Abstract
Second-generation antipsychotics (SGAs) are increasingly prescribed to treat psychiatric symptoms in pediatric patients infected with HIV. We examined the relationship between prescribed SGAs and physical growth in a cohort of youth with perinatally acquired HIV-1 infection. Pediatric AIDS Clinical Trials Group (PACTG), Protocol 219C (P219C), a multicenter, longitudinal observational study of children and adolescents perinatally exposed to HIV, was conducted from September 2000 until May 2007. The analysis included P219C participants who were perinatally HIV-infected, 3–18 years old, prescribed first SGA for at least 1 month, and had available baseline data prior to starting first SGA. Each participant prescribed an SGA was matched (based on gender, age, Tanner stage, baseline body mass index [BMI] z score) with 1–3 controls without antipsychotic prescriptions. The main outcomes were short-term (approximately 6 months) and long-term (approximately 2 years) changes in BMI z scores from baseline. There were 236 participants in the short-term and 198 in the long-term analysis. In linear regression models, youth with SGA prescriptions had increased BMI z scores relative to youth without antipsychotic prescriptions, for all SGAs (short-term increase = 0.192, p = 0.003; long-term increase = 0.350, p < 0.001), and for risperidone alone (short-term = 0.239, p = 0.002; long-term = 0.360, p = 0.001). Participants receiving both protease inhibitors (PIs) and SGAs showed especially large increases. These findings suggest that growth should be carefully monitored in youth with perinatally acquired HIV who are prescribed SGAs. Future research should investigate the interaction between PIs and SGAs in children and adolescents with perinatally acquired HIV infection.
Introduction
The number of children and adolescents with perinatally acquired HIV-1 infection is growing at alarming rates worldwide.1 It is estimated that 5%–10% of all persons living with HIV are children, and most of them acquired HIV through perinatal transmission (i.e., either via transplacental transmission during pregnancy, during the delivery, or via breast-feeding).2
In the United States and other parts of the world where highly active antiretroviral therapy (HAART) is readily available, HIV infection is no longer viewed as a fatal, rapidly progressive infection, but rather as a chronic life-limiting disease. This has refocused treatment to addressing the need to adhere to complex medication regimens, anticipating adverse events from treatment, and maintaining the quality of life, including the mental health of people living with HIV.3–6
Children and adolescents with perinatally acquired HIV-1 infection face multiple risks to their mental health, possibly starting with in utero exposure to the antiretroviral (ARV) medications taken during pregnancy.7,8 In addition, there is the ongoing exposure of the developing brain to the slow but progressive immune dysregulation that characterizes HIV infection9,10 and the required ARV medications, some of which appear to have significant central nervous system (CNS)-related side effects (e.g., efavirenz).11
The risk of mental health problems continues through childhood due to potential prior or current parental mental illness and substance abuse, as well as environmental factors that frequently co-occur within the context of HIV infection, such as poverty, inadequate social support, and unstable housing.12 Indeed, several studies13–19 have reported high combined rates of psychiatric symptoms, cognitive disorders and school problems in this population.
Second-generation antipsychotics (SGAs) are prescribed frequently to children and adolescents,20–22 including those who are HIV-infected, to treat psychiatric symptoms: in a large cohort of U.S. children and adolescents with HIV, 2% of the subjects had prescriptions for risperidone.17 This prescribing trend is likely to continue following the U.S. Food and Drug Administration's (FDA) approval of risperidone use for the symptomatic treatment of irritability in children and adolescents with autism,23 for treatment of schizophrenia in adolescents age 13 to 17 years, and for short-term treatment of manic or mixed episodes of type I bipolar disorder in youth age 10 to 17 years.24
A major advantage of SGAs over conventional antipsychotics is their very low association with extrapyramidal symptoms,25 however, growing evidence suggests an association between SGA treatment and excessive weight gain. Comprehensive reviews of randomized controlled pediatric trials suggest that the weight gain is particularly prominent in children and adolescents treated with olanzapine or risperidone, followed by quetiapine.18,26 In a recent clinical trial,27 there were significant increases in body mass index (BMI) z scores in children and adolescents after 6 months of treatment with olanzapine or risperidone. Bell et al.28 demonstrated an independent relationship between BMI z score as a continuous variable and multiple health risks in children and adolescents (including significant relationship to systolic and diastolic blood pressure, insulin during oral glucose tolerance test, total cholesterol, high-density lipoprotein, triglycerides, and alanine aminotransferase as well as rates of musculoskeletal pain, obstructive sleep apnea symptoms, headaches, depression, anxiety, bullying, and acanthosis nigricans) either in linear- or curvilinear fashion, suggesting the risks increase across the entire range of BMI z score values, rather than increasing only upon crossing a certain threshold value.
Taken together, this evidence gives rise to safety concerns for youth with perinatally acquired HIV and comorbid psychiatric disorders treated with SGAs who are already exposed to risk factors independently affecting growth and metabolic status and salient to HIV infection. For example, HIV infection has been associated with growth retardation both in the developed and developing world,29,30 and treatment with protease inhibitors (PIs) has been strongly associated with marked increases in total cholesterol levels in youth with HIV.31
The primary objective of the present study was to examine the relationship between prescribed SGAs (as a class and individually) and changes in physical growth (as measured by changes in BMI z scores) in a cohort of children and adolescents with perinatally acquired HIV infection. We hypothesized that children and adolescents with perinatally acquired HIV infection who are treated with SGAs will experience significantly higher increases in BMI z score than their counterparts not treated with antipsychotics.
Methods
This study evaluated data from the Pediatric AIDS Clinical Trials Group (PACTG), Protocol 219C cohort study (P219C), a multicenter, longitudinal observational study of children and adolescents perinatally exposed to HIV, conducted from September 2000 until May 2007. P219C was a revision of PACTG protocol 219, initiated in 1993 to study long-term effects of in utero exposure to antiretroviral (ARV) medications and complications of HIV infection. P219C was approved by local Institutional Review Boards at over 80 participating sites in the United States and Puerto Rico. Informed consent and assent were obtained according to local institutional guidelines. Upon enrollment, study nurses abstracted participants' medical records to obtain medical and treatment histories, including diagnoses and ARV and concomitant medications. Follow-up visits included physical examinations, laboratory studies, and self-reports from children and parents or caregivers to provide updated demographic information, medical history (including psychiatric and neurologic diagnoses), and quality-of-life information.
Participants
Among the participants with prescriptions for SGAs, we included those who started their first SGA between the ages of 3 and 18 years, continued that SGA for at least 1 month, and had an available baseline visit prior to starting the first SGA. Each participant with SGA prescriptions was then matched with up to 3 perinatally HIV-infected controls without prescriptions for any antipsychotics (i.e., neither conventional nor SGA). The matching was based on the following criteria: gender, birth date within 1 year, baseline Tanner stage category (1, 2–3, or 4–5), and baseline BMI z score. Study nurses recorded medication dates as reported by parents or older participants or as documented in the medical records available to the study.
Procedures
Height and weight measurements were obtained every 3 months (±1 month) from evaluations performed in routine clinical care or during scheduled study visits, according to the standardized protocol in P219C. Children had standing heights measured to the nearest 0.1 cm using a stadiometer; weights were taken without shoes. One height and weight measurement was recorded at each visit. The baseline visits for the participants with SGA prescriptions and matching participants without antipsychotic prescriptions were required to be within 3 months of each other. For participants with SGA prescriptions, the baseline BMI z score measurement was the latest measurement prior to the first medication start date. The short-term follow-up measurement was the measurement taken closest to 6 months after the baseline visit. This measurement was required to be obtained no less than 1 month after starting antipsychotic drugs and within 1 year after the baseline visit, and to occur while the participant was still taking the first SGA. The long-term follow-up measurement was the measurement taken closest to 2 years after the baseline visit and between 1 year and 3 years after the baseline visit. In contrast to the short-term follow-up measurement, the patient was not required to still be taking the first drug at the time of the long-term follow-up measurement. For the controls, the baseline measurement was defined as the measurement taken closest to the baseline visit for the matching participant with SGA prescription. The short-term follow-up measurement was the measurement taken closest to 6 months after the baseline visit and between 1 month and 1 year after the baseline visit. The long-term follow-up measurement was the measurement taken closest to 2 years after the baseline visit and between 1 year and 3 years after the baseline visit.
The outcomes of interest were short-term and long-term change in BMI z score as measured from a baseline visit to a follow-up visit. Height-for-age and weight-for-age z scores were calculated with reference to national norms for U.S. children and adolescents.32 The BMI z-score was chosen as the primary measure of physical growth in order to adjust for the age- and gender-appropriate developmental changes in height and growth, as recommended in recent literature reviews.26,33
Antipsychotic medications
The initial review of data identified children and adolescents enrolled in P219C who had prescriptions for at least one of the following SGAs: aripiprazole, quetiapine, risperidone, olanzapine, ziprazidone, clozapine and paliperidone. We originally intended to include conventional antipsychotics in the analyses, defined as follows: haloperidol, loxapine, perphenazine, thioridazine, thiothixene, chlorpromazine, fluphenazine, pimozide, trifluoperazine, molindone, and droperidol. This intent, however, was severely limited by the small number of participants on such medications in P219C. There were only three patients on conventional antipsychotics that had data available for analysis of short-term change in BMI z cores and only two patients had data for analysis of long-term change in BMI z scores. Thus, participants with a history of treatment with a conventional antipsychotic prior to or concurrent with SGA therapy were excluded from the analysis. The analysis of individual SGAs was limited to risperidone alone due to the relatively small number of participants with prescriptions for individual SGAs other than risperidone (Table 1).
Table 1.
Frequency Distribution of the Second-Generation Antipsychotics Prescribed to Study Participants
| |
Short-term follow-up |
Long-term follow-up |
||||||
|---|---|---|---|---|---|---|---|---|
| |
First-prescribed |
Ever-prescribed |
First-prescribed |
Ever-prescribed |
||||
| Antipsychotic | n | %a | n | %a | n | %a | n | %a |
| Risperidone | 40 | 67% | 41 | 68% | 34 | 67% | 35 | 69% |
| Olanzapine | 8 | 13% | 9 | 15% | 6 | 12% | 7 | 14% |
| Aripiprazole | 6 | 10% | 8 | 13% | 5 | 10% | 8 | 16% |
| Quetiapine | 5 | 8% | 9 | 15% | 5 | 10% | 9 | 18% |
| Ziprasidone | 1 | 2% | 3 | 5% | 1 | 2% | 2 | 4% |
| Total (n) | 60 | 100% | 51 | 100% | ||||
Percentages were taken out of the total n for each respective group.
Statistical methods
For each outcome of interest (short- and long-term change in BMI z score), we used linear regression models to evaluate the effect of any SGA versus no SGA and risperidone alone versus no SGA, for a total of four sets of analyses. The participant characteristics shown in Table 2 were considered as potential covariates in the model selection process in all four sets of analyses. In selecting a reduced model, all covariates with p values <0.25 in either the univariate model or the full model were considered for inclusion. These covariates were then removed using backward elimination with a significance level of 10%. To maintain consistency, covariates that were significant in either of the two short-term models were included in the final models. Similarly, the long-term final models were adjusted for all covariates that were significant in either of the two long-term, significant-covariate models. Stimulant use was included in each of the four final models as a potential confounder because of the significant imbalance in stimulant use by SGA exposure status (Table 2) and because of reported associations between stimulant use and weight change (see Sirois et al.34 for example). Similarly, we controlled for length of follow-up since the univariate analysis showed some variation in follow-up time by SGA prescription status. All of the short-term final models were adjusted for race, stimulant use, PI use, primary caregiver (PCG), PCG education level, and length of follow-up. The final long-term models were adjusted for CDC class, stimulant use, PI use, PCG, PCG education level, participation in school sports, and length of follow-up. All analyses used data submitted to the P219C data management center by May 2007 and were conducted using SAS 9.1 software (SAS Institute Inc., Cary, NC).
Table 2.
Baseline Characteristics of Subjects Included in the Short-Term Analysis
| Characteristic | Overall (n = 236) | With prescriptions (n = 60) | Without prescriptions (n = 176) | p value |
|---|---|---|---|---|
| Race/Ethnicity | 0.80a | |||
| White, non-Hispanic | 26 (11%) | 8 (13%) | 18 (10%) | |
| Black, non-Hispanic | 136 (58%) | 36 (60%) | 100 (57%) | |
| Hispanic | 69 (29%) | 16 (27%) | 53 (30%) | |
| Other | 5 (2%) | 0 (0%) | 5 (3%) | |
| CD4 Percentb | 0.14c | |||
| 0–<15 | 27 (12%) | 5 (8%) | 22 (13%) | |
| 15–25 | 62 (27%) | 13 (22%) | 49 (28%) | |
| >25 | 144 (62%) | 42 (70%) | 102 (59%) | |
| HIV RNA (copies/mL)b | 0.06c | |||
| 0–400 | 116 (50%) | 35 (58%) | 81 (47%) | |
| >400–10000 | 66 (28%) | 17 (28%) | 49 (28%) | |
| >10,000 | 50 (22%) | 8 (13%) | 42 (24%) | |
| Primary caregiver (PCG) | 0.11a | |||
| Self | 1 (0%) | 0 (0%) | 1 (1%) | |
| Biological parent | 93 (39%) | 16 (27%) | 77 (44%) | |
| Relative | 53 (22%) | 14 (23%) | 39 (22%) | |
| Other adult | 80 (34%) | 26 (43%) | 54 (31%) | |
| Shelter/home | 9 (4%) | 4 (7%) | 5 (3%) | |
| CDC Class Ce | 60 (25%) | 18 (30%) | 42 (24%) | 0.39d |
| At least one PI in ARV regimen | 182 (77%) | 48 (80%) | 134 (76%) | 0.60d |
| Stimulant use | 49 (21%) | 25 (42%) | 24 (14%) | < 0.001d |
| PCG with high school educational level | 63 (27%) | 16 (27%) | 47 (27%) | 0.76d |
| Recent stressful life event | 100 (44%) | 22 (38%) | 78 (46%) | 0.36d |
| Activities limited by health | 37 (17%) | 10 (18%) | 27 (16%) | 0.84d |
| Participated in sports | 139 (63%) | 32 (57%) | 107 (65%) | 0.34d |
| At least one neurologic or psychiatric diagnosis | 94 (40%) | 38 (63%) | 56 (32%) | <0.001d |
Pearson's χ2 test.
Data is closest to and within 6 months of baseline visit.
Mantel-Haenszel χ2 test.
Fisher's exact test.
Data is closest to and before baseline visit.
PI, protease inhibitor; ARV, antiretroviral.
Results
Among the 2589 perinatally infected participants in P219C, 119 (5%) had a history of SGA use. Of these, 45 were excluded for the following reasons: incomplete drug history (n = 5), started SGA prior to study enrollment (n = 34), used first SGA for less than 1 month (n = 5), or was prescribed a conventional antipsychotic medication prior to or concurrently with an SGA (n = 1). The remaining 74 participants were considered potentially eligible for inclusion in the analysis.
Short-term change in BMI z score
Of these 74 participants, 11 did not have either a baseline measurement or a follow-up measurement within the specified time windows, 2 discontinued the first SGA before a follow-up measurement could be taken, and 1 had a questionable baseline BMI z score value. A sensitivity analysis determined that the impact of excluding the questionable observation was minimal. The remaining 60 participants with SGA prescription history had evaluable short-term growth data that were used in the primary short-term analysis (Table 1). The total sample size, including controls, for the primary short-term analysis was 236 (60 participants prescribed SGAs and 176 controls). The risperidone-alone short-term analysis is based on the 40 participants who were prescribed risperidone and their 118 matching controls (n = 158).
The overall distribution of the matched characteristics was as follows: the plurality of participants (37%) were in the 12–15 years age category, followed by 9–12 years (27%), 6–9 years (17%), 15–18 years (13%), 18–21 years (4%) and 3–6 years (3%); the majority of participants were male (71%); the plurality of participants (47%) were in the Tanner stage I at the time of their baseline visit; baseline BMI z score values were of highest frequency in the range 0 to 1 (35%), followed by 1 to 3 (30%), −1 to 0 (27%), and −3 to −1 (8%).
Participant baseline characteristics and the corresponding frequency distributions (overall and by SGA prescription status) are shown in Table 2. Of note, there was a significant difference between participants with and without SGA prescriptions with respect to the proportion who were prescribed stimulants during the growth observation period (42% versus 14%, p < 0.001). There was also a significantly larger proportion of SGA-prescribed participants with at least one recorded neurologic (documented as: “encephalopathy/cerebral palsy,” “hypotonia,” “microcephaly/failure to thrive,” or “epilepsy/seizure/infantile spasm”) or psychiatric diagnosis (documented as: “ADHD/behavior disorder,” “depression/anxiety/bipolar disorder,” or “intellectual disabilities/developmental disorder”) at baseline than participants without antipsychotic prescriptions (63% versus 32%, p < 0.001).
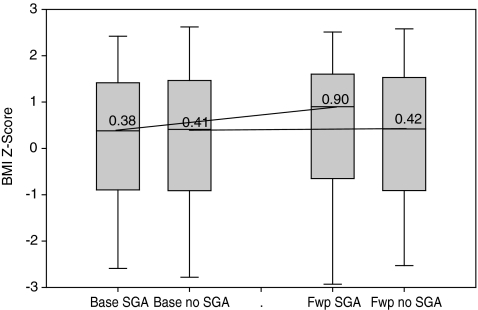
The average duration of short-term follow-up was 6 months (standard deviation [SD] = 1.3) among the participants prescribed SGAs, and 5.7 months (SD = 1.5) among those not prescribed SGAs (p = 0.1). The average short-term change in BMI z score was 0.222 (SD = 0.546) among the SGA-prescribed and −0.003 (SD = 0.236) among the participants without SGA prescriptions (p = 0.002). Figure 1 summarizes the distribution of BMI z scores at baseline and follow-up measurements, showing trends in short-term growth with respect to prescribed SGA treatment.
FIG. 1.
Change in body mass index (BMI) z-scores from baseline to short-term follow-up (approximately 6 months) by second-generation antipsychotics (SGA) exposure (n = 184). Labels on x-axis: Base SGA, baseline measurement in subjects exposed to SGA; Base no SGA, baseline measurement in subject unexposed to SGA; Fwp SGA, follow-up measurement in subjects exposed to SGA; Fwp no SGA, follow-up measurement in subjects unexposed to SGA.
In the final models (Table 3), positive short-term BMI z-score changes were found to be independently predicted by prescribed SGA treatment (slope = 0.193, p = 0.003) as well as by prescribed risperidone (slope = 0.244, p = 0.002). Concomitantly prescribed stimulant medications were not found to be significantly predictive of the short-term BMI z score changes in either case. Supplemental modeling that investigated potential interaction effect showed the presence of a significant short-term interaction between SGAs and PIs (slope = 0.230, p = 0.03), as well as between risperidone and PIs (slope = 0.369, p = 0.01) in predicting change in BMI z scores.
Table 3.
Multiple Regression of Short-Term Change in Body Mass Index z Score on Second-Generation Antipsychotics Adjusted for Selected Covariates (n = 236)
| Predictor | Slope | 95% LCI | 95% UCI | p value |
|---|---|---|---|---|
| Intercept | −0.184 | −0.349 | 0.019 | 0.03 |
| SGA history | 0.193 | 0.064 | 0.321 | 0.003 |
| Stimulant use | 0.059 | −0.081 | 0.199 | 0.41 |
| PI use | 0.098 | 0.025 | 0.171 | 0.008 |
| Biological parent PCG | −0.056 | −0.137 | 0.026 | 0.18 |
| PCG high school graduate | 0.053 | −0.018 | 0.124 | 0.15 |
| Length of follow-up | 0.015 | −0.006 | 0.036 | 0.16 |
Note: Risperidone use was significant in the multiple regression model of short-term change in BMI Z-score on risperidone (p = 0.002), adjusted for covariates listed above; PI use and biological parent as PCG were significant (p = 0.002 and p = 0.003, respectively), and PCG high school graduate and length of follow-up were marginally significant (p = 0.08 and p = 0.07, respectively) in this model.
PCG, primary caregiver; SGA, second-generation antipsychotics; PI, protease inhibitor.
Long-term change in BMI z score
Of the 74 potentially eligible participants, 51 participants with SGA treatment history were included in the primary long-term analysis. The total sample size, including 147 controls, for the primary long-term analysis was 198. The sample size for the final model was 184 due to missing observations in some of the covariates. The baseline characteristics of participants included in the long-term change analysis (not shown) were very similar to those in the short-term analysis, including the significantly higher representation of participants with SGA prescriptions who were also prescribed stimulants (p < 0.001) and those with at least one neurologic or psychiatric diagnosis (p = 0.001). The risperidone long-term analysis is based on 34 patients who were first prescribed risperidone and their 98 matching controls, for a total sample size of 132. The sample size for the final model was 122 due to missing observations in some of the covariates.
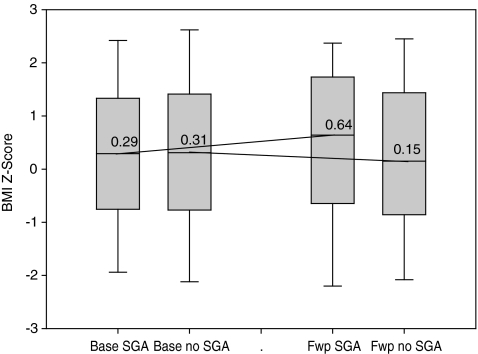
The average duration of the long-term follow-up was 21.9 months (SD = 3.6) among the participants with SGA prescriptions, and 22.0 months (SD = 4.0) among those without SGA prescriptions (p = 0.94). The average long-term change in BMI z score was 0.302 (SD = 0.668) among the participants with SGA prescriptions, and −0.024 (SD = 0.302) among the participants without SGA prescriptions (p = 0.001). Figure 2 summarizes the distribution of BMI z-scores at baseline and follow-up measurements, showing trends in long-term growth with respect to SGA treatment.
FIG. 2.
Change in body mass index (BMI) z-scores from baseline to long-term follow-up (approximately 2 years) by second-generation antipsychotics (SGA) exposure (n = 236). Labels on x-axis: Base SGA, baseline measurement in subjects exposed to SGA; Base no SGA, baseline measurement in subject unexposed to SGA; Fwp SGA, follow-up measurement in subjects exposed to SGA; Fwp no SGA, follow-up measurement in subjects unexposed to SGA.
In the final models (Table 4), positive long-term BMI z-score changes were independently predicted by prescribed SGA treatment and by risperidone as a first-prescribed SGA. This finding was associated with SGA class (slope = 0.350, p < 0.001), and risperidone alone (slope = 0.360, p = 0.001). Concomitantly prescribed stimulant medications were not significantly predictive of the long-term changes in BMI z score for either SGA or risperidone alone (p = 0.22 and 0.38, respectively). Supplementary modeling that investigated a potential interaction effect showed the presence of a significant interaction between SGAs and PIs (slope = 0.323, p = 0.03) and between risperidone and PIs (slope = 0.577, p = 0.005) in predicting change in BMI z scores.
Table 4.
Multiple Regression of Change in Long-term Body Mass Index z Score on Second-Generation Antipsychotics Adjusted for Selected Covariates (n = 184)
| Predictor | Slope | 95% LCI | 95% UCI | p value |
|---|---|---|---|---|
| Intercept | −0.195 | −0.562 | 0.172 | 0.30 |
| SGA history | 0.350 | 0.166 | 0.533 | < 0.001 |
| CDC class C | 0.079 | −0.037 | 0.195 | 0.18 |
| Stimulant use | −0.101 | −0.262 | 0.060 | 0.22 |
| PI use | 0.127 | 0.005 | 0.248 | 0.04 |
| Biological parent PCG | −0.124 | −0.243 | −0.006 | 0.04 |
| PCG high school graduate | 0.129 | 0.010 | 0.249 | 0.03 |
| Participated in sports | 0.104 | −0.019 | 0.227 | 0.10 |
| Length of follow-up | −0.005 | −0.020 | 0.010 | 0.52 |
Note: Risperidone was significant in multiple regression model of long-term change in BMI z score on Risperidone (p = 0.001), adjusted for covariates listed above; CDC Class C, biological parent PCG and PCG high school graduate were significant in this model.
PCG, primary caregiver; SGA, second-generation antipsychotic; CDC, Centers for Disease Control and Prevention; PI, protease inhibitor; BMI, body mass index.
Discussion
In this study, we evaluated the relationship between prescribed SGA treatment and short- and long-term changes in BMI z scores relative to a pretreatment baseline in children and adolescents with perinatally acquired HIV infection from the PACTG 219c cohort. Sociodemographic characteristics of the cohort were representative of the US children and adolescents with perinatal HIV infection. We found that prescribed SGA treatment in general and risperidone specifically, were associated with significant increase in both short- and long-term changes in BMI z scores. The increase in the adjusted mean change in BMI z score (slope) of 0.192 over an average follow-up period of approximately 6 months in risperidone-treated participants was particularly prominent. Since this adjusted score already accounts for confounding factors associated with change in BMI z scores (i.e., race, stimulant use, PI use, PCG, education level of PCG, and length of follow-up), this finding suggests that the increase is likely attributable to prescription of risperidone alone.
HIV disease severity indicators were equally distributed between both groups, suggesting the findings were not significantly confounded by the severity of physical symptoms. Because the participants were matched on pretreatment baseline Tanner stages and BMI z scores, the growth observed in the SGA-prescribed group cannot be attributed to normal development alone, suggesting excessive growth independently associated with prescribed SGA treatment in general and specifically with risperidone treatment. Thus, by controlling for age, gender, and general health and demographic factors, our findings add further credibility to the reports of treatment-emergent excessive weight gain in non-HIV–infected pediatric populations treated with risperidone or olanzapine.20
In the clinical setting, the findings of this study should be interpreted cautiously and within the broader context of the child's specific HAART regimen, their nutritional needs, and current and evolving health status. For example, would selected children with HIV who are significantly undernourished, should they develop a psychiatric disorder where an antipsychotic is indicated, actually benefit from the SGA-associated weight gain? Furthermore, the potential for treatment-associated adverse effects of SGAs should always be weighed against the risks of untreated psychiatric disorders. Realistically, there will be clinical situations where an antipsychotic (SGA or other) is a standard of care with no real alternative, such as childhood schizophrenia. There will also be situations where an SGA is indicated, and, due to limited resources, no other alternative is available. In such situations, clinicians should carefully proceed with prescribing the available antipsychotic, while the child's metabolic status is being monitored routinely, and communication between child psychiatrists, pediatric HIV specialist, and primary care provider tightly integrated into the child's clinical care. In most other cases, however, evidence-based treatments with greater safety records in pediatric populations should be considered;, for example, conventional antipsychotic pimozide or α2- agonist clonidine in the treatment of tic disorders, lithium carbonate in the treatment of bipolar disorder, or applied behavioral analysis for disruptive behaviors in children with autism.20
Due to the low number of participants treated with conventional antipsychotics, we were unable to examine the differential association with changes in BMI z-scores between the two classes of antipsychotics. Thus, it would be premature to recommend preferential prescribing of the conventional antipsychotics, especially because the latter class of antipsychotics has its own safety risks, such as extrapyramidal symptoms and hyperprolactinemia.20
The main effect of PI use was significant in all final models (except for the long-term model for risperidone, which may be a function of small sample size) and is consistent with the findings in other studies.35,36 However, the presence of a significant interaction between PI use and prescribed SGA/risperidone that was found in investigative modeling of both the short-term and long-term models provides some evidence that the effect of simultaneous SGA and PI exposure on change in BMI z score may be stronger than the sum of the individual effects of these two classes of drugs. This enhanced effect of the two medication classes, with known metabolic abnormalities in pediatric populations, might be due to CYP 450 interaction between the PIs and SGAs, particularly risperidone, which is metabolized primarily through the CYP 450 2D6, an enzyme blocked by the PIs indinavir and ritonavir. Such interaction may have led to significantly increased peripheral blood levels of, risperidone, resulting in its increased impact on growth. Adult case reports also suggest that these drug-drug interactions may enhance other adverse events and toxicities, potentially resulting in medical emergencies, such as reversible coma and extrapyramidal symptoms.37,38 This study was not powered for effects in an interaction model, however, and the finding warrants further investigation, preferably in pediatric pharmacokinetic studies of specific ARV medications and SGAs as well as the potential for drug–drug interactions. Interestingly, the increases in BMI z score were relatively more pronounced for risperidone alone than for SGAs as a class. One possible explanation of this finding is that the weight-promoting effect is specific to individual antipsychotic drugs, rather than the entire class. Indeed, 20% of the participants with SGA prescriptions in the short-term analysis and 22% in the long-term analysis had prescriptions for aripiprazole or ziprazidone, which might be weight-neutral, or for quetiapine, which has limited and somewhat inconsistent data regarding weight gain in children and adolescents.20,26 Furthermore, the gap between the weight-promoting effects of risperidone versus SGAs as a class was particularly prominent in the short-term analysis, where the method of evaluating the change in BMI z scores was more rigorous (in that participants were required to have stayed on their initial SGA medication) than in the long-term analysis. Thus, the presence of aripiprazole, ziprazidone, and quetiapine in the analyses of SGAs as a class could have offset the weight gain associated with risperidone and olanzapine. Another possible explanation is that risperidone was dosed comparably higher than the other SGAs evaluated in the present study (either via prescribed high doses or possibly inadvertently, via CYP 450 interaction with the PIs). We could not evaluate the latter two possibilities because of the small individual numbers of participants treated with SGAs other than risperidone and because P219C did not collect dosing information on non-ARV medications. Finally, while the short-term length of follow-up approximately reflects the time the patient was prescribed the first SGA, this was not necessarily the case in the long-term analysis; thus, the corresponding model reflects long-term impact of the first medication regardless of how long the patient was on that medication.
This study has several limitations. First, SGA treatment was not randomly assigned. Thus, one could speculate, for example, that weight gain in children on SGAs was secondarily aggravated by behavioral factors leading to increased food intake in psychiatrically impaired children. It seems unlikely that this would be a plausible sole explanation for the observed difference, given its magnitude, but a randomized controlled trial would have minimized its possible confounding effect. Second, P219C did not measure adherence to medications other than ARVs, so we do not know to what extent the prescribed SGAs were actually taken (as prescribed). Given the direction of the observed associations, one might assume that, with perfect SGA adherence, the increases in BMI z scores in SGA-exposed youth in this study would have been even higher, not lower. Still, because of this limitation, we can only attribute the observed association to the prescription of SGAs and refrain from attributing it to actual SGA exposure. Future studies of this type should monitor adherence to concomitant non-ARV medications. Finally, the study was not designed to examine the relationship between stimulant use and weight gain as the primary goal. As a result, our choice of cohorts did not allow for optimal study of stimulants (both in terms of maximizing sample size as well as choice of periods of observation). Thus, we were only able to look at stimulant use as a potential confounder in our exploration of association between SGAs and weight gain, but cannot claim that coadministration of stimulants does not have impact on the SGA-associated growth changes.
Conclusion
This extensive but selective review of data from the PACTG 219C long term outcomes study has demonstrated a strong positive association between prescribed SGA treatment, particularly risperidone, and both short- and long-term changes in BMI z scores in children and adolescents with perinatally acquired HIV infection. Clinicians treating children with perinatally acquired HIV infection and comorbid psychiatric disorders requiring SGA treatment should routinely monitor growth and metabolic parameters utilizing a careful history and physical, standardized height and weight assessments, and treatment-directed laboratory studies, and maintain communication with other prescribers involved in the child's clinical care.
Treatment-associated adverse effects should be weighed against the risks of untreated psychiatric disorders. When possible, alternative evidence-based treatments should be considered. Pharmacokinetic studies should be designed to examine the presence of significant drug–drug interactions in children with perinatally acquired HIV infection that are simultaneously treated with HAART and SGAs. This will be particularly important as children and adolescents receive both HAART for their primary HIV infection and other medications for the complications seen in this difficult and increasingly complex disease.
Acknowledgments
The following institutions and individuals participated in the PACTG Protocol 219C, in order of enrollment: Baylor Texas Children's Hospital: F. Minglana, M.E. Paul, and C.D. Jackson; University of Florida, Jacksonville: M.H. Rathore, A. Khayat, K. Champion, and S. Cusic; Chicago Children's Memorial Hospital: R. Yogev and E. Chadwick; University of Puerto Rico, University Children's Hospital AIDS Program: I. Febo-Rodriguez and S. Nieves; Bronx Lebanon Hospital Center: M. Purswani, S. Baksi, E. Stuard, and M. Dummit; San Juan Hospital: M. Acevedo, M. Gonzalez, L. Fabregas, and M.E. Texidor; University of Miami: G.B. Scott, C.D. Mitchell, L. Taybo, and S. Willumsen; University of Medicine and Dentistry of New Jersey: L. Bettica, J. Amour, B. Dashefsky, and J. Oleske; Charity Hospital of New Orleans and Earl K. Long Early Intervention Clinic: M. Silio, T. Alchediak, C. Boe, and M. Cowie; University of California San Diego Mother, Child, and Adolescent HIV Program: S.A. Spector, R. Viani, M. Caffery, and L. Proctor; Howard University: S. Rana, D. Darbari, J.C. Roa, and P.H. Yu; Jacobi Medical Center: M. Donovan, R. Serrano, M. Burey, and R. Auguste; St Christopher's Hospital for Children, Philadelphia: J. Chen and J. Foster; Baystate Medical Center Children's Hospital: B.W. Stechenberg, D.J. Fisher, A.M. Johnston, and M. Toye; Los Angeles County Medical Center/University of Southern California: J. Homans, M. Neely, L.S. Spencer, and A. Kovacs; Children's Hospital Boston: S. Burchett and N. Karthas; Children's Hospital of Michigan: E. Moore and C. Cromer; St Jude Children's Research Hospital, Memphis: P.M. Flynn, N. Patel, M. Donohoe, and S. Jones; New York University School of Medicine/Bellevue Hospital: W. Borkowsky, S. Chandwani, N. Deygoo, and S. Akleh; Children's Hospital at Downstate: E. Handelsman, H.J. Moallem, D.M. Swindell, and J.M. Kaye; Columbia Presbyterian Medical Center and Cornell University New York Presbyterian Hospital: A. Higgins, M. Foca, P. LaRussa, and A. Gershon; Children's Hospital of Philadelphia: R.M. Rutstein, C.A. Vincent, S.D. Douglas, and G.A. Koutsoubis; Children's Hospital of Oakland: A. Petru and T. Courville; University of California San Francisco, Moffitt Hospital: D. Wara and D. Trevithick; Children's Hospital, University of Colorado, Denver: E. McFarland and C. Salbenblatt; Johns Hopkins University Pediatrics: N. Hutton, B. Griffith, M. Joyner, and C. Kiefner; Children's Hospital and Regional Medical Center, Washington: M. Acker, R. Croteau, C. McLellan, and K. Mohan; Metropolitan Hospital Center: M. Bamji, I. Pathak, S. Manwani, and E. Patel; Children's National Medical Center: H. Spiegel and V. Amos; University of Massachusetts Medical School: K. Luzuriaga and A. Sharples; University of Alabama at Birmingham: R. Pass and M. Crain; University of Maryland Medical Center: J. Farley and K. Klipner; Schneider Children's Hospital: V.R. Bonagura, S.J. Schuval, C. Colter, and L. Campbell; Boston Medical Center: S.I. Pelton and A.M. Reagan; University of Illinois: K.C. Rich, K. Hayani, and M. Bicchinella; State University of New York Stony Brook: S. Nachman, D. Ferraro, and S. Madjar; North Broward Hospital District: A. Puga; Duke University: F. Wiley, K. Whitfield, O. Johnson, and R. Dizney; Harlem Hospital: S. Champion, M. Frere, M. DiGrado, and E.J. Abrams; Cook County Hospital: J. Martinez; University of South Alabama: M. Mancao; Connecticut Children's Medical Center: J. Salazar and G. Karas; University of North Carolina at Chapel Hill: T. Belho, B. Pitkin, and J. Eddleman; Ruiz Arnau University Hospital: W. Figueroa and E. Reyes; State University of New York Upstate Medical University: L.B. Weiner, K.A. Contello, W.A. Holz, and M.J. Famiglietti; Children's Medical Center of Dallas; University of Florida at Gainesville: R. Lawrence, J. Lew, C. Delany, and C. Duff; Children's Hospital at Albany Medical Center: A.D. Fernandez, P.A. Hughes, N. Wade, and M.E. Adams; Lincoln Medical and Mental Health Center and Phoenix Children's Hospital: J.P. Piatt, J. Foti, and L. Clarke-Steffen; Public Health Unit of Palm Beach County: J. Sleasman and C. Delaney; Medical College of Georgia: C.S. Mani; Yale University School of Medicine: W.A. Andiman, S. Romano, L. Hurst, and J. de Jesus; Vanderbilt University Medical Center: G. Wilson; University of Rochester Medical Center: G.A. Weinberg, F. Gigliotti, B. Murante, and S. Laverty; St. Joseph's Hospital and Medical Center, New Jersey: N. Hutchcon and A. Townley; Emory University Hospital: S. Nesheim and R. Dennis; University of South Florida: P. Emmanuel, J. Lujan-Zilberman, C. Graisberry, and S. Moore; Children's Hospital of the King's Daughters: R.G. Fisher, K.M. Cunnion, T.T. Rubio, and D. Sandifer; Medical University of South Carolina: G.M. Johnson; University of Mississippi Medical Center: H. Gay and S. Sadler; Harbor-University of California Los Angeles Medical Center: M. Keller, J. Hayes, A. Gagajena, and C. Mink; Mount Sinai Medical Center: D. Johnson; Children's Hospital of Los Angeles: J. Church, T. Dunaway, and C. Salata; Long Beach Memorial: A. Deveikis and L. Melton; Robert Wood Johnson Medical School: S. Gaur, P. Whitley-Williams, A. Malhotra, and L. Cerracchio; Sinai Children's Hospital: M. Dolan, J. D'Agostino, and R. Posada; Medical Center, Pediatric Columbus, Georgia: C. Mani and S. Cobb; Medical College of Virginia: S.R. Lavoie and T.Y. Smith; Cooper Hospital-University Medical Center: A. Feingold and S. Burrows-Clark; University of Cincinnati: J. Mrus and R. Beiting; Columbus Children's Hospital: M. Brady, J. Hunkler, and K. Koranyi; Sacred Heart Children's Medical Services of Florida: W. Albritton; St Luke's/Roosevelt Hospital Center: R. Warford and S. Arpadi; Incarnation Children's Center, New York: A. Gershon and P. Miller; Montefiore Medical-Albert Einstein College of Medicine: A. Rubinstein and G. Krienik; Children's Hospital of Los Angeles: A. Kovacs and E. Operskalski; San Francisco General Hospital: D. Wara, A. Kamrin, and S. Farrales; Cornell University New York Presbyterian: R. Johan-Liang and K. O'Keefe; St. Louis Children's Hospital: K.A. McGann, L. Pickering, and G.A. Storch; North Shore University Hospital: S. Pahwa and L. Rodriquez; and Oregon Health and Science University: P. Lewis and R. Croteau.
We are grateful for the contributions of Joyce Kraimer, Barbara Heckman, Shirley Traite, and Nathan Tryon. We also thank the children and families for their participation in PACTG 219C and the individuals and institutions involved in the conduct of 219C.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Monasch R. Mahy M. Young people: The centre of the HIV epidemic. World Health Organ Tech Rep Ser. 2006;938:15–41. ; discussion 317–341. [PubMed] [Google Scholar]
- 2.Saharan S. Lodha R. Agarwal R. Deorari AK. Paul VK. Perinatal HIV. Indian J Pediatr. 2008;75:359–362. doi: 10.1007/s12098-008-0039-0. [DOI] [PubMed] [Google Scholar]
- 3.Lee GM. Gortmaker SL. McIntosh K. Hughes MD. Oleske JM. Quality of life for children and adolescents: Impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117:273–283. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 4.Storm DS. Boland MG. Gortmaker SL, et al. Protease inhibitor combination therapy, severity of illness, and quality of life among children with perinatally acquired HIV-1 infection. Pediatrics. 2005;109:e61. doi: 10.1542/peds.2004-1693. [DOI] [PubMed] [Google Scholar]
- 5.Butler A. Williams P. Howland L. Hutton A. Storm D. Seage GR., III Disclosure of HIV infection status and health-related quality of life in children with HIV infection. Pediatrics. 2009;123:935–943. doi: 10.1542/peds.2008-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf. [Feb 22;2009 ]; July 29, 2008; pp. 1–134.
- 7.Blanche S. Tardieu M. Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 8.Venerosi A. Calamandrei G. Alleva E. Animal models of anti-HIV drugs exposure during pregnancy: Effects on neurobehavioral development. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:747–761. doi: 10.1016/s0278-5846(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 9.Mekmullica J. Brouwers P. Charurat M, et al. Early immunological predictors of neurodevelopmental outcomes in HIV-infected children. Clin Infect Dis. 2009;48:338–346. doi: 10.1086/595885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs A. Early immune activation predicts central nervous system disease in HIV-infected infants: Implications for early treatment. Clin Infect Dis. 2009;48:347–349. doi: 10.1086/595886. [DOI] [PubMed] [Google Scholar]
- 11.Forstein M. Cournos F. Douaihy A. Goodkin K. Wainberg ML. Wapenyi KH. Guideline Watch: Practice Guideline for the Treatment of Patients with HIV/ AIDS. Arlington, VA: American Psychiatric Association; 2006. [Google Scholar]
- 12.Donenberg GR. Pao M. Youth and HIV/AIDS: Psychiatry's role in a changing epidemic. J Am Acad Child Adolesc Psychiatry. 2005;44:728–747. doi: 10.1097/01.chi.0000166381.68392.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellins CA. Smith R. O'Driscoll MS, et al. NIH/HIAID/NICHD/NIDA-Sponsored Women and Infant Transmission Study Group. High rates of behavioral problems in perinatally HIV-infected children are not linked to HIV disease. Pediatrics. 2003;111:384–393. doi: 10.1542/peds.111.2.384. [DOI] [PubMed] [Google Scholar]
- 14.Nozyce ML. Lee SS. Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 15.Malee K. Williams P. Montepiedra G, et al. The role of cognitive functioning in medication adherence of children and adolescents with HIV infection. J Pediatr Psychol. 2009;34:164–175. doi: 10.1093/jpepsy/jsn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaughan DM. Hughes MD. Oleske JM, et al. Psychiatric hospitalizations among children and youth with HIV infection. Pediatrics. 2004;113:e544–551. doi: 10.1542/peds.113.6.e544. [DOI] [PubMed] [Google Scholar]
- 17.Chernoff M.Nachman S.Williams PL, et al. Mental health interventions in HIV-Infected and uninfected children and adolescents enrolled in PACTG P1055 Pediatrics 2009124627–636.19596734 [Google Scholar]
- 18.Martinez J. Hosek SG. Carleton RA. Screening and assessing violence and mental health disorders in a cohort of inner city HIV-positive youth between 1998–2006. AIDS Patient Care STDs. 2009;23:469–475. doi: 10.1089/apc.2008.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellins CA. Brackis-Cott E. Leu CS, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry. 2009;50:1131–1138. doi: 10.1111/j.1469-7610.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapetanovic S. Simpson GM. Review of antipsychotics in children and adolescents. Expert Opin Pharmacother. 2006;7:1871–1885. doi: 10.1517/14656566.7.14.1871. [DOI] [PubMed] [Google Scholar]
- 21.Patel NC. Crismon ML. Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44:548–556. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- 22.Olfson M. Blanco C. Liu L. Moreno C. Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration: FDA approves the first drug to treat irritability associated with autism, Risperdal. FDA News October 6, 2006. www.fda.gov/bbs/topics/news/2006/new01485.html. [Jul 13;2009 ];
- 24.U.S. Food and Drug Administration: FDA approves Risperdal for two psychiatric conditions in children and adolescents. FDA News August 22, 2007. www.fda.gov/bbs/topics/news/2007/NEW01686.html. [Jul 13;2009 ];
- 25.Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7:268–274. doi: 10.4088/pcc.v07n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: A systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry. 2007;46:687–700. doi: 10.1097/chi.0b013e318040b25f. [DOI] [PubMed] [Google Scholar]
- 27.Fraguas D. Merchán-Naranjo J. Laita P, et al. Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. J Clin Psychiatry. 2008;69:1166–1175. doi: 10.4088/jcp.v69n0717. [DOI] [PubMed] [Google Scholar]
- 28.Bell LM. Byrne S. Thompson A, et al. Increasing body mass index z score is continuously associated with complications of overweight in children, even in the healthy weight range. J Clin Endocrinol Metab. 2007;92:517–222. doi: 10.1210/jc.2006-1714. [DOI] [PubMed] [Google Scholar]
- 29.Foster C. Judd A. Tookey P, et al. Young people in the United Kingdom and Ireland with perinatally acquired HIV: The pediatric legacy for adult services. AIDS Patient Care STDs March. 2009;23:159–166. doi: 10.1089/apc.2008.0153. [DOI] [PubMed] [Google Scholar]
- 30.Kabue MM. Kekitiinwa A. Maganda A. Risser JM. Chan W. Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDs. 2008;22:245–251. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 31.Tassiopoulos K. Williams PL. Seage GR., 3rd Crain M. Oleske J. Farley J. International Maternal Pediatric Adolescent AIDS Clinical Trials 219C Team. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–614. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Z-score data files. 2000. www.cdc.gov/nchs/about/major/nhanes/growthcharts/zscore/zscore.htm. [Jul 13;2009 ];
- 33.Correll CU. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- 34.Sirois PA. Montepiedra G. Kapetanovic S, et al. Impact of Medications Prescribed for Treatment of Attention Deficit-Hyperactivity Disorder on Physical Growth in Children and Adolescents with HIV. J Dev Behav Pediatr. (in press). [DOI] [PMC free article] [PubMed]
- 35.Buchacz K. Cervia JS. Lindsey JC, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108:E72. doi: 10.1542/peds.108.4.e72. [DOI] [PubMed] [Google Scholar]
- 36.Miller TL. Mawn BE. Orav EJ. Wilk D, et al. The effect of protease inhibitor therapy on growth and body composition in human immunodeficiency virus type 1-infected children. Pediatrics. 2001;107:E77. doi: 10.1542/peds.107.5.e77. [DOI] [PubMed] [Google Scholar]
- 37.Jover F. Cuadrado JM. Andreu L. Merino J. Reversible coma caused by risperidone-ritonavir interaction. Clin Neuropharmacol. 2002;25:251–253. doi: 10.1097/00002826-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DV. Béïque LC. Bowmer MI. Extrapyramidal symptoms with ritonavir/indinavir plus risperidone. Ann Pharmacother. 2002;36:827–830. doi: 10.1345/aph.1A335. [DOI] [PubMed] [Google Scholar]