Abstract
Radioactive O-phosphoryl-L-serine was detected after alkaline deacylation of rat and rooster liver [3H]seryl-tRNA acylated in vitro with homologous synthetases. Ribonuclease treatment of this tRNA yielded a compound with the properties of phosphoseryl-adenosine. Benzoylated DEAE-cellulose chromatography of seryl-tRNA yielded four distinct peaks, only one of which contained phosphoserine. A unique fraction for phosphoserine was also found on chromatography of nonacylated tRNA. In ribosome binding studies, this fraction responded very slightly with poly(U,C), but not with any of the known serine trinucleotide codons. Substantial incorporation of [3H]-serine into protein from this tRNA species was observed in an aminoacyl-tRNA dependent polysomal system derived from chick oviducts. No phosphoserine was found in Escherichia coli or yeast seryl-tRNA acylated with homologous enzymes, nor in E. coli seryl-tRNA acylated with liver synthetase. In the absence of tRNA, free phosphoserine was not formed in reaction mixtures, which suggests that phosphoseryl-tRNA arises by phosphorylation of the unique seryl-tRNA species. These results demonstrate a discrete tRNASer species in rat and rooster liver containing phosphoserine and suggest that this tRNA is involved in ribosomal polypeptide synthesis.
Full text
PDF

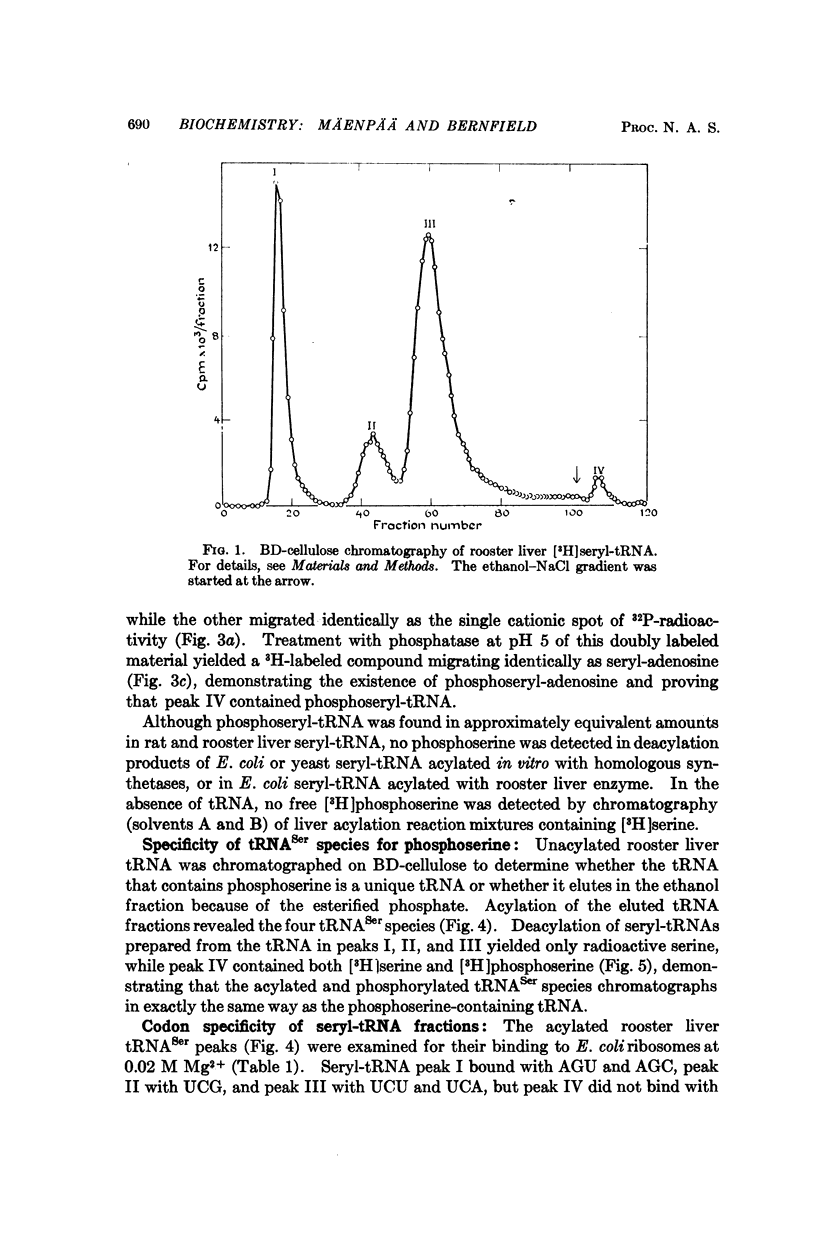
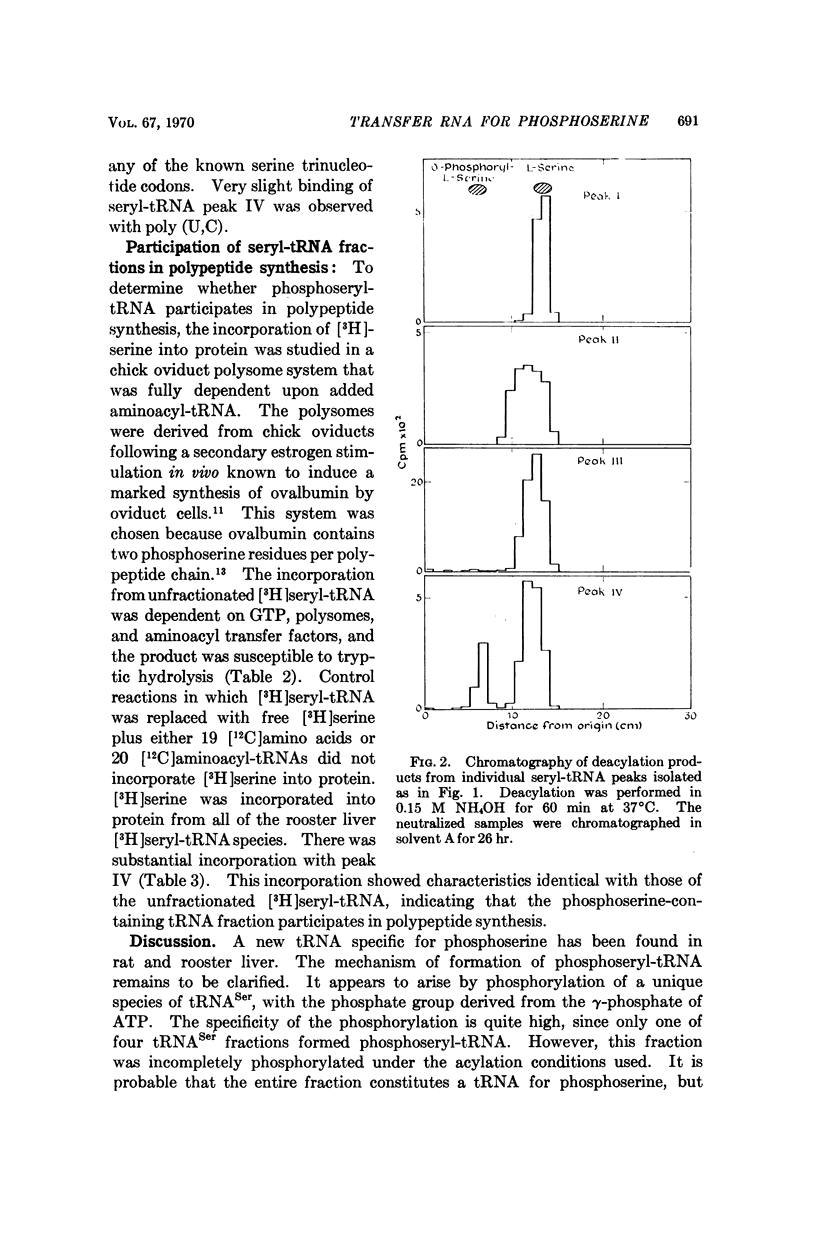
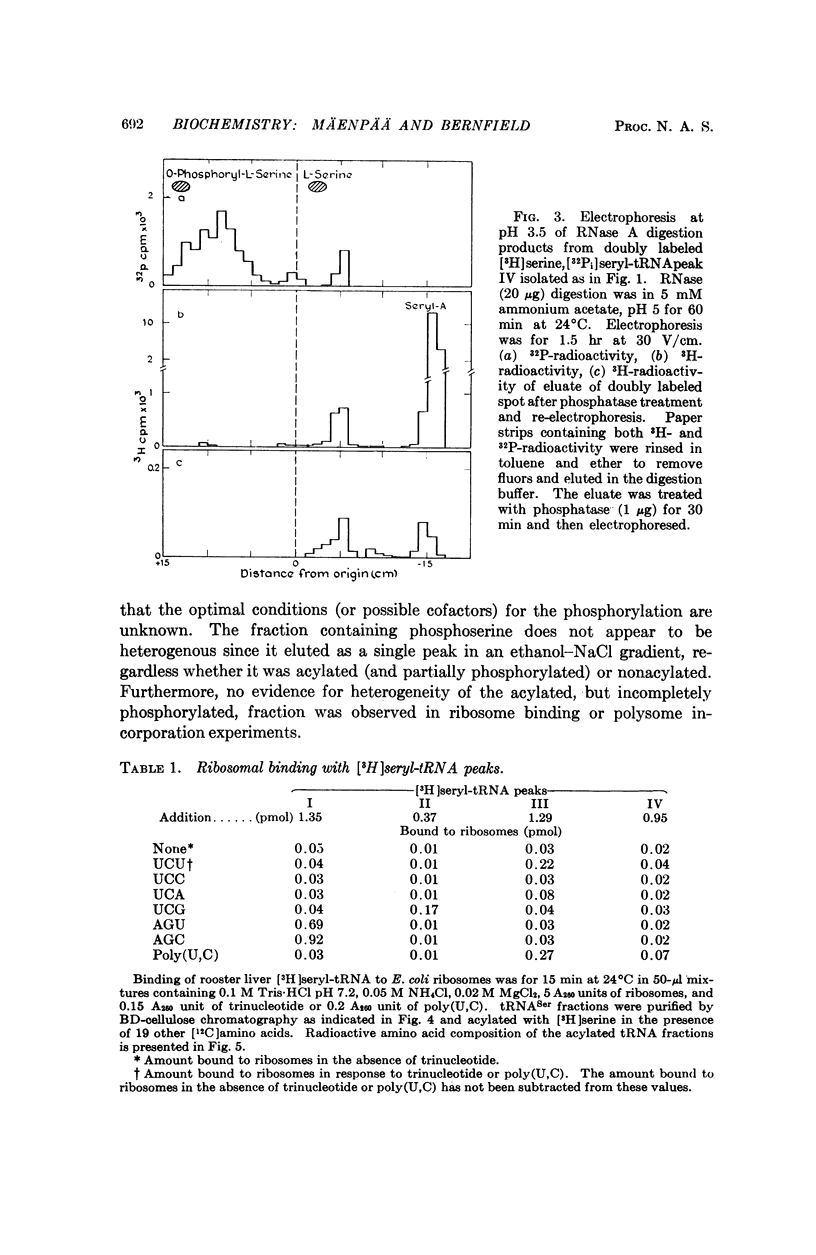
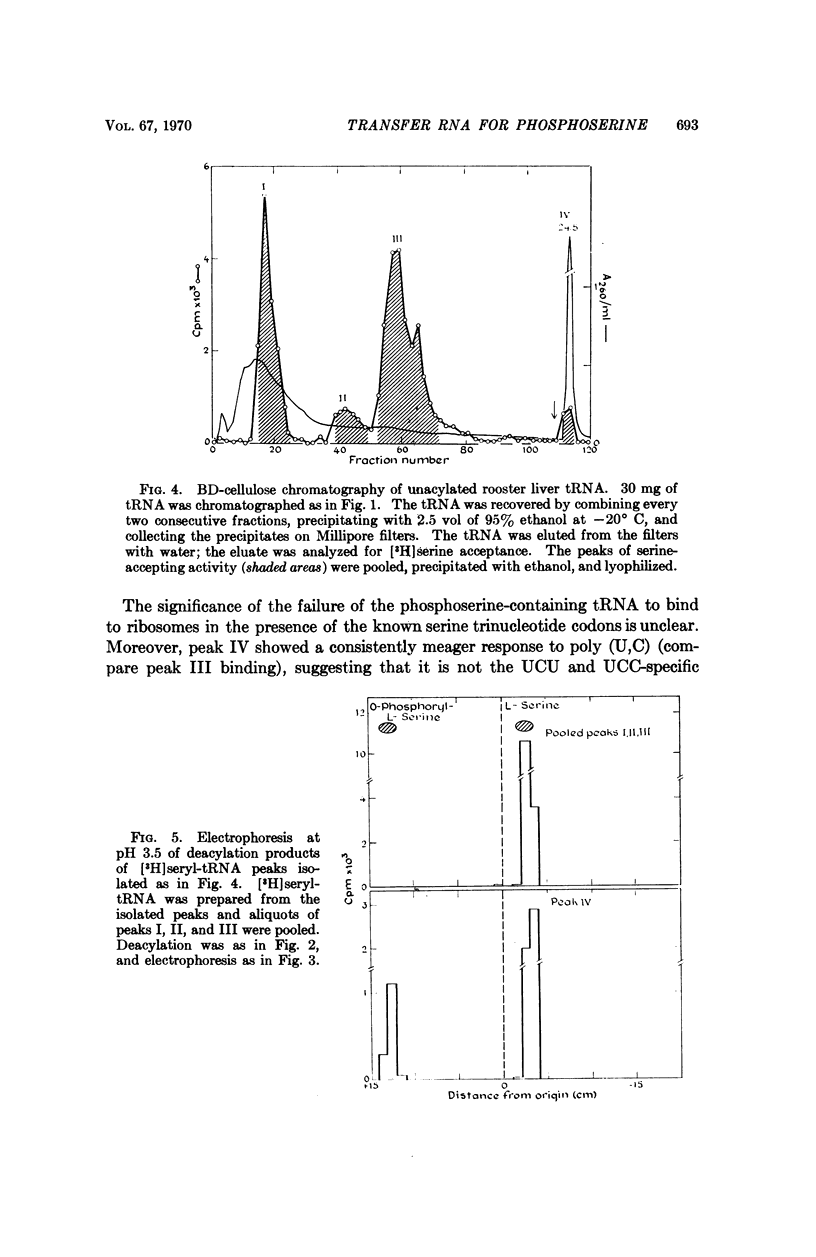


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bumsted R. M., Dahl J. L., Söll D., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. X. Further study of the glycyl transfer ribonucleic acids active in peptidoglycan synthesis in Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):779–782. [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A., Nirenberg M. RNA codons and protein synthesis. 15. Dissimilar responses of mammalian and bacterial transfer RNA fractions to messenger RNA codons. J Mol Biol. 1968 Oct 14;37(1):99–118. doi: 10.1016/0022-2836(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- FLAVIN M. The linkage of phosphate to protein in pepsin and ovalbumin. J Biol Chem. 1954 Oct;210(2):771–784. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. M., Thornton M. P., Liepkalns V., Lennarz W. J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. II. Specificity of alanyl phosphatidylglycerol synthetase. J Biol Chem. 1968 Jun 10;243(11):3096–3104. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Bernfield M. R. Quantitative variation in serine transfer ribonucleic acid during estrogen-induced phosphoprotein synthesis in rooster liver. Biochemistry. 1969 Dec;8(12):4926–4935. doi: 10.1021/bi00840a041. [DOI] [PubMed] [Google Scholar]
- Nesbitt J. A., 3rd, Lennarz W. J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. I. Specificity of lysyl phosphatidylglycerol synthetase. J Biol Chem. 1968 Jun 10;243(11):3088–3095. [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Petit J. F., Strominger J. L., Söll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VII. Incorporation of serine and glycine into interpeptide bridges in Staphylococcus epidermidis. J Biol Chem. 1968 Feb 25;243(4):757–767. [PubMed] [Google Scholar]
- Rosenberg E., Elson D. The formation of hydroxypyruvyl-tRNA. FEBS Lett. 1969 Aug;4(3):222–226. doi: 10.1016/0014-5793(69)80240-1. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Söll D. Purification of five serine transfer ribonucleic acid species from Escherichia coli and their acylation by homologous and heterologous seryl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Mar 25;245(6):1394–1400. [PubMed] [Google Scholar]
- Walsh D. A., Sallach H. J. Comparative studies on the pathways for serine biosynthesis in animal tissues. J Biol Chem. 1966 Sep 10;241(17):4068–4076. [PubMed] [Google Scholar]
- Wilcox M. Gamma-glutamyl phosphate attached to glutamine-specific tRNA. A precursor of glutaminyl-tRNA in Bacillus subtilis. Eur J Biochem. 1969 Dec;11(3):405–412. doi: 10.1111/j.1432-1033.1969.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc Natl Acad Sci U S A. 1968 Sep;61(1):229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]



