM and attentional processes (Wager and Smith, 2003; Wager et al., 2004). Research on parietal functioning during working memory among individuals with substance use disorders has been somewhat inconsistent. Using the same SWM task, we previously failed to observe parietal abnormalities among adolescent users of alcohol and marijuana (Schweinsburg et al., 2005b), though teens with alcohol use disorders alone showed increased posterior parietal brain response compared with controls (Tapert et al., 2004) despite similar task performance between groups. Greater fMRI activation in posterior parietal cortex has also been observed during SWM among adult marijuana users (Kanayama et al., 2004) as well as during verbal working memory among adolescent marijuana users experiencing nicotine withdrawal (Jacobsen et al., 2007).
Heightened activation among individuals with substance use disorders may be associated with compensatory neural responding to perform well on a task (e.g., Kanayama et al., 2004; Tapert et al., 2004; Schweinsburg et al., 2005b; Chang et al., 2006; Jacobsen et al., 2007). MJ teens in the current study displayed increased response in parietal cortex, yet diminished activation in prefrontal cortex, both of which play important roles in SWM (Wager and Smith, 2003). Frontal cortex may be primarily involved in general executive functioning components of working memory tasks, while superior parietal cortex may more specifically subserve attentional allocation and visuospatial rehearsal demands of SWM (Diwadkar et al., 2000; Wager and Smith, 2003; Wager et al., 2004). Thus, abstinent MJ teens may rely more on spatial rehearsal and attention rather than general executive abilities to perform the task, resulting in increased recruitment of posterior parietal cortex, but decreased right dorsolateral prefrontal activity. This altered pattern is consistent with previous evidence of reorganized attentional networks in MJ users (Chang et al., 2006). Further, estimates of typical blood-alcohol concentration achieved were negatively associated with parietal response among MJ teens, which could indicate that heavier drinking MJ teens may demonstrate less neural compensation or be less likely to utilize spatial strategies relative to those with lighter alcohol use histories. This is consistent with previous findings of diminished parietal activation during SWM among young adults with alcohol use disorders (Tapert et al., 2001), and suggests a potential interaction between heavy alcohol and marijuana use in youth (Schweinsburg et al., 2005b).
We previously characterized the relationship between age and fMRI response among 49 typically developing teens ages 12–17 using the same SWM task (Schweinsburg et al., 2005a). Younger teens evidenced increased response in superior portions of posterior parietal cortex, while older teens utilized more inferior aspects of posterior parietal cortex. This shift in localization of parietal response across adolescence indicates a change in strategy, with younger teens relying on rote spatial rehearsal and older teens implementing more spatial storage. MJ teens in the current study demonstrated increased SWM activity relative to controls in superior portions of right parietal cortex, paralleling response patterns of younger adolescents. Thus, MJ teens may employ spatial rehearsal strategies more consistent with those used by younger youths. This may suggest the possibility of altered neuromaturation among adolescent marijuana users, which could implicate an adverse influence of marijuana on the developing brain or preexisting neural differences that may have contributed to the initiation of substance use.
MJ teens demonstrated increased vigilance response and reduced SWM response compared with controls in two regions of medial occipital cortex: superior portions of the cuneus, and lingual gyrus/inferior cuneus. This could be related to performance differences between task conditions, as reaction times were slower during the SWM condition than during vigilance, despite better accuracy during vigilance. This is consistent with our previous findings (Schweinsburg et al., 2005a), and suggests that the efficiency with which correct responses can be implemented may differ between task conditions. The visual discrimination necessary for the vigilance condition may be more time-consuming, and may therefore differentially recruit visual cortex between groups. In addition, occipital cortex has been associated with visual attention, and may become more active as attentional capacity is reached (Marois and Ivanoff, 2005) yet less active during practiced tasks (Weissman et al., 2002). Greater occipital vigilance response among MJ teens may indicate less efficient processing and greater attentional demand during vigilance blocks. Such occipital hyperactivation among MJ teens was not observed during SWM blocks, during which attentional resources were not focused solely on visual selective attention, but allocated to accommodate working memory processing. Previous studies have implicated diminished attentional capacity in heavy marijuana-using adults (Solowij et al., 1991, 1995; Chang et al., 2006) and adolescents (Tapert et al., 2002; Schweinsburg et al., 2005b). During SWM, MJ teens may allocate limited attentional resources to spatial processing, depriving attentional input to executive systems, resulting in increased parietal and decreased frontal activation. A SWM task with greater executive demand may require more attentional input to frontal systems, diminishing response capability in the parietal cortices.
Among adolescent marijuana users, those who began regular use earlier in adolescence demonstrated greater abnormalities in occipital brain response. This could indicate greater decrements in spatial attention associated with early marijuana use. Similarly, adults who began using marijuana in early adolescence showed greater neural dysfunction during spatial attention (Chang et al., 2006) and poorer functioning on tests of attention (Ehrenreich et al., 1999), and verbal abilities and short-term memory (Pope et al., 2003). Animal models also indicate that cannabinoid exposure during adolescence is associated with greater impairments in working memory and spatial learning than adult exposure (Stiglick and Kalant, 1982, 1985; O’Shea et al., 2004). Thus, evidence suggests that marijuana use during adolescence may influence the course of brain development, and those who begin using marijuana at a younger age may be more susceptible to dysfunction with continued use.
Brain response differences between groups may also relate to aberrant cerebral blood flow among MJ teens. Adult marijuana users have demonstrated reduced resting frontal and cerebellar blood flow during short-term abstinence (Loeber and Yurgelun-Todd, 1999; Block et al., 2000; Lundqvist et al., 2001). Further, elevated cerebrovascular resistance and systolic blood flow remained high after a month of abstinence, suggesting lasting blood flow abnormalities (Herning et al., 2005). These blood flow abnormalities could affect the magnitude of the observed BOLD response (Cohen et al., 2002). Specifically, reductions in frontal blood flow may contribute to diminished frontal SWM activation among MJ teens. Future investigations could more closely account for resting perfusion when examining BOLD response among marijuana users.
This study raises several questions to be addressed in future studies. First, MJ teens demonstrated altered neural activation patterns, despite similar task performance as controls, and task performance was generally not related to group differences in fMRI response. Thus, fMRI differences between groups may represent different strategic approaches to the task that are not related to behavioral performance. Altering task difficulty may elicit alternate performance and brain response patterns between marijuana users and controls. Further, a task with more equivalent demands on processing speed between task conditions should be implemented in future studies.
Another factor to consider is that most MJ users in this study were moderate to heavy drinkers, and had more experience with other drugs (i.e., opiates and hallucinogens) than controls. Though this is representative of adolescent marijuana users (Agosti et al., 2002; Stinson et al., 2006), and cigarette and other drug use were not related to brain response in the current study, the functional impact of marijuana use alone is still difficult to determine. Our previous research identified altered fMRI response among youths with comorbid alcohol and marijuana use disorders compared with teens with alcohol use disorders alone, suggesting a unique contribution of marijuana above and beyond the effects of heavy drinking (Schweinsburg et al., 2005b). In the current study, brain response differences among MJ teens remained significant after excluding the four participants with alcohol use disorders, suggesting that meeting criteria for an alcohol use disorder per se did not influence fMRI patterns. However, a higher typical blood-alcohol concentration was associated with less superior parietal brain response in the MJ teens, which may indicate that the degree of heavy alcohol use may be related to aberrant brain response. Studies with larger samples and variability in drinking and other drug use patterns will help clarify the substance-specific neurocognitive effects.
MJ and control teens were comparable on demographics, behavior, personality and intellectual functioning, and differed slightly on mood, but none of these measures accounted for group differences in brain response. However, altered activation patterns among MJ teens may relate to preexisting traits that were not measured. Future studies might attempt to identify potential premorbid characteristics that contribute to brain function abnormalities among marijuana users, such as additional facets of personality and psychological functioning, genetic markers, and hormonal influences. In particular, sex differences in cannabinoid receptor binding and interactions between cannabinoids and sex hormones have been observed (Rodriguez De Fonseca et al., 1993, 1994), raising the possibility of gender differences in neural response to cannabinoids. In support of this, previous studies have identified gender differences in the neurocognitive impact of marijuana use among humans (Skosnik et al., 2006) and rodents (O’Shea et al., 2004, 2006). Gender differences in the rate and timing of neurodevelopment (Giedd et al., 1999, 2006) may also contribute to gender differences in sensitivity to marijuana in adolescence. Although the current study had limited power to detect gender differences, this is an important line for future research.
In addition, emerging evidence suggests shifts in neural processing during the initial stages of abstinence, yet the small sample size in the current study limited our ability to detect relationships to recency of marijuana use. Longitudinal examinations with larger sample sizes are needed to elucidate the time course and pattern of response through different stages of sobriety, which may inform treatment program design. Further, longitudinal follow-ups are needed to determine whether observed group differences will resolve with more extended abstinence.
Finally, assessing marijuana users with additional neuroimaging techniques should be a goal for future research endeavors. Diffusion tensor imaging and functional connectivity analyses could better delineate the neural networks and neurocognitive strategies influenced by marijuana use, while magnetic resonance spectroscopy could describe the cellular and metabolic changes associated with marijuana use.
In summary, adolescent marijuana users demonstrated different patterns of fMRI brain response during spatial working memory after 28 days of monitored abstinence, despite similar task performance to that of non-using controls. MJ teens showed decreased right dorsolateral prefrontal response and increased right posterior parietal activation during SWM, as well as increased vigilance response in medial occipital regions. Together, these results point to increased spatial rehearsal and attention, but decreased executive strategies among MJ teens. These findings suggest the possibility that heavy marijuana use in adolescence may be associated with persisting neurocognitive abnormalities, which could have important implications for future functioning among these youths.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants DA15228 and DA021182 to S. F. Tapert. We thank Valerie Barlett, Christina Burke, Lisa Caldwell, Tim McQueeny and Dr. M.J. Meloy for their assistance with this project.
Footnotes
Portions of this study were presented at the annual meeting of the International Neuropsychological Society, February 2–5, 2005, St. Louis, Missouri.
References
- Achenbach TM, Rescorla LA. Research Center for Children, Youth, and Families. Burlington, VT: University of Vermont; 2001. Manual for the ASEBA School-age Forms and Profiles. [Google Scholar]
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. American Journal of Drug and Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) San Antonio, TX: Psychological Corporation; 1978. [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypoactivity in frequent marijuana users. NeuroReport. 2000;11:749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology, Biochemistry, and Behavior. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. Journal of Cerebral Blood Flow and Metabolism. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Costa PTJ, McCrae RR. Professional Manual: Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI) Lutz, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology. 2001;153:373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Carpenter PA, Just MA. Collaborative activity between parietal and dorsolateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. NeuroImage. 2000;12:85–99. doi: 10.1006/nimg.2000.0586. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ellis GM, Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clinical Pharmacology and Therapeutics. 1985;38:572–578. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF. Intoxication Test Evidence. Deerfield, IL: Clark Boardman Callaghan; 1995. [Google Scholar]
- Fraser AD, Coffin L, Worth D. Drug and chemical metabolites in clinical toxicology investigations: the importance of ethylene glycol, methanol and cannabinoid metabolite analyses. Clinical Biochemistry. 2002;35:501–511. doi: 10.1016/s0009-9120(02)00325-9. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana — a comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. 2005;64:488–493. doi: 10.1212/01.WNL.0000150882.69371.DD. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Annals of the New York Academy of Sciences. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology. 2006;185:358–368. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. The Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2005. Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology. 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophrenia Research. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Yurgelun-Todd DA. Human neuroimaging of acute and chronic marijuana use: implications for frontocerebeller dysfunction. Human Psychopharmacology: Clinical and Experimental. 1999;14:291–304. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicology and Teratology. 2001;23:437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT. Neuropsychological consequences of regular marijuana use: a twin study. Psychological Medicine. 2004;34:1239–1250. doi: 10.1017/s0033291704002260. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends in Cognitive Sciences. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Millsaps CL, Azrin RL, Mittenberg W. Neuropsychological effects of chronic cannabis use on the memory and intelligence of adolescents. Journal of Child and Adolescent Substance Abuse. 1994;3:47–55. [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of Psychopharmacology. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats. Journal of Psychopharmacology. 2006;19:19. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association. 1996;275:521–527. [PubMed] [Google Scholar]
- Pope HG, Jr, Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuo-spatial memory. Psychotherapy and Psychosomatics. 1997;66:179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sciences. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases in Children. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescent development. Journal of the International Neuropsychological Society. 2005a;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005b;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Vohs JL, O’Donnell BF. The effect of cannabis use and gender on the visual steady state evoked potential. Clinical Neurophysiology. 2006;117:144–156. doi: 10.1016/j.clinph.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press, Inc; 1992. pp. 41–72. [Google Scholar]
- Solowij N, Michie PT, Fox AM. Effects of long-term cannabis use on selective attention: an event-related potential study. Pharmacology, Biochemistry, and Behavior. 1991;40:683–688. doi: 10.1016/0091-3057(91)90382-c. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biological Psychiatry. 1995;37:731–739. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stiglick A, Kalant H. Residual effects of prolonged cannabis administration on exploration and DRL performance in rats. Psychopharmacology. 1982;77:124–128. doi: 10.1007/BF00431933. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology. 1985;85:436–439. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychological Medicine. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. Coplanar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown GG, Brown SA, Frank LR, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Varma VK, Malhotra AK, Dang R, Das K, Nehra R. Cannabis and cognitive functions: a prospective study. Drug and Alcohol Dependence. 1988;21:147–152. doi: 10.1016/0376-8716(88)90061-0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Ward BD. Deconvolution Analysis of FMRI Time Series Data. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin; 2002. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weissman DH, Woldorff MG, Hazlett CJ, Mangun GR. Effects of practice on executive control investigated with fMRI. Brain Research: Cognitive Brain Research. 2002;15:47–60. doi: 10.1016/s0926-6410(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank LR. Single slab high-resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society for Magnetic Resonance in Medicine. 2000;8:683. [Google Scholar]