Abstract
Guanosine 3′,5′-cyclic monophosphate (cGMP) and small GTPase Rac are critical regulators of cell functions. Recently, Rac has been shown to use its downstream effector p21-activated kinase (PAK) to directly activate transmembrane guanylyl cyclases (GCs). This novel Rac/PAK/GC/cGMP signaling pathway bridges Rac and cGMP, and provides a general molecular mechanism for diverse receptors to regulate physiological functions such as cell migration through elevating the cellular cGMP level.
Keywords: Guanylyl cyclase, Rac, PAK, cGMP
Introduction
cGMP is a common second messenger regulating diverse cell functions [1–4]. The production of cGMP is catalyzed by guanylyl cyclases (GCs). There are two types of GCs, soluble and transmembrane. The soluble GC is activated through the binding of nitric oxide to its heme group. There are seven mammalian transmembrane GCs. Transmembrane GCs are regulated through both extracellular and intracellular signaling pathways. GC-A and GC-B are natriuretic peptide receptors (also named natriuretic factor receptor guanylyl cyclases). GC-C can be activated by bacterial enterotoxins, guanylin, and uroguanylin. GC-E, GC-F, and GC-G are without known extracellular ligands. GC-E and GC-F, mainly found in retina, can be modulated by a group of retinal-specific cellular proteins named GCAPs (guanylyl cyclase activating proteins) in a calcium-dependent manner [1]. GC-D, specifically expressed in a subset of olfactory neurons in mammals, could be activated by guanylin and uroguanylin, as well as by bicarbonate ions [5–8]. Upon the binding of the extracellular ligands or the intracellular activators, GCs go through conformational changes to become active. The positioning of the two cyclase domains from the GC homodimer is critical to the activation state of the enzyme.
Rac belongs to the Rho GTPases, a family of proteins best known for their regulation of cytoskeleton and cell migration [9–11]. Rac can be activated by extracellular signals through receptor tyrosine kinases such as the PDGF receptor and through G protein-coupled receptors. Rac, as well as its downstream effectors such as PAK and WAVE proteins, play key roles in regulating actin polymerization and promoting lamellipodial formation at the front edges of migrating cells.
PAK is one of the best characterized downstream effectors of Rac. PAK family members regulate cellular proliferation, differentiation, transformation, and survival [12–14]. Recent studies in cancer cells, Dictyostelium, neuronal cells, and other systems indicate that PAKs play important parts in cytoskeletal rearrangement and cell migration [12–16]. From the crystal structure of PAK1 [17], the inactive state of PAK is maintained by the N-terminal auto-regulatory region binding to and inhibiting the catalytic kinase domain. Direct binding of GTP-bound Rac (or Cdc42) releases PAK kinase domain from this inhibition. PAK then autophosphorylates to stabilize the active state [17]. Active PAK triggers various physiological responses by phosphorylating target proteins at their serine and/or threonine residues. However, a phosphorylation-independent function of PAK has also been reported. The molecular mechanism for such a function was not known. Our discovery of a phosphorylation-independent function, i.e., GC activation by PAK, provides insights in the diverse functions of PAKs [18].
cGMP plays a role in the cytoskeletal reorganization and cell movement. For example, elevation of the cGMP concentration disrupts stress fibers through phosphorylation and inactivation of RhoA by cGMP-dependent protein kinase (PKG) [19]. In platelets, cGMP regulates cytoskeleton and cell motility by activating PKG and its phosphorylation on the actin-binding protein VASP [20]. In Dictyostelium amoebae, cGMP mediates myosin filament formation in the posterior of the cell [21], a crucial step for chemotaxis. cGMP production in Dictyostelium can also be stimulated at the leading edge and could be involved in the stimulation of pseudopod extension [22]. cGMP is also involved the migration process in other cell types such as endothelial cells [23–25], vascular smooth muscle cells [26], and neutrophils [27]. Moreover, a properly controlled cGMP gradient and/or a cGMP/cAMP ratio may be critical in neuronal cell migration and axonal guidance [28, 29]. These findings not only suggest potential significant roles of cGMP played in the regulatory pathways of cytoskeleton and cell motility, but also raise the possibility of a crosstalk between GCs and other cell migration regulators such as Rac and PAK. In fact, Rac1 could be transiently activated by cGMP in HEK293 cells [30]. Our studies showed that transmembrane GCs could be directly activated by PAK, providing the missing link between cGMP and Rac, two important regulators of cellular functions (Fig. 1).
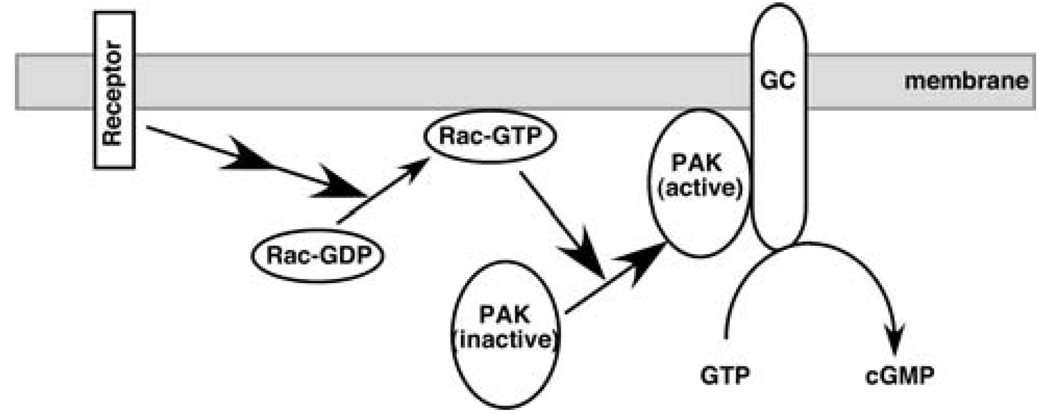
Fig. 1.
Schematic diagram of the activation of membrane-bound GCs by intracellular signals. Upon ligand binding and activation, receptors transmit the signals to Rac via guanine nucleotide-exchange factors. Activated Rac is translocated to the membrane. Rac, in turn, activates PAKs and translocates PAKs to the membrane where PAKs directly contact GCs to cause a conformational change and to activate GCs, leading to the production of cGMP
Activation of transmembrane GCs by Rac and PAK
In our studies of the regulation of transmembrane GCs, we found that Rac1 could increase the GC activity in cells. Expression of a constitutively activated mutant of Rac1 in CHO-GC cells (CHO cells stably expressing different GCs) led to a significant increase of cellular cGMP levels. This stimulatory effect is specific to Rac1 since the other two members of the Rho-family small GTPases, RhoA, and Cdc42, did not increase the cellular cGMP levels when their constitutively activated mutant forms were transiently expressed in CHO-GC cells. The Rac1 regulation appears to be a general phenomenon to the transmembrane GCs since Rac1 had no effect on the activity of soluble GC [18].
Membrane signaling receptors can use this Rac-mediated pathway to modulate the cellular cGMP level. In fibroblast cells, PDGF binds and stimulates PDGFR, resulting in the activation of Rac. In serum-starved cells expressing membrane GCs, PDGF treatment led to higher cellular cGMP levels [18]. This increase of cGMP level could be blocked by the expression of a dominant-negative Rac1 mutant [Rac1 (T17N)] in these cells [18]. Moreover, in Rac1-deficient mouse embryonic fibroblast (MEF) cells, PDGF-induced increase of cellular cGMP was demolished. Re-expression of Rac1 in these Rac1-deficient cells restored the cGMP response. These data further demonstrated that Rac relays the PDGF signal to increase GC activity.
In vitro GC activity assay with purified proteins indicated that Rac is not a direct activator of GC. Analyses of known downstream effectors of Rac1 demonstrated that PAK is required for the activation of GC. Expression of a constitutively active mutant of PAK1 in CHO-GC cells increased the cellular cGMP levels [18]. More importantly, transient expression of the PAK1 autoinhibitory domain PAK1-(83–149), which inhibits PAK function [31], blocked the stimulation of GCs by Rac1(G12V). On the other hand, expression of PAK1-(83–149)L107F, which lacks the inhibitory effect on PAK1, did not block Rac1(G12V)-induced cellular cGMP accumulation [18]. These findings demonstrate that active PAK acts downstream of Rac1 in stimulating GC.
PAK is a downstream effector for both Rac and CDC42. In an in vitro kinase assay, PAK could be activated by Rac and CDC42 to a similar extent. In our studies of the activation of GC by PAK, we observed that both purified Rac1 and CDC42 could activate PAK, and in turn increase the activity of purified GC protein. However, measurements of cGMP levels in cultured cells showed that only constitutively active Rac was able to increase cGMP production. Constitutively active CDC42 failed to do so. Although this is consistent with the fact that Rac and CDC42 play distinctively different physiological roles, the reason for their differences in the regulation of GC is not clear at the present time.
Phosphorylation-independent activation of GC by PAK
GC activity assays with purified PAK proteins (PAK1 and PAK2) and intracellular domains of GC-D and GC-E (GC-D-intra and GC-E-intra) demonstrate that PAK is a direct activator of GC. Purified PAK (in the presence of Mg2+-ATP) by itself increased the activity of purified GC-intra by ~ twofold. Addition of Rac1-GTPγS to activate PAK led to more than an eightfold increase of GC-intra activity [18]. Moreover, the activation of GC-E by PAK2 (in the presence of Rac1-GTPγS) was concentration-dependent with an EC50 value of ~ 42 nM [18]. Since PAK is a protein kinase, a logical scenario would be that PAK activates GC through phosphorylation of GC leading to its subsequent conformational change. However, our studies showed that this activation of GC by PAK is phosphorylation-independent.
In vitro phosphorylation assays showed that GC-E-intra was not phosphorylated by active PAK. However, kinase-dead mutants of PAK could not stimulate GC in cells or in in vitro assays with purified proteins. These data suggest that although the phosphorylation of GC by PAK is not required for its activation, the kinase activity of PAK is needed. PAK1 can autophosphorylate itself on several serine and threonine residues including Thr423 (Thr402 in PAK2) at the activation loop of the catalytic domain. Phosphorylation of Thr423 is critical for the conformational change of the activation loop, assembly of the active configuration of the catalytic domain, and activation of PAK1. The phosphorylation of PAK is essential for its ability to activate GC. Wild-type PAK proteins could no longer activate GC after they were de-phosphorylated with alkaline phosphatase [18]. The de-phosphorylated PAK could still be activated by Rac1-GTPγS in the presence of ATP leading to auto-phosphorylation; this re-phosphorylated PAK regained its ability to stimulate GC [18]. Furthermore, when Thr423 of PAK1(K298A) was mutated to glutamine to mimic the auto-phosphorylation, the kinase-dead protein was able to activate GC in vitro [16]. Moreover, a pre-phosphorylated PAK1(K298A) also increased the activity of GC-intra. There fore, although PAK does not phosphorylate GC, its own phosphorylation is required for its ability to activate GC.
PAK activates GC through direct interaction with the cyclase domain by its kinase domain
Several lines of evidence confirmed the direct interaction of PAK and GC-E-intra. These include the co-immunoprecipitation of PAK and GC (endogenous or epitope-tagged) from mammalian cells, the co-expression and co-purification of His-tagged GC-E-intra and GST-tagged PAK in Sf9 cells, and the direct interaction of purified GST-PAK and His-GC-E-intra proteins [18]. Consistent with the GC activation assays, only the PAK with an active conformation can interact with GC. Further binding studies showed the interaction between the two proteins is through the kinase domain of PAK and the cyclase domain of GC [18]. These data indicated that PAK is likely to activate GC by direct contact with the cyclase domain to induce a conformational change. This is actually similar to the allosteric activation of transmembrane adenylyl cyclases by G proteins, suggesting a general mechanism in the regulation of cyclase activity by intracellular factors. Furthermore, our results revealed a new, phosphorylation-independent role of PAK kinase domain in regulating cellular functions. A recent report showed that the phosphorylated kinase domain of PAK2 dimerizes. The interaction between two kinase domains stabilizes the active conformation of the enzyme, leading to a full activation through trans-autophosphorylation [32]. A similar mechanism has also been reported in the activation of EGFR [33]. These studies, plus the fact that the cyclases, which contain highly conserved catalytic domains, are activated through conformational changes induced either by ligand binding or by interactions with intracellular factors, led us to propose a similar allosteric activation process in the activation of GCs by PAK (Fig. 1).
Role of GC in PDGF-induced lamellipodial formation and cell migration
Rac1 and PAK are known to be critical players in cytoskeletal reorganization and cell migration. Rac1/PAK could use multiple pathways or feedback loops to control cell migration. Our results demonstrated that Rac1/PAK signaling through GC/cGMP is one of them. Both the treatment by PDGF and the expression of constitutively active Rac1 or PAK1 proteins could increase cellular cGMP levels in mouse embryonic fibroblast (MEF) cells. GC-A is the major transmembrane GC in MEF. When we knocked down GC-A protein level in MEF cells by RNA interference, the PDGF-induced increase in cGMP production was reduced [18]. More importantly, PDGF-induced cell migration was impaired (Fig. 2). In addition, GC-A RNAi inhibited active Rac1-induced cell migration. ANP stimulation alone or treatment with a membrane-permeant cGMP analog was not sufficient to induce MEF cell migration. Thus, an increased cGMP level (or a gradient) is required, but by itself not sufficient, for cell migration. Nonetheless, our data show that GCs, like Rac and PAK, play a critical role in cell migration.
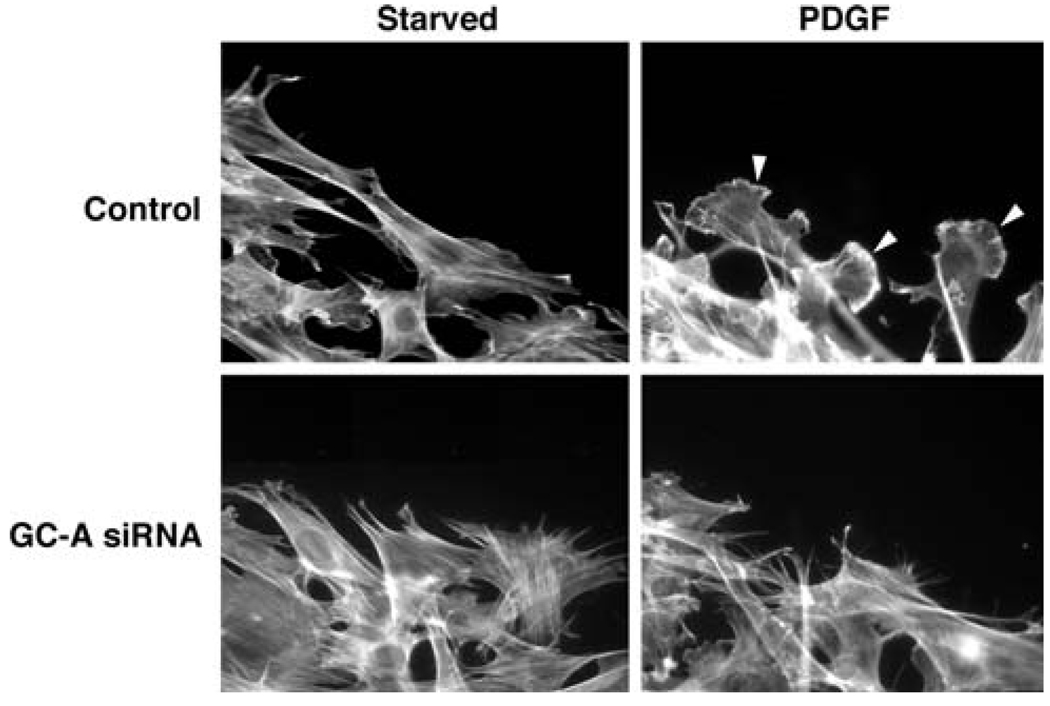
Fig. 2.
GC-A is required for PDGF-induced lamellipodium formation. PDGF (20 ng/ml for 30 min) induced the formation of lamellipodia (indicated by arrowheads) in control siRNA-treated cells at the edge of the wound. GC-A siRNA treatment blocked PDGF-induced lamellipodium formation
The molecular mechanism by which GC is required for PDGF-induced fibroblast cell migration was further explored. We showed that the Rac/cGMP pathway plays an important role in lamellipodial formation [18]. Lamellipodia are protruding membrane structures at the leading edge of migrating cells. Rac1 and PAK have been shown to mediate PDGF-induced lamellipodial formation [34–37]. GC-A siRNA treatment led to a reduction of lamellipodia in the presence of PDGF. Furthermore, constitutively active Rac1 induced formation of lamellipodia which was also inhibited by GC-A RNAi treatment [18]. Moreover, although ANP alone was not sufficient to induce MEF cell migration, it induced transient formation of lamellipodia (Fig. 3). These data suggest that GC is involved in PDGF and Rac1 induced lamellipodial formation.
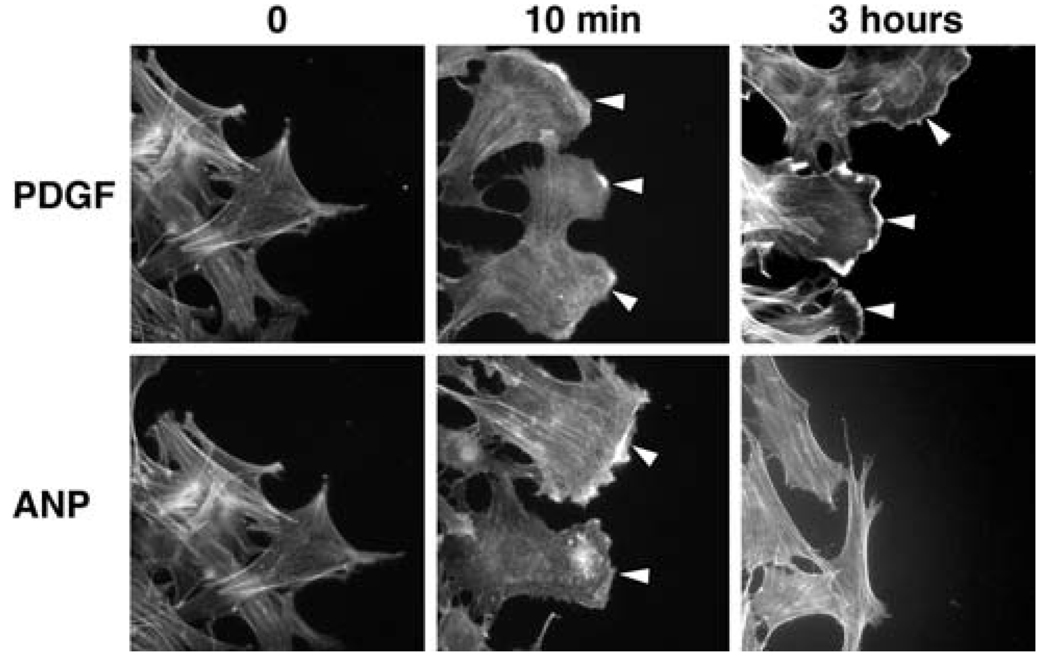
Fig. 3.
While PDGF (20 ng/ml) induced persistent lamellipodia (indicated by arrowheads), ANP (10 µM) induced transient formation of lamellipodia (at 10 min)
Rac could down regulate Rho activity at the front in order for the cell to migrate forward [38]. The mechanisms for this regulation are not entirely clear. Production of oxygen radicals as a result of Rac activation has been shown to inactivate Rho through p190RhoGAP [39]. In our analysis of cytoskeletal reorganization in GC-A RNAi cells, we observed that, in addition to the lack of lamellipodial formation induced by PDGF, the RNAi-treated cells showed persistent formation of stress fibers. In contrast, stress fibers quickly disappeared in wild-type cells after PDGF treatment, indicating a decrease in RhoA activity. These results suggest that cGMP plays a role linking Rac and Rho, balancing the activities of the two small GTPases during the course of cell migration.
Conclusion
In summary, we have discovered a new Rac/PAK/GC/cGMP signaling pathway that provides a general mechanism for diverse signaling receptors to increase the cellular concentrations of cGMP. This new pathway could be essential for various biological processes such as cell migration and axonal guidance. Our findings also provide new insights in the modulation of cyclase activity. For example, PAK kinase domain may act as an allosteric activator to activate GC. This in turn will further our understanding of the functions and regulations of both GC and Rac/PAK pathways.
Acknowledgments
Research projects on guanylyl cyclases in our laboratory were supported by a grant from the NIH (GM84191).
References
- 1.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52(3):375–414. [PubMed] [Google Scholar]
- 2.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 3.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283(5410):2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 4.Finn JT, Grunwald ME, Yau KW. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- 5.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci. 2007;104(36):14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci. 2009;106(6):2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo D, Zhang J, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265(1):23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 11.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 12.Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, Konicek S, Hom J, Marshall M, Graff JR. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- 13.Edwards DC, Sanders LC, Bokoch GG, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 14.Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17(3):1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller-Taubenberger A, Bretschneider T, Faix J, Konzok A, Simmeth E, Weber I. Differential localization of the Dictyostelium kinase DPAKa during cytokinesis and cell migration. J Mus Res Cell Motil. 2002;23(7–8):751–763. doi: 10.1023/a:1024475628061. [DOI] [PubMed] [Google Scholar]
- 16.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 17.Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102(3):387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 18.Guo D, Tan YC, Wang D, Madhusoodanan KS, Zheng Y, Maack T, Zhang JJ, Huang XY. A Rac-cGMP signaling pathway. Cell. 2007;128:341–355. doi: 10.1016/j.cell.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275(28):21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 20.Butt E, Gambaryan S, Göttfert N, Galler A, Marcus K, Meyer HE. Actin binding of human LIM and SH3 protein is regulated by cGMP- and cAMP-dependent protein kinase phosphorylation on serine 146. J Biol Chem. 2003;278:15601–15607. doi: 10.1074/jbc.M209009200. [DOI] [PubMed] [Google Scholar]
- 21.Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, Van Haastert PJ. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002;21:4560–4570. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veltman DM, Roelofs J, Engel R, Visser AJ, Van Haastert PJ. Activation of soluble guanylyl cyclase at the leading edge during Dictyostelium chemotaxis. Mol Biol Cell. 2005;16:976–983. doi: 10.1091/mbc.E04-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kook H, Itoh H, Choi BS, Sawada N, Doi K, Hwang TJ, Kim KK, Arai H, Baik YH, Lee S, Rivero F, Park KC, Huang E, Funamoto S, Firtel RA. Dictyostelium PAKc is required for proper chemotaxis. Mol Biol Cell. 2004;15(12):5456–5469. doi: 10.1091/mbc.E04-04-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda M, Kohno M, Takeda T. Inhibition by cardiac natriuretic peptides of rat vascular endothelial cell migration. Hypertension. 1995;26:401–405. doi: 10.1161/01.hyp.26.3.401. [DOI] [PubMed] [Google Scholar]
- 25.Nakao K. Physiological concentration of atrial natriuretic peptides induces endothelial regeneration in vitro. Am J Physiol Heart Circ Physiol. 2003;284:H1388–H1397. doi: 10.1152/ajpheart.00414.2002. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, et al. Insulin-stimulated cyclic guanosine mono-phosphate inhibits vascular smooth muscle cell migration by inhibiting Ca/calmodulin-dependent protein kinase II. Circulation. 2003;107:1539–1544. doi: 10.1161/01.cir.0000056766.45109.c1. [DOI] [PubMed] [Google Scholar]
- 27.Kumada T, Lakshmana MK, Komuro H. Reversal of neuronal migration in a mouse model of fetal alcohol syndrome by controlling second-messenger signalings. J Neurosci. 2006;26(3):742–756. doi: 10.1523/JNEUROSCI.4478-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 29.Ayoob JC, Yu HH, Terman JR, Kolodkin AL. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci. 2004;24:6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou Y, Ye RD, Browning DD. Activation of the small GTPase Rac1 by cGMP-dependent protein kinase. Cell Signal. 2004;16:1061–1069. doi: 10.1016/j.cellsig.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J Biol Chem. 1998;273(43):28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- 32.Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, Wemmer DE, Kuriyan J. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J Mol Biol. 2006;361:312–326. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 35.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 36.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145(4):837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase 1 (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7(3):202–217. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 38.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147(5):1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nimnual AS, Yatsula BA, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]