Abstract
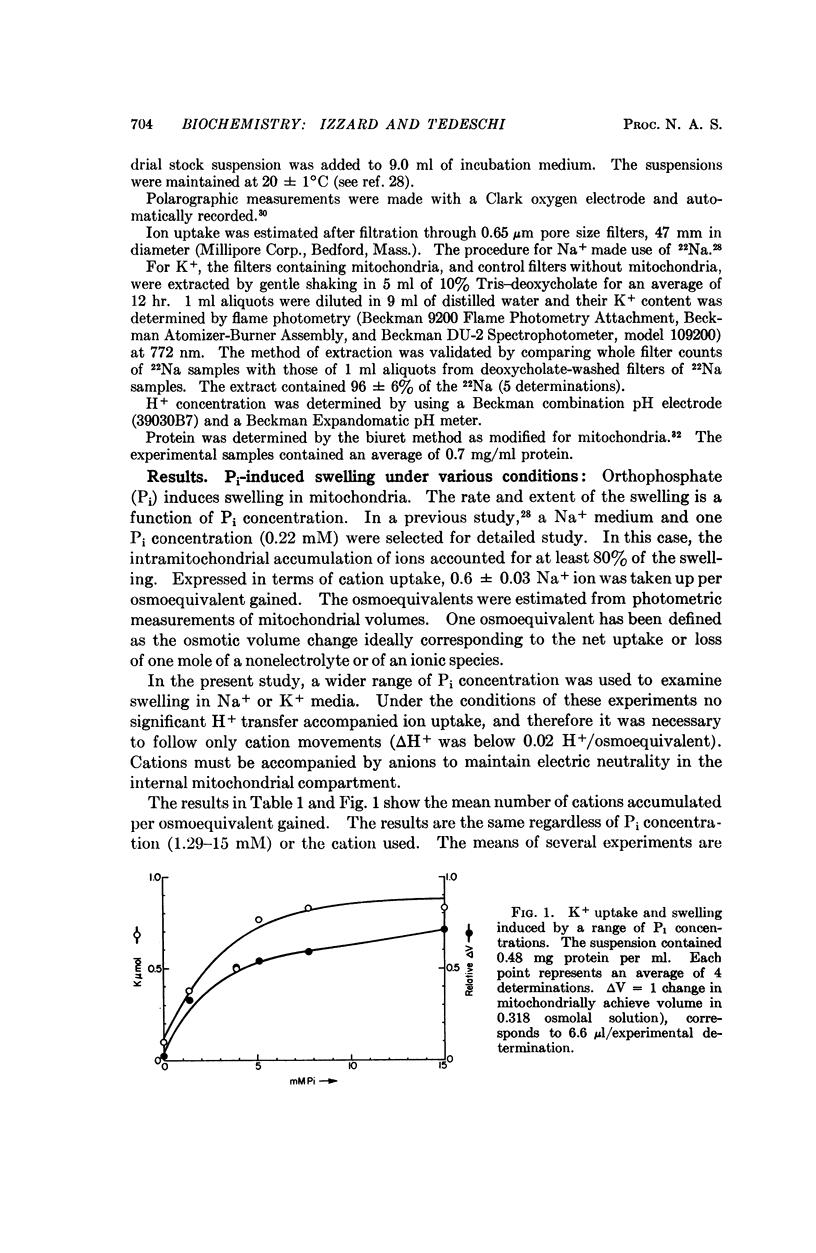
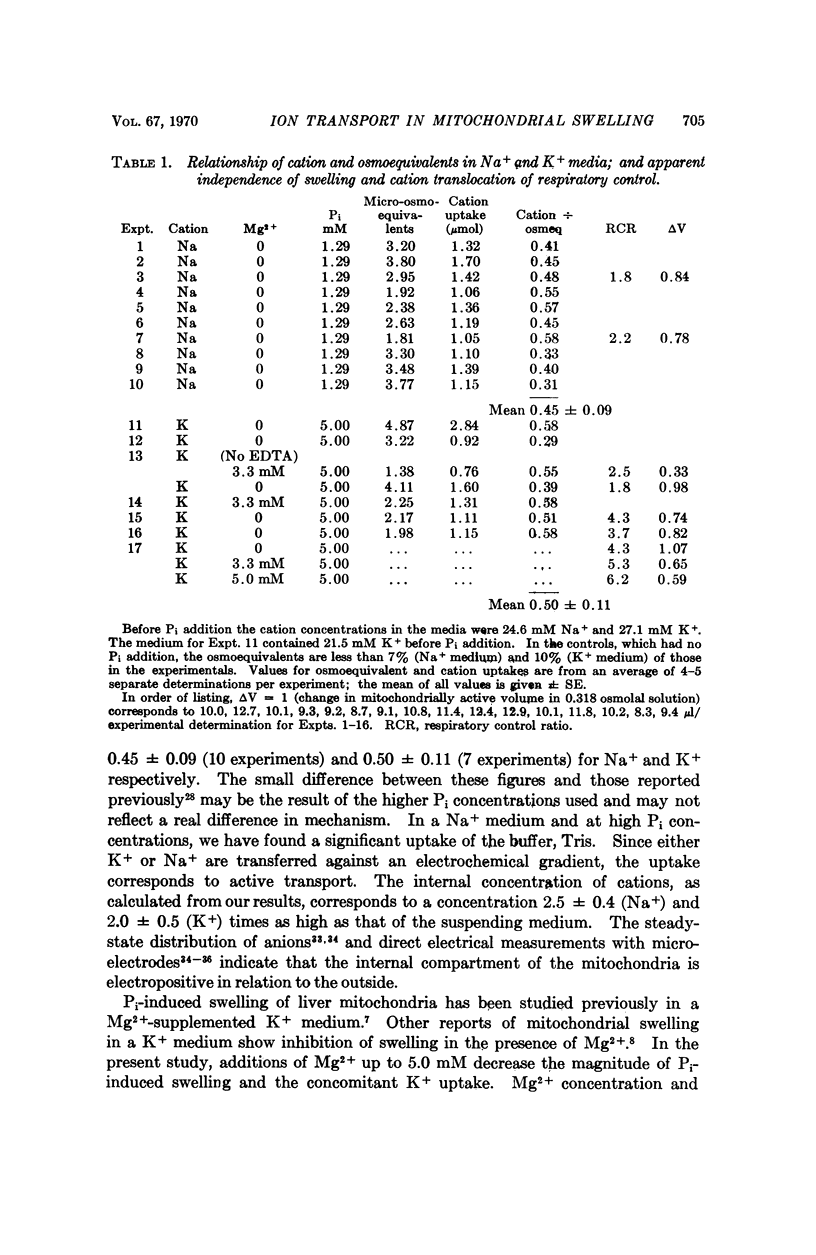
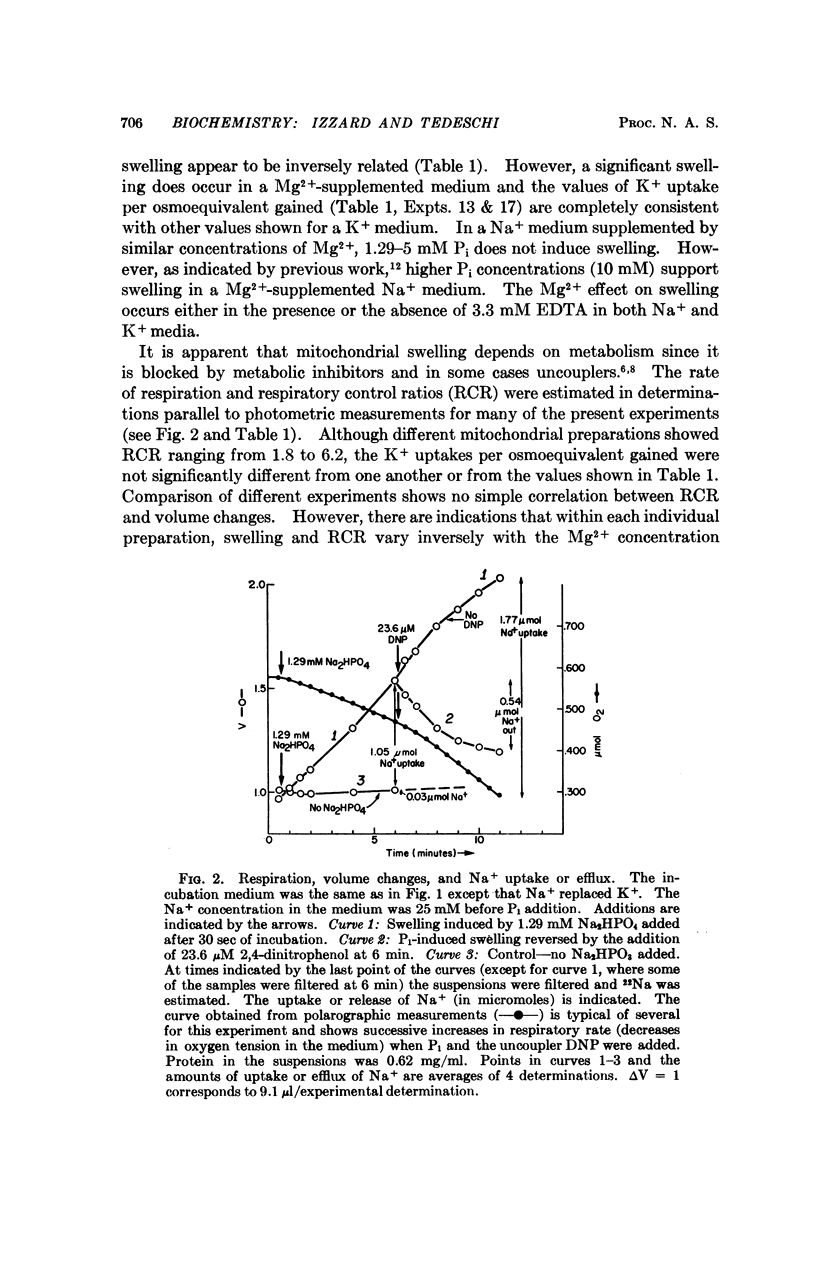
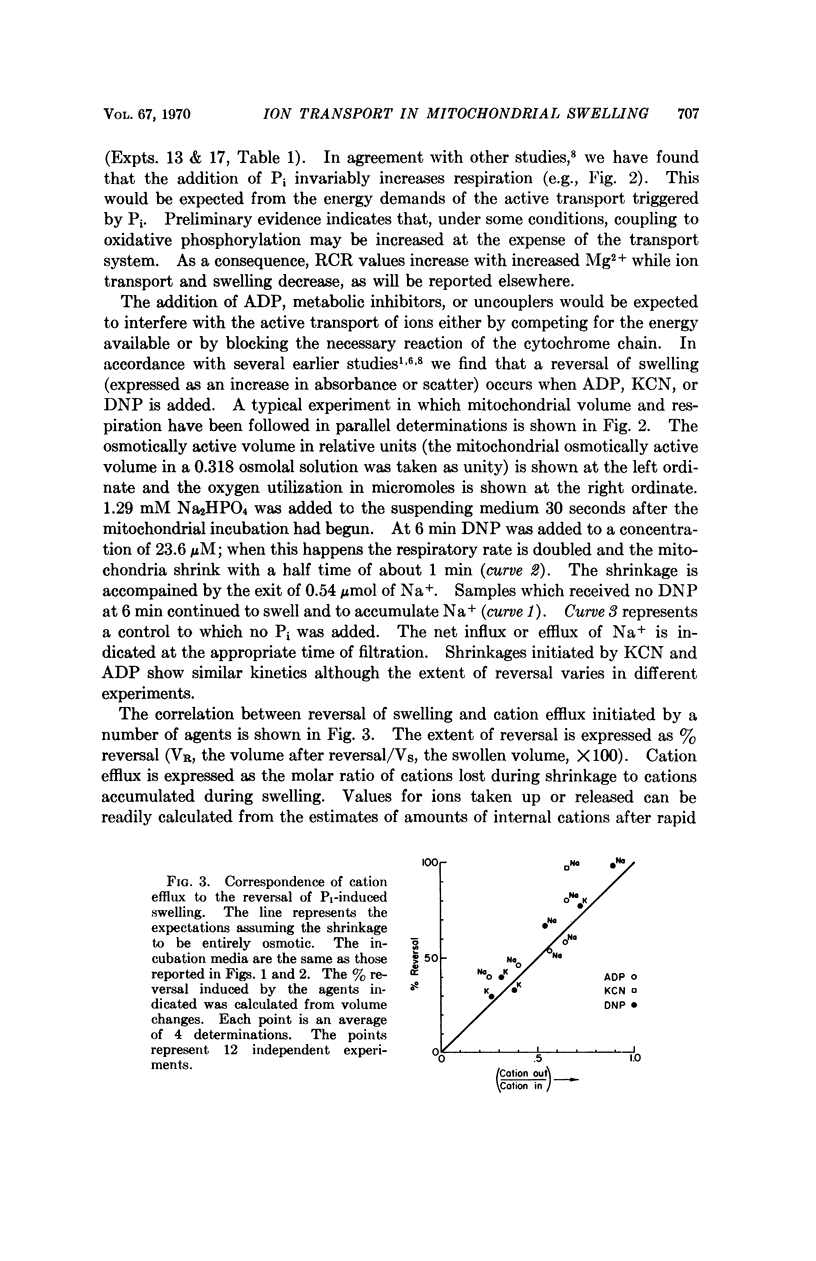
An earlier study has indicated that the swelling of isolated mitochondria induced by inorganic phosphate (Pi) can be accounted for largely or completely by the accumulation of ions. In the present study, a similar uptake was shown to be induced by a wider range of Pi concentrations. Addition of ADP, KCN, or 2,4-dinitrophenol initiates a shrinkage which can be accounted for by the efflux of ions. The results are consistent with the explanations that (a) Pi induces a transport of ions and a concomitant osmotic swelling, and (b) the addition of substances which compete or interfere with the energy available for transport results in ion efflux and a corresponding mitochondrial shrinkage. The results are not consistent with proposals that the changes in the light scattered by mitochondrial suspensions with alterations in metabolic states reflect a mechanochemical coupling phenomenon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai J., Blondin G. A., Vail W. J., Green D. E. The mechanism of mitochondrial swelling. IV. Configurational changes during swelling of beef heart mitochondria. Arch Biochem Biophys. 1969 Jul;132(2):524–544. doi: 10.1016/0003-9861(69)90396-8. [DOI] [PubMed] [Google Scholar]
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. II. Low amplitude swelling-shrinkage cycles. Biochim Biophys Acta. 1965 Aug 24;105(2):265–278. doi: 10.1016/s0926-6593(65)80151-5. [DOI] [PubMed] [Google Scholar]
- Blondin G. A., Green D. E. Mechanism of mitochondrial swelling. 3. Two forms of energized swelling. Arch Biochem Biophys. 1969 Jul;132(2):509–523. doi: 10.1016/0003-9861(69)90395-6. [DOI] [PubMed] [Google Scholar]
- Blondin G. A., Vail W. J., Green D. E. The mechanism of mitochondrial swelling. II. Pseudoenergized swelling in the presence of alkali metal salts. Arch Biochem Biophys. 1969 Jan;129(1):158–172. doi: 10.1016/0003-9861(69)90162-3. [DOI] [PubMed] [Google Scholar]
- Buffa P., Guarriera-Bobyleva V., Muscatello U., Pasquali-Ronchetti I. Conformational changes of mitochondria associated with uncoupling of oxidative phosphorylation in vivo and in vitro. Nature. 1970 Apr 18;226(5242):272–274. doi: 10.1038/226272a0. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Utsumi K., Packer L. Oscillatory states of mitochondria. 3. Ultrastructure of trapped conformational states. Arch Biochem Biophys. 1967 Sep;121(3):641–651. doi: 10.1016/0003-9861(67)90049-5. [DOI] [PubMed] [Google Scholar]
- Green D. E., Asai J., Harris R. A., Penniston J. T. Conformational basis of energy transformations in membrane systems. 3. Configurational changes in the mitochondrial inner membrane induced by changes in functional states. Arch Biochem Biophys. 1968 May;125(2):684–705. doi: 10.1016/0003-9861(68)90626-7. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968 May;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Pressman B. C. The direction of polarity of the mitochondrial trans-membrane potential. Biochim Biophys Acta. 1969 Jan 14;172(1):66–70. doi: 10.1016/0005-2728(69)90092-9. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Asbell M. A., Asai J., Jolly W. W., Green D. E. The conformational basis of energy transduction in membrane systems. V. Measurement of configurational changes by light scattering. Arch Biochem Biophys. 1969 Jul;132(2):545–560. doi: 10.1016/0003-9861(69)90397-x. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Penniston J. T., Asai J., Green D. E. The conformational basis of energy conservation in membrane systems. II. Correlation between conformational change and functional states. Proc Natl Acad Sci U S A. 1968 Mar;59(3):830–837. doi: 10.1073/pnas.59.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER L. Metabolic and structural states of mitochondria. I. Regulation by adenosine diphosphate. J Biol Chem. 1960 Jan;235:242–249. [PubMed] [Google Scholar]
- PACKER L. Metabolic and structural states of mitochondria. II. Regulation by phosphate. J Biol Chem. 1961 Jan;236:214–220. [PubMed] [Google Scholar]
- Packer L., Utsumi R., Mustafa M. G. Oscillatory states of mitochondria. 1. Electron and energy transfer pathways. Arch Biochem Biophys. 1966 Nov;117(2):381–393. doi: 10.1016/0003-9861(66)90426-7. [DOI] [PubMed] [Google Scholar]
- Packer L., Wrigglesworth J. M., Fortes P. A., Pressman B. C. Expansion of the inner membrane compartment and its relation to mitochondrial volume and ion transport. J Cell Biol. 1968 Nov;39(2):382–391. doi: 10.1083/jcb.39.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T., Harris R. A., Asai J., Green D. E. The conformational basis of energy transformations in membrane systems. I. Conformational changes in mitochondria. Proc Natl Acad Sci U S A. 1968 Feb;59(2):624–631. doi: 10.1073/pnas.59.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikmenspoel R. Oxygen electrode chamber for biological suspensions. Anal Biochem. 1969 Aug;30(2):292–295. doi: 10.1016/0003-2697(69)90403-5. [DOI] [PubMed] [Google Scholar]
- Sordahl L. A., Blailock Z. R., Kraft G. H., Schwartz A. The possible relationship between ultrastructure and biochemical state of heart mitochondria. Arch Biochem Biophys. 1969 Jul;132(2):404–415. doi: 10.1016/0003-9861(69)90382-8. [DOI] [PubMed] [Google Scholar]
- Stoner C. D., Sirak H. D. Passive induction of the "energized-twisted" conformational state in bovine heart mitochondria. Biochem Biophys Res Commun. 1969 Apr 10;35(1):59–66. doi: 10.1016/0006-291x(69)90482-3. [DOI] [PubMed] [Google Scholar]
- TEDESCHI H., HARRIS D. L. Some observations on the photometric estimation of mitochondrial volume. Biochim Biophys Acta. 1958 May;28(2):392–402. doi: 10.1016/0006-3002(58)90487-6. [DOI] [PubMed] [Google Scholar]
- TEDESCHI H., HEGARTY H. J. OSMOTIC REVERSAL OF CA2+ INDUCED MITOCHONDRIAL SWELLING. Biochem Biophys Res Commun. 1965 May 3;19:558–564. doi: 10.1016/0006-291x(65)90162-2. [DOI] [PubMed] [Google Scholar]
- Tedeschi H., Hegarty H. J., James J. M. Osmotic reversal of phosphate-induced mitochondrial swelling. Biochim Biophys Acta. 1965 Jul 8;104(2):612–615. doi: 10.1016/0304-4165(65)90372-7. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Microelectrode studies on the membrane properties of isolated mitochondria. II. Absence of a metabolic dependence. Proc Natl Acad Sci U S A. 1969 Jul;63(3):713–717. doi: 10.1073/pnas.63.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Microelectrode studies on the membrane properties of isolated mitochondria. Proc Natl Acad Sci U S A. 1969 Jun;63(2):370–377. doi: 10.1073/pnas.63.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Mitochondrial membrane potentials measured with microelectrodes: probable ionic basis. Science. 1969 Dec 19;166(3912):1539–1540. doi: 10.1126/science.166.3912.1539. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Observations on low amplitude Ca2+-induced swelling in mitochondria. Life Sci. 1967 Oct 1;6(19):2021–2028. doi: 10.1016/0024-3205(67)90220-2. [DOI] [PubMed] [Google Scholar]
- Utsumi K., Packer L. Oscillatory states of mitochondria. II. Factors controlling period and amplitude. Arch Biochem Biophys. 1967 May;120(2):404–412. doi: 10.1016/0003-9861(67)90257-3. [DOI] [PubMed] [Google Scholar]
- Weber N. E., Blair P. V. Ultrastuctural studies of beef heart mitochondria. I. Effects of adenosine diphosphate on mitochondrial morphology. Biochem Biophys Res Commun. 1969 Sep 10;36(6):987–993. doi: 10.1016/0006-291x(69)90301-5. [DOI] [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J., Sheffield H. G. Morphology of mitochondria in the coupled, uncoupled and recoupled states. Exp Cell Res. 1967 Apr;46(1):129–143. doi: 10.1016/0014-4827(67)90415-6. [DOI] [PubMed] [Google Scholar]



