Abstract
fMRI studies of early abstinence cocaine users offer information about the state of the brain when most cocaine users seek treatment. This study examined the relationship between pretreatment brain function and subsequent treatment response in 19 treatment-seeking early abstinence cocaine dependent (CD) subjects. These subjects and 14 non-drug using control subjects underwent fMRI while performing a working memory task with three levels of difficulty. CD subjects were then randomized to treatment studies. Results showed CD subjects had significantly lower (random effects, corrected for multiple comparisons) brain activation in caudate, putamen, cingulate gyrus, middle and superior frontal gyri, inferior frontal gyrus pars triangularis and pars opercularis, precentral gyrus, and thalamus compared to non-drug using controls. Within CD subjects, thalamic activation significantly correlated with treatment response. This study shows CD subjects in early abstinence have alteration of brain function in frontal, striatal, and thalamic brain regions known to be part of a circuit associated with motor control, reward, and cognition. Subjects with pretreatment thalamic deactivation showed the poorest treatment response, possibly related to thalamic involvement in mesocortical and mesolimbic dopamine projections.
Keywords: fMRI, Cocaine, Working Memory, Thalamus, Treatment Response
1. Introduction
Research on cocaine abuse has used brain imaging to examine differences in brain function in cocaine dependence subjects. Brain imaging studies in cocaine dependent subjects that focused on the long-term effects of cocaine use have studied cocaine users after a period of abstinence ranging from one week to several months, and have shown differences in dopamine receptor availability and lower metabolic activity in the frontal cortex that persist for months (Volkow et al., 1992; Volkow et al., 1993). Other studies measuring brain function within minutes after a dose of cocaine have shown that cocaine acutely affects brain function in a wide distribution of brain regions including, but not limited to, regions involved in reward, memory, and decision making (Breiter et al., 1997; Breiter and Rosen, 1999). Studies focusing on the state of brain function in cocaine users during the period of early abstinence (ranging from hours to days after last use of cocaine), in which the subjects were not intoxicated with cocaine but often had urine drug screens that were positive for the cocaine metabolite benzoylecgonine, have also shown differences in brain function between chronic cocaine users and controls (Volkow et al., 1991; Kaufman et al., 2003; Tomasi et al., 2007a; 2007b). Brain function measured in this early abstinence period after cocaine use may be most relevant for treatment studies, since a majority of outpatient treatment-seeking cocaine users present for treatment research in early abstinence (Sayre et al., 2004). Using positron emission tomography (PET), Volkow et al., (1991) showed that chronic cocaine users who were within one week of cocaine withdrawal had higher rates of regional brain metabolism in the basal ganglia and orbitofrontal cortex than normal control subjects. These differences were not found in subjects who were studied after 2 to 4 weeks of cocaine withdrawal (Volkow et al., 1991). Studies using functional magnetic resonance imaging (fMRI) have also shown differences in brain function in cocaine users who were in early abstinence. fMRI activation in the cingulate, presupplementary motor cortex, and insula was significantly reduced in cocaine users while performing a go-nogo task compared to controls (Kaufman et al., 2003a). More recently, using a sustained visual attention task, Tomasi et al. (2007a) showed that cocaine users in early abstinence had hypoactivation in the thalamus and hyperactivation in occipital and frontal cortices compared to controls. One of the few studies to examine brain function during working memory in cocaine users showed that cocaine users in early abstinence had lower activation in the mesencephalon and thalamus compared to controls (Tomasi et al., 2007b). The authors pointed out that these are brain regions where either dopamine neurons are located (mesencephalon) or are the target of dopamine pathways (thalamus) (Tomasi et al., 2007b).
In parallel to brain imaging studies in cocaine users, a separate line of research has measured baseline behavioral characteristics as potential predictors of treatment response in cocaine dependence. Moeller et al., (2001), showed that impulsivity as measured by the Barratt Impulsiveness Scale (BIS-11) was a significant predictor of treatment retention; cocaine dependent subjects who had scores above the median on the BIS-11 were significantly more likely to drop-out of treatment than cocaine dependent subjects who had scores below the median. This finding was replicated by Patkar et al., (2004). More recently, other behavioral and cognitive measures have been examined as predictors of treatment response in cocaine dependence. Aharonovich et al., (2006) examined the relationship between baseline performance on a cognitive assessment battery and subsequent treatment response in cocaine dependent subjects. Results of that study were that subjects who dropped out of treatment had significantly lower scores on a computerized MicroCog than subjects who remained in treatment for 12 or more weeks (Aharonovich et al., 2006). Green et al., (2009) examined the relationship between treatment outcome and baseline performance on a measure of decision making, the Iowa Gambling Task (IGT) in cocaine dependent subjects. Findings of that study were that cocaine dependent subjects who had better decision making as measured by the IGT were more likely to show a reduction in cocaine positive urines when treated with citalopram (Green et al., 2009).
Although there have been several studies showing differences in brain function between cocaine users and non-drug using controls, and some studies showing that baseline cognitive function predicts treatment response in cocaine users, to date there have been few studies showing a relationship between differences in brain activation and subsequent treatment response in cocaine users. To our knowledge, the only published study linking brain activation on fMRI and subsequent treatment response was published by Brewer et al. (2008), comparing cocaine dependent subjects in early abstinence to controls while performing a Stroop color word interference task. Results of that study showed that cocaine dependent subjects had a significant correlation between pretreatment activation in the right putamen and subsequent percent negative cocaine urine drug screens (Brewer et al., 2008b).
In order to further examine pretreatment brain function in cocaine dependent subjects who are in early abstinence, fMRI was conducted on treatment-seeking cocaine users and non-drug using controls while performing a working memory task. A working memory task was chosen for use in the scanner, since the relationship between working memory and dopamine is well established (Goldman-Rakic, 1996), and chronic cocaine users have been shown to have alteration in dopamine function in previous imaging studies (Volkow et al., 2004), which is predictive of choice of cocaine over money in the laboratory (Martinez et al., 2007a). The hypotheses of the study were: 1) cocaine users would show differences in brain activation in brain regions known to be associated with dopamine function compared to controls while performing a working memory task; 2) These differences would be correlated with subsequent treatment response in cocaine users; cocaine dependent subjects showing the greatest differences from controls would show the worst treatment outcome.
2. Materials and Methods
2.1. Subjects
This study was approved by the Institutional Review Board (IRB) for the University of Texas Health Science Center-Houston, and was in accordance with the 1964 Declaration of Helsinki. Subjects were recruited prior to treatment initiation in two IRB-approved treatment studies at the UT-Houston Treatment Research Clinic. Subjects were recruited using IRB-approved advertisements. Subjects were fully informed of the procedures, risks, and benefits of all studies, and written informed consent was obtained for all subjects prior to their participation. Subjects completed a structured psychiatric interview (SCID) (First et al. 1996), medical history and physical examination, blood chemistry, complete blood count, and urine pregnancy test (females). All subjects were assessed via breathalyzer for alcohol on the day of scanning. Subjects were required to have a 0.00 breath alcohol level at the time of scanning. Subjects are required to abstain from nicotine and caffeine for at least one hour prior to the scanning session. Two groups of subjects were recruited: (1) cocaine-dependent subjects (CD) who met Diagnostic and Statistical Manual Fourth Edition (DSM-IV) (American Psychiatric Association, 1994) criteria for cocaine-dependence as determined by a board-certified psychiatrist and confirmed by SCID, and (2) normal control subjects (NORM) who had no current or lifetime history of any DSM-IV substance or psychiatric disorder. Subjects were excluded if they had non-psychiatric illness or were taking any medications that could affect the brain (other than drugs of abuse in the CD group) or had a positive pregnancy test. None of the subjects had any clinically significant abnormalities on MRI as determined by a radiologist (LAK). Breath alcohol and urine drug screening were conducted on all subjects immediately prior to scanning. All subjects had negative breath alcohol screens and all NORM subjects had negative urine drug screens.
2.2. Treatment
After fMRI scanning, CD subjects were randomized to treatment in one of two ongoing studies. Nine subjects were included in a 16-week study in which they could have received, in addition to cognitive behavioral therapy, 60 mg d-amphetamine, 400 mg modafinil, combination 30 mg d-amphetamine and 200 mg modafinil, or placebo. Ten subjects were included in a 16-week study in which they could have received 800/200 mg levodopa/carbidopa, 50 mg naltrexone, 400 mg modafinil, or placebo. Urine drug screens were measured three times weekly. Treatment outcome was calculated using the Treatment Effectiveness Score (TES) (Ling et al., 1997), which assigns one point for each cocaine-negative urine sample, and no points for positive or missing samples. In a 16-week trial, TES could range from 0 to 48. Since these studies are ongoing, the blind was not broken to determine the treatment that subjects were receiving.
2.3. IMT/DMT Behavioral protocol
The delayed memory (DMT) condition is a delayed matching to sample task to retain a visual stimulus in working memory (Dougherty et al. 1998; Dougherty et al. 2002). Each stimulus consists of a string of numbers that are displayed simultaneously in a horizontal array in black font on a white background for 0.5 s, followed by an inter-stimulus interval of blank white screen for 0.5 s, at a rate of 1 stimulus per s. The target and probe stimuli are separated by distracter stimuli, consisting of a string of all zeros (e.g., 00000) that is repeated three times at the same rate and duration as the target and probe stimuli. Thus the memory delay between the end of the target stimulus and beginning of the probe stimulus is 3.5 s. Subjects are instructed to press a button when the probe matches the target. The probability of a match is 50%, and the probability of a catch trial is 50%, in which the probe differs from the target by only 1 of the digits. For example, one possible DMT trial would be: 38963, 00000, 00000, 00000, 38963. Subjects are instructed to ignore the distracter stimuli and to remember only the target (e.g., first occurrence of 38963) and to identify only the probe (e.g., second occurrence of 38963). Each trial consists of a different set of targets and probes. The “immediate memory” (IMT) condition is a control condition and similar to DMT in rate, duration, and type of stimuli except there are no distracter stimuli, and thus the memory delay between target and probe is 0.5 s. A stimulus consisting of all 1’s is presented once after each DMT trial and 4 times after each IMT trial. Thus the sum of non-salient intertrial and distracter stimuli is the same (four) during DMT and IMT conditions. The A’ score (Donaldson 1992) is used as an accuracy measure, ranging from 0.5 (chance) to 1.0 (perfect discriminability). Recall failures or errors, as well as correct responses, are taken into account in the formula for calculating the A’ accuracy score (Donaldson 1992).
The IMT/DMT fMRI protocol is a block design. The number of digits in the stimulus string can be 3, 5, or 7 digits and is constant within each block. All digit conditions are presented within each run. An IMT block is always followed by a DMT block with the same number of digits. There are 6 IMT blocks and 6 DMT blocks within each run. The order of digit conditions is counterbalanced between runs and subjects. The duration of each block is 42.5 s; there is 10 s rest between blocks and 20 s rest at the beginning of each run. Run duration is 10 min 47 s. Before scanning, each subject practiced at least one run in an MRI Simulator while listening to a recording of MRI scanning sounds. During Simulator sessions, the IMT/DMT task was projected by a liquid-crystal display projector on a screen that the subject viewed using mirror-prism glasses. The subject listened to a recording of MRI sounds through earphones during the Simulator sessions. Stimulus presentation and recording of behavioral performance data during fMRI scanning were managed through the IFIS-SA fMRI System (Invivo Corporation, Orlando, Florida) that used fiberoptic transmission of all signals between the magnet and control rooms. The subject viewed the task on an liquid-crystal displayvideo unit that is built into the head coil. The subject responded on the IFIS-SA button response unit by pressing a key with the index finger of the right hand. The behavioral protocol was written in the IFIS-SA implementation of E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA), and controlled and run on the integrated IFIS-SA dual PC computers.
2.4. Statistical analysis of demographic and behavioral data
Statistical analysis of demographic data used SAS 9.1.3 (SAS Institute Inc., Cary, NC) TTEST procedure to compare differences between groups for continuous data, and Fisher’s exact test with FREQ procedure for categorical data. Differences in behavioral performance were analyzed with the SAS 9.1.3 MIXED procedure implementation of repeated measures ANOVA, after graphical inspection of the residuals (e.g. via Normal-Quantile Plot) indicated that they were distributed normally. Spearman non-parametric correlations were computed using CORR procedure.
2.5. MRI scans
FMRI data were acquired on a Philips 3.0 T Intera system with a six-channel receive head coil (Philips Medical Systems, Best, Netherlands). Stimulus presentation and recording of performance used the Integrated Functional Imaging System-Stand Alone (IFIS-SA) (Invivo Corporation, Orlando, Florida). 3D-SPGR (resolution=0.938 mm × 0.938 mm × 1 mm) and Fluid Attenuation Inversion Recovery (FLAIR) scans were also acquired. The fMRI pulse sequence in this study used spin-echo EPI, rather than gradient echo EPI, to avoid signal losses caused by through-slice dephasing in regions (e.g., medial orbitofrontal cortex) that are affected by strong susceptibility gradients at 3 T magnetic field strength (Kruger et al., 2001; Norris et al., 2002; Wang et al., 2004), and which is sensitive for cognitive fMRI at 3 T (Norris et al, 2002) using the Blood Oxygen Level Dependent effect (BOLD) (Ogawa et al., 1990). Images were acquired in the transverse plane using single shot spin-echo EPI with SENSE factor=2.0, repetition time=2212 ms, echo time=75 ms (optimized for spin echo), flip angle=90 degrees, number of axial slices=22, field-of-view=240 mm × 240 mm, in-plane resolution=3.75 mm × 3.75 mm, slice thickness=3.75 mm, gap=1.25 mm, repetitions=294 after 10 dummy acquisitions, run duration=10 min 47 s. Each subject had 2 runs, separated by 1 min rest.
2.6. fMRI Processing
Processing of the fMRI data was conducted with Statistical Parametric Mapping (SPM2) software from the Wellcome Department of Cognitive Neurology, London, UK, implemented in Matlab (Mathworks Inc. Sherborn MA, USA). After slice-timing correction, each fMRI series was realigned to correct head motion, and the two runs were realigned to each other. Series with head motion greater than 2.0 mm translation or 2.0 degrees rotation were eliminated from analysis. After coregistering the 3D-SPGR to the fMRI images, the 3D-SPGR was transformed to the coordinates of the Montreal Neurological Institute (MNI) standard space (Collins et al., 1995; Mazziotta et al., 2001) using the SPM2 normalization procedure. This transformation was applied to the fMRI images to convert them to MNI space, which were then resliced to 2 mm isotropic resolution and spatially smoothed with a Gaussian filter of 8 mm isotropic full width at half maximum.
2.7. fMRI Statistical Analysis
Voxel-wise analysis of the fMRI data was computed with SPM2. The IMT and DMT blocks for each digit condition were modeled by boxcar functions convolved with the SPM2 hemodynamic response function. The parameters for each condition were estimated using the General Linear Model (Friston et al., 1995) at each voxel without global normalization. The fMRI time series was high-pass filtered with a cut-off period of 330 s determined by Fourier transformation of each condition’s time model, which showed that signal from the experimental condition was retained at this value, whereas shorter cut-off periods would eliminate most of the signal from the experimental condition. Activation for each digit condition was defined as the contrast of DMT minus IMT parameter estimates for that condition.
Planned comparison of activation between groups was conducted at the second level of analysis (Random Effects) by entering one DMT_minus_IMT contrast image per subject (mean over 2 runs) into the SPM2 Random Effects procedure (Holmes and Friston 1998). A separate two-sample t-test analysis was computed for each digit condition using non-sphericity correction over observations. Examination of the interaction of the categorical factors group x gender was conducted with the SPM2 Basic models ANOVA procedure with non-sphericity correction over observations. The examination of the interaction of the continuous factor age with group was conducted with the SPM2 Basic models multiple regression procedure. The non-sphericity correction used in the SPM2 group comparison corrected for possible differences in variance between the groups, for example due to unequal sample size or other possible causes (Glaser and Friston, 2004). In addition, planned comparisons between groups were similarly conducted for the contrast of 5-digit minus 3-digit activation, and between the contrast of 7-digit minus 3-digit activation. For all analyses, the cluster-defining threshold was t = 2.0, and all P values resulting from the fMRI analysis reported in this paper are corrected cluster p values that were corrected for multiple comparisons using Random Field Theory (Adler 1981) computed by SPM2 to control the family-wise error rate to be less than 0.05 (Friston et al. 1994; Friston et al. 1996). Because we examined two comparisons for each condition: CD activation greater than and less than NORM activation, we conservatively report the two-tailed probability (P) values obtained by multiplying by 2 the corrected one-tailed cluster P values that were computed by SPM2.
The mean activation across all voxels within a cluster was computed using Marsbar Toolbox (Brett et al., 2002). MNI coordinates were converted to coordinates of the Talairach Atlas (Talairach & Tournoux, 1988) using the Brett (1999) mni2tal Matlab script. Approximate anatomical and Brodmann area labels for regions of activation were determined using Talairach Daemon (Lancaster et al. 2002), Talairach atlas (Talairach and Tournoux, 1988), Anatomical Automatic Labeling (Tzourio-Mazoyer et al., 2002), and SPM Anatomy Toolbox (Eickhoff et al., 2005; Eickhoff et al., 2007; Toga et al., 2006).
3. Results
3.1. Demographics
The final sample consisted of 19 CD and 14 NORM subjects. Two NORM subjects and 6 CD subjects were included whose FLAIR MRI brain scans showed a few small white matter hyperintensities that were judged to be clinically insignificant by the radiologist (LAK) and the other physician coauthors (FGM and JLS) prior to the experimental analysis. None of the other subjects had any brain abnormalities on FLAIR scans. All the CD subjects met DSM-IV criteria for both current and past cocaine dependence. Seven CD subjects had no diagnosis of substance use disorder other than cocaine dependence, 4 CD subjects had 1 other substance use disorder, and 8 CD subjects had 2 or more other substance use disorders (range 2 to 5). One CD subject had a DSM-IV diagnosis of past sedative abuse, 1 past sedative dependence, 1 current cannabis dependence, 6 past cannabis abuse, 3 past cannabis dependence, 1 past opiate abuse, 1 current alcohol abuse, 2 current alcohol dependence, 3 past alcohol abuse, 4 past alcohol dependence, 1 past hallucinogen abuse, 1 current ecstasy abuse, 1 past ecstasy dependence, 2 current stimulant abuse (other than cocaine), and 2 past stimulant dependence (other than cocaine). All CD subjects had at least 9 hours of abstinence (mean hours 47.0 ± standard deviation 43.6, range 9 to 168) from cocaine use prior to scanning, except for 1 subject for whom this information was missing. None of the subjects had any symptoms of cocaine intoxication as assessed by study physician at the time of scanning.
The mean age (years) ± standard deviation for CD was 40.8 ± 8.4 (range 24.1 to 52.4); and for NORM 34.5 ± 11.8 (19.7 to 54.1), which was a trend significant difference between groups (t [31] = 1.696, P = 0.084). The number of years education for CD was 13 ± 2 (11 to 17); and for NORM 14 ± 2 (11 to 16), which was not statistically significant between groups (t [31] = 1.462, P = 0.154). There were 16 males and 3 females in the CD group, and 7 males and 7 females in NORM group, which was trend significantly different between groups (Fishers exact test P = 0.057). All the subjects in both groups were right-handed except for 1 left-handed CD and 1 left-handed NORM subject (not statistically significant between groups: Fishers exact test P = 1.000). Urine drug screening (UDS) immediately prior to MRI scanning was positive for cocaine in 14 CD subjects and negative for cocaine in 5 CD subjects. Two CD subjects who had positive UDS for cocaine also had positive UDS for cannabinoids. One CD subject who had a negative UDS for cocaine had a positive UDS for THC. There were no other positive UDS for any other drugs in the CD subjects. None of the NORM subjects here positive for any drugs of abuse at the time of scanning.
3.2. Behavioral results during fMRI scanning
Repeated measures mixed-model ANOVA for the main effects of Group (CD vs. NORM) on A’ across all conditions on the IMT/DMT during the fMRI scanning, resulted in mean A’ for CD = 0.855 ± 0.114, and for NORM = 0.889 ± 0.093, which was trend significantly different between groups (F [1, 31.9] = 3.12, P = 0.087). There was no significant difference between the two groups for the effects of number of digits, i.e., 3, 5, or 7 digits per stimulus (interaction of Digits x Group F [2, 49]= 1.11, P = 0.336). Similarly, there was no significant difference between the two groups for the effects of memory delay, i.e., IMT 0.5 s delay and DMT 3.5 s delay (interaction of Memory Delay x Group F [1, 53.2] = 0.29, P = 0.596). The three-way interaction of Memory Delay x Digits x Group was also not statistically significant (F [2, 44.1] = 1.10, P = 0.341). The main effects of digits on A’ across both groups combined were significant: F (2, 49) = 34.72, P < 0.001 (mean 3-digit A’= 0.934 ± 0.067, 5-digit A’ = 0.877 ± 0.082, and 7-digit A’ = 0.798 ± 0.117). Likewise, the main effects of memory delay across both groups combined were also significant: F (1, 53.2) = 20.40, P < 0.001 (mean A’ for IMT = 0.892 ± 0.078, and mean A’ for DMT = 0.847 ± 0.125). In addition, the two-way interaction of Digits x Memory Delay across both groups combined was significant: F (2, 44.1) = 5.15, P = 0.010 (significantly lower A’ scores for increasing number of digits during DMT compared to IMT for both groups combined). The addition of gender as a factor in the repeated-measures mixed model showed that there were no significant 2, 3, or 4-way interactions of gender with group (F < 1.58, P > 0.219). The addition of age as a covariate in the repeated-measures ANOVA showed that there was no significant regression with age (F[1, 29.7] = 0.20, P = 0.658), and no significant 2, 3, or 4-way interactions of age with group (F < 1.91, P > 0.160).
3.3. Comparison of BOLD Activation Between Groups
The coordinates of the center of mass of each cluster and the locations of the three relative maximal voxel t values that are at least 8 mm apart within each significant cluster are reported in Tables 1, 2, and 3. Since each significant cluster often extended into more than one brain region that may not always coincide with the center of mass or the location of the three relative maximal t values within each cluster, the following text describes all the brain regions where each significant cluster was found. For the 3-digit condition, 2 clusters were found (Tabel 1) in which NORM had significantly greater activation than CD (corrected cluster 2-tailed P < 0.05). Cluster 1 was in left (L) anterior cingulate gyrus (g) in the vicinity of Brodmann Area 32 (BA32), right (R) anterior cingulate g, bilateral (LR) middle cingulate g, LR superior frontal gyrus (fg), LR middle fg, LR superior medial fg, LR superior orbital fg, LR mid orbital fg, LR rectus g, L medial orbital fg, R medial fg (BA9), L inferior fg, and LR caudate nucleus. Cluster 2 was in L superior fg (BA6), R superior fg (BA6 and 8), LR superior medial fg, L middle fg (BA9 and 10), R middle fg, and L precentral g (BA9 and 44).
Table 1.
Group comparison of 3-digit DMT-IMT activation. Within each significant cluster, the three relative maximal voxel t values that are greater than 8 mm apart and their approximate anatomical locations within 3 mm radius are listed
| Contrast | Cluster label (center of mass X Y Z) |
Two- tailed corrected cluster P |
Number of voxels in cluster (volume mL) |
Mean difference in activation between groups across all voxels in cluster (± 90% CI) [% whole brain BOLD] |
Voxel t value |
Voxel t X Y Z |
Voxel t location |
|---|---|---|---|---|---|---|---|
| 3-digit NORM > COC |
1 (2 41 11) |
P < 0.002 |
3260 (26.080) |
0.857 (0.345) |
4.81 | −4 23 28 | L cing g (BA32) |
| 4.15 | 10 46 22 | R medial fg (BA9) |
|||||
| 3.79 | −2 41 9 | L ant cing g (BA32) |
|||||
| 2 (−12 32 36) |
0.024 | 1065 (8.520) |
0.728 (0.384) |
3.33 | 24 33 46 | R sup fg (BA8) |
|
| 3.20 | −30 46 22 | L middle fg | |||||
| 3.20 | −46 17 34 | L precentral g (BA9) |
|||||
| 3-digit COC > NORM |
P > 0.05 | No significant clusters |
CI=confidence interval. X, Y, and Z=Talairach standard space coordinates (mm). Negative X=Left hemisphere. BA=approximate Brodmann Area. L=Left. R=Right. cing=cingulate. g=gyrus. f=frontal. sup=superior. ant=anterior. Smoothness of residual field (Full Width at Half Maximum “FWHM”) = [10.3 10.1 7.8] mm. Search volume = 169685 voxels = 1547.4 resolution elements (resels).
Table 2.
Group comparison of 5-digit DMT activation. Within each significant cluster, the three relative maximal voxel t values that are greater than 8 mm apart and their approximate anatomical locations within 3 mm radius are listed
| Contrast | Cluster label (center of mass X Y Z) |
Two- tailed corrected cluster P |
Number of voxels in cluster (volume mL) |
Mean difference in activation between groups across all voxels in cluster (± 90% CI) [% whole brain BOLD] |
Voxel t value |
Voxel t X Y Z |
Voxel t location |
|---|---|---|---|---|---|---|---|
| 5-digit NORM > COC |
1 (35 −60 37) |
P < 0.002 |
1641 (13.128) |
0.534 (0.233) |
3.71 | 48 43 30 | R supramarginal g |
| 3.65 | 38 −74 28 | R sup occ g (BA19) |
|||||
| 3.54 | 38 −56 36 | R angular g | |||||
| 5-digit COC > NORM |
P > 0.05 | No significant clusters |
CI=confidence interval. X, Y, and Z=Talairach standard space coordinates (mm). Negative X=Left hemisphere. BA=approximate Brodmann Area. R=Right. g=gyrus. sup=superior. inf=inferior. par=parietal. occ=occipital. Smoothness of residual field (FWHM) = [9.4 9.3 7.4] mm. Search volume = 169685 voxels = 1943.8 resolution elements (resels).
Table 3.
Group comparison of 7-digit DMT activation. Within each significant cluster, the three relative maximal voxel t values that are greater than 8 mm apart and their approximate anatomical locations within 3 mm radius are listed
| Contrast | Cluster label (center of mass X Y Z) |
Two- tailed corrected cluster P |
Number of voxels in cluster (volume mL) |
Mean difference in activation between groups across all voxels in cluster (± 90% CI) [% whole brain BOLD] |
Voxel t value |
Voxel t X Y Z |
Voxel t location |
|---|---|---|---|---|---|---|---|
| 7-digit NORM>COC |
1 (−26 17 21) |
P < 0.002 |
1849 (14.792) |
0.493 (0.179) |
4.72 | −16 12 10 | L caudate body |
| 4.26 | −20 11 20 | L sublobar extranuclear |
|||||
| 4.19 | −14 19 36 | L cing g | |||||
| 2 (24 24 28) |
0.008 | 993 (7.944) |
0.4437 (0.171) |
4.40 | 22 32 24 | R ant cing g | |
| 4.30 | 32 15 29 | R middle fg | |||||
| 3.87 | 40 5 26 | R inf fg (BA9) |
|||||
| 3 (5 −13 1) |
0.016 | 910 (7.280) |
1.051 (0.475) |
3.92 | 6 −12 −1 | R thalamus | |
| 3.02 | −12 −14 −1 | L thalamus | |||||
| 2.84 | 10 −15 10 | R medial dorsal thalamus |
|||||
| 7-digit COC>NORM |
P > 0.05 | No significant clusters |
CI=confidence interval. X, Y, and Z=Talairach standard space coordinates (mm). Negative X=Left hemisphere. BA=approximate Brodmann Area. L=Left. R=Right. g=Gyrus. cing=cingulate. f=frontal. ant=anterior. inf=inferior. subthal=subthalamic nucleus. Smoothness of residual field (FWHM) = [9.4 9.2 7.2] mm. Search volume = 169685 voxels = 2012.4 resolution elements (resels).
For the 5-digit condition, 1 cluster was found (Table 2) in which NORM had significantly greater activation than CD (corrected cluster 2-tailed P < 0.05): R supramarginal g, R superior parietal lobule, R inferior parietal lobule, R superior occipital g, R middle occipital g (BA 19 and 39), R middle temporal g, R angular g, R precuneus, and R cuneus.
For the 7-digit condition, 3 clusters were found (Table 3 and Figure 1) in which NORM had significantly greater activation than CD (corrected cluster 2-tailed P < 0.05). Cluster 1 was in left caudate body, L putamen, L cingulate g, L middle fg, L superior fg, L superior medial fg, L inferior fg pars triangularis (BA45) and pars opercularis (BA44), and L precentral g. Cluster 2 was in R anterior cingulate g, R middle cingulate g (BA44), R middle fg, R superior fg, R superior medial fg, R inferior fg (BA9), R inferior fg pars triangularis and pars opercularis (BA44), and R precentral g (BA44). Cluster 3 was in LR thalamus and L subthalamic nucleus.
Figure 1.
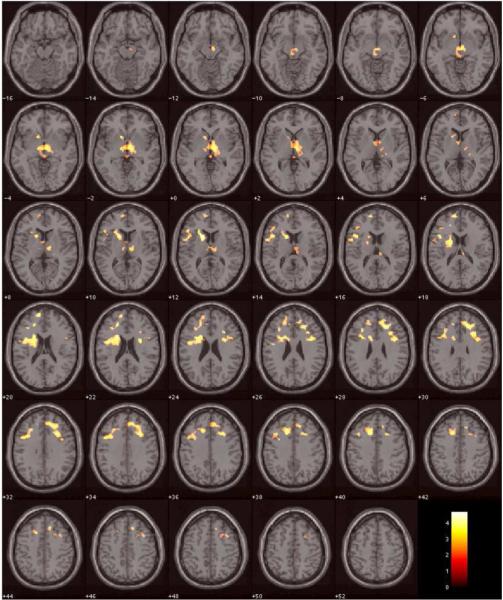
Brain regions, where cocaine dependent subjects showed significantly lower BOLD activation in DMT during the 7-digit condition compared to normal controls, are overlayed in color on axial slices of the MNI single-subject template brain. The number below each slice indicates slice location (mm) of MNI Z coordinate. Scale on color bar represents t values.
For 3-digit, 5-digit, and 7-digit conditions, no significant clusters were found in which CD had greater activation than NORM (corrected cluster two-tailed P > 0.05).
For the contrast of 5-digit minus 3-digit activation, there were no clusters in which NORM had significantly greater or less activation than CD. For the contrast of 7-digit minus 3-digit activation, 2 clusters were found in which NORM had significantly greater activation than CD (corrected cluster 2-tailed P < 0.05). Cluster 1 (center of mass Talairach coordinates x, y, z [mm] = −34 −47 36) was in L inferior parietal lobule (relative maximal t location = −59, −35, 31) and L sub-gyral parietal lobe (relative maximal t location = −24, −45, 39). Cluster 2 (center of mass = 32 −43 34) was in R subgyral parietal lobe (relative maximal t location = 36, −33, 33) and R precuneus (relative maximal t location = 26, −62, 34). For the contrast of 7-digit minus 3-digit activation, there were no clusters in which CD had significant greater activation than NORM.
Within each cluster that was significantly different between NORM and CD, there was no significant difference at either the cluster or voxel level of inference (family-wise-error [FWE]-corrected two-tailed P > 0.05 using SPM2 Small Volume Correction [cluster volumes used in this analysis are given in Tables 1, 2, and 3]) in BOLD activation between the CD subjects who had pre-scan UDS positive for cocaine and the CD subjects who had pre-scan UDS negative for cocaine. SPM2 Basic models regression analysis within each cluster showed that there was no significant regression of age with BOLD activation and no significant interaction of age x group for BOLD activation at either the cluster or voxel level of inference (FWE-corrected two-tailed P > 0.05 using SPM2 Small Volume Correction). In addition, SPM2 Basic models ANOVA within each cluster showed that there was no significant interaction between gender and group for BOLD activation at either the cluster or voxel level of inference (FWE-corrected two-tailed P > 0.05 using SPM2 Small Volume Correction), except for one voxel in cluster 2 of the 7-digit load condition (out of 993 voxels examined in this cluster) which was found in frontal lobe sub-gyral white matter (Talairach X Y Z [mm] = 32 7 25).
3.4. Treatment Response in COCAINE subjects
The mean TES score across all 19 CD subjects was 7.0 ± 10.5 (0 to 37). The mean number of weeks retention in the treatment protocol was 11.3 ± 5.6 (2 to 16). There was no significant difference (t [17] = 0.448, P = 0.660) in TES score between the 14 CD subjects who had pre-scan UDS positive for cocaine (7.7 ± 12.0 [0 to 37]) and the 5 CD subjects who had pre-scan UDS negative for cocaine (5.2 ± 5.1 [0 to 11]). In addition, there was no significant difference (t [17] = 1.620, P = 0.124) in the number of weeks retention in the treatment protocol between the CD subjects who had pre-scan UDS positive for cocaine (12.5 ± 5.1 [2 to 16]) and the CD subjects who had pre-scan UDS negative for cocaine (8.0 ± 6.1 [3 to 16]).
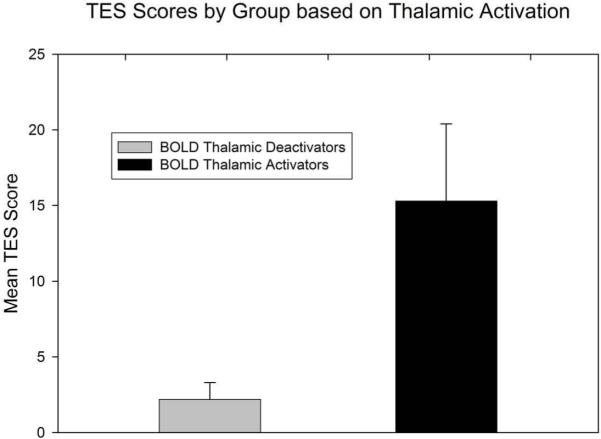
Within each of the clusters in which NORM had significantly greater activation than CD, the BOLD activation (unit is percent of whole brain BOLD) was averaged (“MEAN_BOLD”) across all of the voxels within that cluster for each CD subject. For each cluster, the Spearman correlation coefficients were computed between the MEAN_BOLD for each CD subject and the TES score for each CD subject, and also between the MEAN_BOLD for each CD subject and the number of weeks retention for each CD subject. The Spearman correlation between MEAN_BOLD and TES was significant (r = 0.642, uncorrected P = 0.003, Bonferroni corrected P = 0.048) for the cluster in bilateral thalamus (i.e., Cluster 3 in Table 3). In this cluster, the 12 CD subjects who deactivated, defined by MEAN_BOLD less than 0 for each of these 12 subjects, had significantly less activation (average MEAN_BOLD = −0.866 ± 0.912 [−3.260 to −0.011] percent of whole brain BOLD signal) compared to the 7 CD subjects who activated in this cluster (average MEAN_BOLD = 0.252 ± 0.226 [0.025 to 0.696]; t(17) = 3.119, two-tailed P = 0.006). The 12 CD subjects who deactivated in this cluster, i.e., who each had MEAN_BOLD less than 0, also had significantly lower TES scores (mean TES = 2.2 ± 1.1 [0 to 11]) than the 7 CD subjects who activated in this cluster (mean TES = 15.3 ± 5.1 [0 to 37]) (Figure 2). This difference in TES scores was statistically significant: t (17) = 3.205, two-tailed P = 0.005. The MEAN_BOLD (0.252 ± 0.085 [0.025 to 0.696]) of the 7 CD subjects who activated in this cluster, i.e., the 7 CD subjects who had relatively good TES, was not significantly different from the MEAN-BOLD (0.596 ± 0.197 [−0.372 to 2.208]) of NORM in this cluster (t [19] = 1.197, two-tailed P = 0.246). The correlation of MEAN_BOLD in this cluster with the number of weeks retention was not significant (r = 0.378, uncorrected P = 0.111, Bonferroni corrected P = 1.000). In addition, there were no significant correlations of TES or number of weeks retention with the MEAN_BOLD of the other clusters (r < 0.35, uncorrected P > 0.146, Bonferroni corrected P = 1.000).
Figure 2.
TES scores in 12 CD subjects who showed a pretreatment BOLD deactivation relative to NORM subjects in the thalamic cluster (Cluster 3 in Table 3) and 7 subjects who showed BOLD activation in the thalamic cluster. Error bars indicate standard deviation.
4. Discussion
CD subjects showed reduced activation relative to non-drug using controls in prefrontal cortex, striatum, and thalamus. These brain regions are part of a cortico-thalamic-striatal circuit, which is associated with both motor and cognitive brain functions, including working memory (reviewed in Haber and McFarland, 2001). Results of this study support previous research showing alteration in brain function in recently abstinent cocaine dependent subjects. Consistent with our results, Tomasi et al. (2007b) reported that cocaine abusers during abstinence showed less activation in the medial prefrontal gyrus and precuneus compared to non-drug using controls when performing a 2-back relative to a 1-back working memory task. These findings by Tomasi et al. (2007b) during “working memory load activation” are similar to our findings of less activation in cortical regions during greater working memory load minus less working memory load conditions (i.e., DMT minus IMT). Other studies in early abstinent cocaine users have shown similar findings. Kaufman et al. (2003) found that cocaine users had significantly less activation in the anterior cingulate and insula during stops in a go-nogo task compared to controls. Kulber et al. (2005) found that cocaine users in early abstinence had less activation compared to controls in several brain regions including the cingulate gyrus, medial frontal gyrus, middle frontal gyrus, thalamus, globus pallidus/putamen, and precuneus while performing an attentional task that involved switching between verbal and visuospatial memory.
In the present study, cocaine-dependent subjects had significantly less thalamic activation compared to controls, and deactivation in thalamus was associated with subsequent poorer treatment outcome. Other fMRI studies have shown differences in thalamic activation between cocaine users in early abstinence and controls. Tomasi et al. (2007a) demonstrated less activation in thalamus in cocaine users in early abstinence compared to controls during a sustained visuo-spatial attention task. As mentioned above, Kubler and colleagues (2005) also demonstrated that cocaine abusers had less activation in thalamus during an attention task.
One possible reason for the association between thalamic activation and subsequent treatment response is related to brain dopamine neurotransmission. In a PET study (Volkow et al., 2005), cocaine addicts but not controls showed a reduction in thalamic [11C]raclopride binding after methylphenidate, which significantly correlated with an increase in methylphenidate induced metabolism in the orbitomedial prefrontal cortex in cocaine users. This response to methylphenidate administration in the thalamus of cocaine abusers was associated with their cocaine craving level, which may trigger cocaine use relapse. The role of mesolimbic and mesocortical dopamine circuits in drug addiction and the alteration of these circuits by chronic drug use is well documented (Reviewed in Goldstein and Volkow 2002). As the thalamus plays a role in both of these circuits, it is possible that the alteration in dopamine function seen in chronic cocaine use (Volkow et al., 2005; Martinez et al., 2007b) is responsible for the differences between cocaine users and controls in thalamic activation and the association between thalamic activation and eventual treatment response.
4.1. Limitations
One limitation of this study is that it was only possible to examine the relationship between baseline brain activation and overall treatment success and not the response to individual treatments. Since these subjects were taking part in ongoing clinical trials that remain blinded, the precise treatment that subjects were receiving is not known. Bearing this limitation in mind, this study does support a relationship between pretreatment brain function and subsequent clinical improvement. Brewer et al. (2008) also showed an association between baseline brain function and overall treatment response in subjects who were undergoing several different treatments. That study found that subjects with less fMRI activation in the caudate while performing a Stroop task had a lower percentage of cocaine negative urines in subsequent treatments using different behavioral and pharmacologic interventions (Brewer et al., 2008a). It is possible that both the findings of the current study and the results of Brewer et al. (2008) are related to the hypothesis that cocaine dependent subjects who have the greatest difference in brain function compared to controls have the poorest treatment response. This premise is supported by behavioral studies showing that cocaine dependent subjects with the poorest performance on behavioral laboratory and neuropsychological tests have the worst treatment response (Aharonovich et al., 2006; Green et al., 2009).
Differences between the brain regions found in the study by Brewer et al. (2008) and the brain regions found in the present study, that showed an association between baseline brain function and subsequent treatment response, could be related to different tasks used in the scanner or to different treatment modalities. Larger scale studies are warranted to assess the association between baseline brain function and specific treatment response.
Another limitation is that the present study used a block fMRI design that included incorrect as well as correct behavioral responses during each block. Because of the fact that errors as well as correct responses were included in each block, the trend significant difference in overall accuracy between the groups (P = 0.087) suggests that the anterior cingulate activation differences are difficult to interpret since these may reflect diminished response to errors in the CD group (Hester et al., 2007). Further studies using event-related fMRI design would be necessary to tease apart the BOLD activation signal during correct vs. incorrect responses.
The inclusion of one left-handed subject in the cocaine group and one left-handed subject in the control group is a confounding factor that needs to be considered in interpreting the results of this study because of the uncertainty in how the processing of the verbal working memory task in these individuals was lateralized. The finding that the main effects of memory delay on accuracy across both groups combined was significant, and the fact that the interaction of Digits x Memory Delay on accuracy across both groups combined was also significant, suggest that the task placed sufficient demands on working memory, especially in the 5- and 7- digit load conditions. However, because of the finding that there was no significant difference between groups in the effect of working-memory delay on accuracy, it is quite possible that the regions that showed differences in activation between groups may not specifically be related to working memory performance, but instead may reflect neuronal differences between cocaine-dependent subjects and non-drug-using controls that might be detected with other tasks.
The fact that the two groups were not matched and trend significantly different with respect to age and gender is problematic regarding the interpretation of the behavioral and fMRI results. Although there was no significant regression with age or interaction effect of age x group for either the behavioral or fMRI data, and no significant interaction of gender x group on either the behavioral or fMRI data (except for one voxel in sub-gyral white matter), the lack of significant interaction effects may be due to low sample size within each cell in the analysis. Thus further studies are necessary with larger numbers of subjects in which age and gender are closely matched in order to verify the present results.
Bearing in mind the limitations, the present study supports a growing body of literature showing deficits in brain function in cocaine dependent subjects in early abstinence and the association between these deficits and subsequent clinical improvement. Larger scale studies of brain function and response to specific treatments may lead to improved outcome in cocaine dependence.
Acknowledgements
This study was supported by grants from the National Institute on Drug Abuse (P50DA009262, and K02DA00403 (FGM)) and CCTS/CRU grant number UL1 RR024148,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflicts of Interest: The authors know of no potential conflicts of interest relevant to the findings in this manuscript.
References
- Adler RJ. The Geometry of Random Fields. John Wiley & Sons; Chichester: 1981. [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Annals of the New York Academy of Sciences. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox (abstract). Neuroimage; Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002; 2002. Available on CD-ROM from Neuroimage. [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. 1999 mni2tal.m. Internet website: http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment Brain Activation During Stroop Task Is Associated with Outcomes in Cocaine-Dependent Patients. Biological Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Holmes C, Peters T, Evans A. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3:190–208. [Google Scholar]
- Donaldson W. Measuring recognition memory. Journal of Experimental Psychology: General. 1992;121:275–277. doi: 10.1037//0096-3445.121.3.275. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behavioral Research Methods Instruments & Computers. 2002;34(3):391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Steinberg JL, Wassef AA, Medearis D, Cherek DR, Moeller FG. Immediate versus delayed visual memory task performance among schizophrenic patients and normal controls. Psychiatry Research. 1998;79:255–65. doi: 10.1016/s0165-1781(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Glaser D, Friston K. Variance components. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W, editors. Human Brain Function Second Edition. Elsevier Academic Press; San Diego, California: 2004. pp. 781–791. [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences U S A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, Moeller FG, Schmitz JM, Lucke JF, Lane SD, Swann AC, Lasky RE, Carbonari JP, Tyson JE. Evaluation of heterogeneity in pharmacotherapy trials for drug dependence: a bayesian approach. American Journal of Drug and Alcohol Abuse. 2009;35(2):95–102. doi: 10.1080/00952990802647503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7(4):315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32(9):1974–84. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Holmes A, Friston KJ. Generalisability, random effects & population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger G, Kastrup A, Glover GH. Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magnetic Resonance in Medical Science. 2001;45:595–604. doi: 10.1002/mrm.1081. [DOI] [PubMed] [Google Scholar]
- Kübler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. European Journal of Neuroscience. 2005;21(7):1984–92. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Research Monographs. 1997;175:208–220. [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. American Journal of Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society London B: Biological Sciences. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21(4):193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. Journal of Addictive Diseases. 2004;23(2):109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Norris DG, Zysset S, Mildner T, Wiggins CJ. An investigation of the value of spin-echo-based fMRI using a stroop color–word matching task and EPI at 3 T. Neuroimage. 2002;15:719–726. doi: 10.1006/nimg.2001.1005. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre SL, Evans M, Hokanson PS, Schmitz JM, Stotts AL, Averill P, Grabowski J. “Who gets in?” Recruitment and screening processes of outpatient substance abuse trials. Addictive Behaviors. 2004;29:389–398. doi: 10.1016/j.addbeh.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 1 ed Thieme Medical Publishers, Inc.; New York: 1988. [Google Scholar]
- Toga AW, Thompson PM, Mori S, Amunts K, Zilles K. Towards multimodal atlases of the human brain. Nature Reviews Neuroscience. 2006;7:952–966. doi: 10.1038/nrn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155(3):189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Research. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labelling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. American Journal of Psychiatry. 1991;148(5):621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J of Neuroscience. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li L, Roc AC, Alsop DC, Tang K, Butlera NS, Schnalla MD, Detrea JA. Reduced susceptibility effects in perfusion fMRI with single-shot spin-echo EPI acquisitions at 1.5 Tesla. Magnetic Resonance Imaging. 2004;22:1–7. doi: 10.1016/S0730-725X(03)00210-8. [DOI] [PubMed] [Google Scholar]