Abstract
Structural properties of articular cartilage such as proteoglycan content, collagen content and collagen alignment are known to vary over length scales as small as a few microns (Bullough and Goodfellow, 1968; Bi et al., 2006). Characterizing the resulting variation in mechanical properties is critical for understanding how the inhomogeneous architecture of this tissue gives rise to its function. Previous studies have measured the depth-dependent shear modulus of articular cartilage using methods such as particle image velocimetry (PIV) that rely on cells and cell nuclei as fiducial markers to track tissue deformation (Buckley et al., 2008; Wong et al., 2008a). However, such techniques are limited by the density of trackable markers, which may be too low to take full advantage of optical microscopy. This limitation leads to noise in the acquired data which is often exacerbated when the data is manipulated. In this study, we report on two techniques for increasing the accuracy of tissue deformation measurements. In the first technique, deformations were tracked in a grid that was photobleached onto each tissue sample (Bruehlmann et al, 2004). In the second, a numerical technique was implemented that allowed for accurate differentiation of optical displacement measurements by minimizing the propagated experimental error while ensuring that truncation error associated with local averaging of the data remained small. To test their efficacy, we employed these techniques to compare the depth dependent shear moduli of neonatal bovine and adult human articular cartilage. Using a photobleached grid and numerical optimization to gather and analyze data led to results consistent with those reported previously (Buckley et al., 2008; Wong et al., 2008a) but with increased spatial resolution and characteristic coefficients of variation that were reduced by up to a factor of 3. This increased resolution allowed us to determine that the shear modulus of neonatal bovine and adult human tissue both exhibit a global minimum at a depth z of around 100 μm and plateau at large depths. The consistency of the depth dependence of |G*| (z) for adult human and neonatal bovine tissue suggests a functional advantage resulting from this behavior.
Keywords: Cartilage mechanics, shear, depth dependence, imaging, photobleaching
Introduction
Measuring the depth-dependent mechanical properties of articular cartilage with a high spatial resolution can help elucidate the functional benefits resulting from the tissue’s complex structure. As such, recent studies have investigated the depth-dependent compressive and shear properties of this tissue (Guilak et al., 1995; Schinagl et al., 1996; Wang et al., 2002; Chahine et al., 2004; Wong et al., 2008a; Wong et al., 2008b; Buckley et al., 2008) using particle image velocimetry (PIV) and other feature-tracking algorithms. Unfortunately, the spatial resolution in these techniques is limited by the density of trackable markers (i.e., cells or cell nuclei). For example, in adult human articular cartilage, where cells are particularly sparse, the depth-dependent shear modulus G(z) has been reported to an accuracy of ~350 μm (Wong et al., 2008a; Wong et al., 2008b). However, near the surface, structural properties can vary over much smaller length scales (Bi et al., 2007).
Here, we describe two techniques for improving measurement resolution in G(z). To increase the spatial accuracy of local displacement measurements, we used grid-resolution automated tissue elastography (GRATE). This technique builds on pioneering efforts for measuring deformation in intervertebral disk under flexion (Bruehlmann et al., 2004) and entails tracking the displacement of gridlines photobleached onto the sample. Since the gridlines are continuous, measurement resolution is limited by diffraction rather than the density of trackable markers. To reduce noise inherent in processing the extracted displacement data, we employ weight-averaged noisy differentiation (WAND). This numerical technique addresses amplification of noise associated with differentiation of discrete experimental data and draws from previously described methods (Anderssen and Bloomfield, 1974; Muller et al., 1987; Anderssen et al., 1996; Carlsson et al., 1992; Anderssen and Hegland, 1999; Chartrand, 2005).
We applied these procedures to neonatal bovine and adult human articular cartilage tested in a tissue deformation imaging stage (TDIS) (Buckley et al., 2008; Michalek et al., 2009). We found that these techniques substantially improve the resolution and accuracy of the measured shear modulus profiles.
Methods
Sample Preparation: Adult Human Tissue
Three 6 mm diameter cylindrical explants of thickness 2–3 mm were harvested from frozen adult human tibial plateus (Musculoskeletal Transplant Foundation). After dissection, samples were bisected into hemi-cylinders and placed into PBS until thawed. Prior to mechanical testing, hemi-cylinders were placed into PBS with 7 μg/mL 5-dichlorotriazinylaminofluorescein (5-DTAF) for 2 hours (Bruehlmann et al, 2004; Michalek et al., 2009). 5-DTAF modifies amines in proteins and fully stains the extracellular matrix. Shear modulus profiles obtained using carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE), a cellular stain, were consistent with those obtained using 5-DTAF, verifying that matrix proteins are not mechanically altered by this stain (data not shown).
Sample Preparation: Neonatal Bovine Tissue
Three 6 mm diameter cylindrical explants of thickness 3–4 mm were harvested from patellofemoral grooves of 1–3 day old calves. Prior to mechanical testing, samples were placed into PBS and 7 μg/mL 5-DTAF for 2 hours.
Mechanical Testing
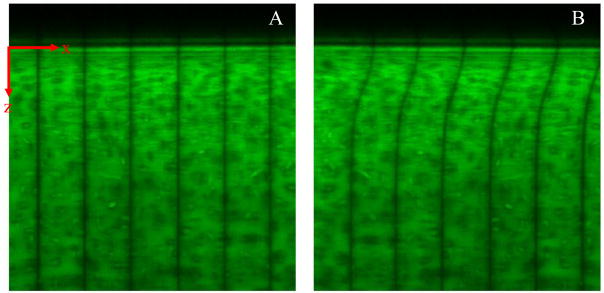
Cartilage hemi-cylinders were placed between two glass shearing plates of a TDIS. Sandblasted protrusions ~10 μm in diameter on the moving plate gripped the surface and prevented slip (Supplementary Section 1). Results were consistent with those obtained using smooth glass (data not shown). The opposing face of the tissue was adhered to the stationary plate using cyanoacrylate glue. For all experiments, the compressive strain on the hemi-cylinder was 10%. After positioning the device onto an inverted Zeiss LSM 510 confocal microscope, five lines spaced by 50 μm were photobleached onto the hemi-cylinder along the z axis (Figure 1A) using a 488 nm laser. Samples were imaged (Figure 1B) during sinusoidal shear with frequency f = 100 mHz and a shearing plate peak-to-peak displacement amplitude of 32 μm.
Figure 1.
Confocal micrographs of 5-DTAF-stained human articular cartilage with vertical photobleached lines (A) before and (B) during application of shear. The photobleached lines are spaced by 50 μm.
Data Analysis: PIV
For PIV, the displacement amplitude at a given depth u0(z) was determined using software adapted from MatPIV (Sveen and Cowen, 2004; Buckley et al., 2008) with a window size of 317 × 20 μm2.
Data Analysis: GRATE
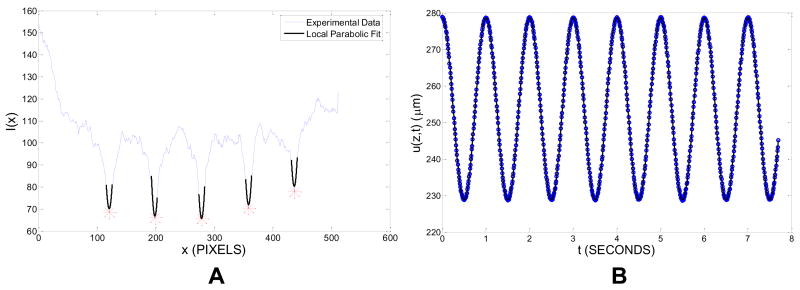
For GRATE, custom MATLAB (The Mathworks, Inc., Natick, MA) software was used to determine u0(z). For an image taken at time t, this software first plots I(x), the average intensity across vertical regions of width w=20 μm centered at a depth z, versus horizontal location x (Fig. 2A). It then determines mn(z,t), the locations of the five local minima of I(x) corresponding to the five photobleached lines indexed by n. To better determine these minima, a parabola is fit to I(x) over 11 pixel-wide regions centered at mn(z,t). The locations Mn(z,t) of the minima of these parabolic fits give the photobleached line locations. The mean photobleached line location u(z,t) is the average of Mn(z,t) over all lines. u(z,t) is then plotted as a function of time, yielding a sinusoidal curve (Fig. 2B). Both the displacement amplitude u0(z) and the displacement phase angle δu(z) are obtained by fitting a cosine to u(z,t).
Figure 2.
(A) Mean intensity I(x) at depth z for a sheared sample of articular cartilage at time t (blue). Also shown are local parabolic fits near each photobleached line location (black) and the calculated photobleached line locations (red stars). (B) Mean photobleached line location u(z,t) at depth z vs. time t for a sheared sample of articular cartilage (blue circles) and sinusoidal fit (black solid line).
Data Analysis: Obtaining |G*| (z) from u0(z)
For a dynamically sheared inhomogeneous material, the shear strain amplitude γ0(z) is given by
| (1) |
if the stress is assumed to be uniform with z. This relation reduces to du0/dz in the limit where δu(z) = 0. To obtain γ0, differentiation of u0 cos δu and u0 sin δu was performed numerically using either five-point linear least-squares fitting (5PLSQ) (Supplementary Section 2) or WAND (see below). The complex shear modulus profile is given by:
| (2) |
where τ0 is the measured stress amplitude.
Data Analysis: WAND
WAND addresses the amplification of noise associated with differentiation of discrete experimental data. To differentiate a function f sampled at depths zi such that fj = f(zj), we employ the finite difference derivative operator:
| (4) |
where the weights wj satisfy
| (5) |
D smoothes the measurement error in the differentiated series by using a weighted average of the standard finite-difference derivative between zi and data points in its neighborhood. We determine wj using the CVXopt Python package to calculate
which minimizes the propagated experimental error σ̄i while ensuring that the smoothing (or truncation) error ēi remains 100 times smaller (Supplementary Section 3).
Results
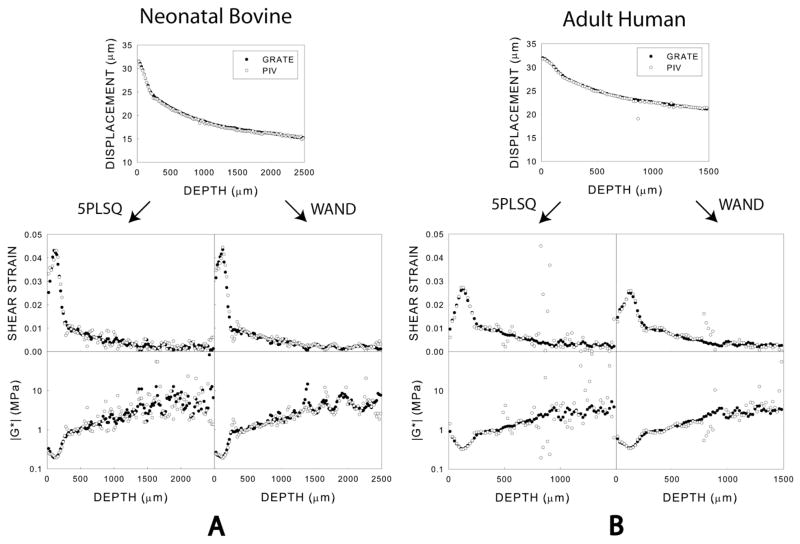
For representative samples of adult human and neonatal bovine articular cartilage, GRATE displacement and shear modulus profiles were consistent with those obtained using PIV (Fig. 3A,B). However, using GRATE decreased scatter in these profiles. WAND further smoothed shear strain and shear modulus profiles in both PIV and GRATE data, particularly the former.
Figure 3.
Depth dependence of peak-to-peak displacement amplitudes and complex shear moduli for single representative samples of (A) neonatal bovine and (B) adult human articular cartilage subject to shear at 100 mHz and analyzed using 5PLSQ and WAND.
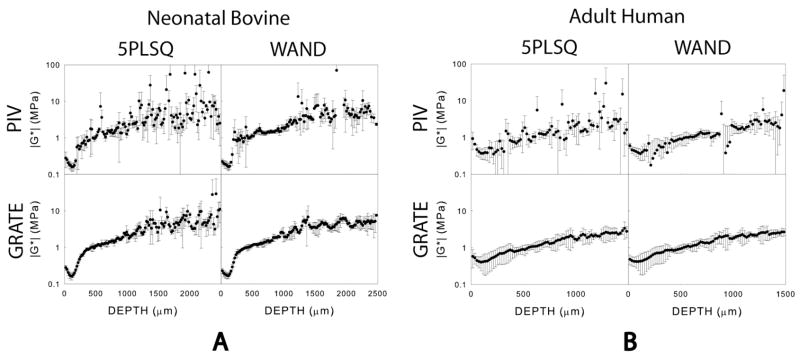
In both neonatal bovine (n=3) and adult human (n=3) articular cartilage, the standard deviation of the local shear modulus was substantially lower when WAND was used instead of 5PLSQ and when GRATE was used instead of PIV (Fig. 4A,B). We define cV as the ratio of the mean standard deviation of |G*| (z) to the mean value of |G*| (z) over the range 0<z<1000 μm. In neonatal bovine tissue, cV was nearly 0.5 for data analyzed with PIV and 5PLSQ. However, cV was 0.26 when PIV and WAND was employed, 0.14 when GRATE and 5PLSQ were used and 0.15 when GRATE and WAND were used. Similarly, in adult human tissue, cV was reduced from 0.70 to 0.58 when implementing WAND instead of 5PLSQ on images analyzed with PIV. cV was lowest (0.36) when both GRATE and WAND were used.
Figure 4.
Depth dependence of complex shear moduli for n=3 samples of (A) neonatal bovine and (B) adult human articular cartilage subject to shear at 100 mHz and analyzed using PIV and GRATE.
The shear modulus of adult human cartilage |G*| (z) depended strongly on z (Fig. 3). In particular, the shear stiffness displayed a global minimum of 0.4 MPa at a depth z~100 μm. The shear modulus profile for neonatal bovine articular cartilage exhibited similar features.
Discussion
In this study, we combined GRATE and WAND to measure the spatially-dependent shear stiffness of articular cartilage with a spatial resolution of less than 20 μm. Given the same set of images, GRATE yields displacement profiles with reduced scatter and higher spatial resolution than PIV, leading to more accurate shear modulus profiles. In addition, we demonstrated that strain profiles obtained using PIV can be smoothed using WAND, yielding shear modulus profiles with substantially reduced scatter and a resolution that approaches the separation between markers. These techniques should also be applicable for mapping shear modulus profiles in other soft biological tissues with small-scale inhomogeneities.
By performing GRATE and WAND on samples of adult human articular cartilage, we found that |G*| (z) exhibits a global minimum at a depth z~100 μm. A similar qualitative behavior was observed in bovine neonatal tissue. The fact that the general shape of the shear modulus profile is maintained across different species implies that such a depth-dependent mechanical response is phylogenetically conserved and therefore may have an important as yet undiscovered functional benefit.
Supplementary Material
Acknowledgments
We thank Harrick Scientific for providing us with a TDIS. This study was supported by NSF IGERT Program, NSF DMR-0606040, NSF CMMI-0726773, NIH R21AR054867 and CCMR MRSEC SEED DMR-0079992.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderssen R, Bloomfield P. Numerical differentiation procedures for non-exact data. Numerische Mathematik 1974 [Google Scholar]
- Anderssen R, de Hoog F, Hegland M. A stable finite difference ansatz for higher order differentiation of non-exact data. 1996 www.maths.anu.edu.au.
- Anderssen R, Hegland M. For numerical differentiation, dimensionality can be a blessing! Mathematics of Computation 1999 [Google Scholar]
- Bi, et al. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochim Biophys Act. 2006;1758:934–941. doi: 10.1016/j.bbamem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Bruehlmann S, Matyas JR, Duncan NA. Collagen Fibril Sliding Governs Cell Mechanics in the Anulus Fibrosus. Spine. 2004;29:2612–2620. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- Buckley MR, Gleghorn JP, Bonassar LJ. Mapping the Depth Dependence of Shear Properties in Articular Cartilage. Journal of Biomechanics. 2008;11:2430–2437. doi: 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Bullough P, Goodfellow J. The significance of the fine structure of articular cartilage. Journal of Bone and Joing Surgery. 1968;50:852–857. [PubMed] [Google Scholar]
- Carlsson B, Sternad M, Ahlem A. Digital differentiation of noisy data measured through a dynamic system. IEE Trans Sig Proc. 1992;40:218–222. [Google Scholar]
- Chahine NO, Wang CC, Hung CT, Ateshian GA. Anisotropic Strain-Dependent Material Properties of Bovine Articular Cartilage in the Transitional Range From Tension to Compression. Journal of Biomechanics. 2004;37:1251–1261. doi: 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand R. Numerical differentiation of noisy data. Mathematical Modeling and Analysis 2005 [Google Scholar]
- Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: A confocal Microscope Study. Journal of Orthopaedic Research. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. Measurement of Local Strains in Intervertebral Disc Anulus Fibrosus Tissue Under Dynamic Shear: Contributions of Matrix Fiber Orientation and Elastin Content. Journal of Biomechanics. 2009 doi: 10.1016/j.jbiomech.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HG, Stadtmuller U, Schmitt T. Bandwidth choice and confidence intervals for derivatives of noisy data. Biometrika. 1987;74:743–749. [Google Scholar]
- Schinagl RM, Ting MK, Price JH, Sah RLY. Video microscopy to quantitate the inhomogeneous equilibrium strain within articular cartilage during confined compression. Annals of Biomedical Engineering. 1996;24:500–512. doi: 10.1007/BF02648112. [DOI] [PubMed] [Google Scholar]
- Sveen JK, Cowen EA. Quantitative Imaging Techniques and their Application to Wavy Flows. In: Grue J, Liu LF, Pederson GK, editors. PIV and Water Waves. Springer; Tokyo: 2004. pp. 1–49. [Google Scholar]
- Wang CC, Deng JM, Ateshian GA, Hung CT. An Automated Approach for Direct Measurement of Two-Dimensional Strain Distributions Within Articular Cartilage Under Unconfined Compression. Journal of Biomechanical Engineering. 2002;124:557–567. doi: 10.1115/1.1503795. [DOI] [PubMed] [Google Scholar]
- Wong BL, Bae WC, Chun J, Gratz KR, Lotz M, Sah RL. Biomechanics of Cartilage Articulation: Effects of Lubrication and Degeneration on Shear Deformation. Arthritis and Rheumatism. 2008a;58:2065–2074. doi: 10.1002/art.23548. [DOI] [PubMed] [Google Scholar]
- Wong BL, Bae, Gratz KR, Sah RL. Shear Deformation Kinematics During Cartilage Articulation: Effect of Lubrication, Degeneration and Stress Relaxation. Molecular and Cellular Biomechanics. 2008b;5:197–206. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.